零价铁/无定形磷酸钙复合物的合成及其对CdTe量子点吸附性能
2016-11-28金丽那丽华刘芳同龚丽莹娄大伟张建坡
金丽 那丽华 刘芳同 龚丽莹 娄大伟 张建坡*,
(1吉林化工学院化学与制药工程学院,吉林132022)
(2中国科学院长春应用化学研究所合成橡胶重点实验室,长春130022)
零价铁/无定形磷酸钙复合物的合成及其对CdTe量子点吸附性能
金丽1那丽华2刘芳同1龚丽莹1娄大伟1张建坡*,1
(1吉林化工学院化学与制药工程学院,吉林132022)
(2中国科学院长春应用化学研究所合成橡胶重点实验室,长春130022)
在氮气保护下利用共沉淀方法成功地合成了零价铁无定形磷酸钙复合物(Fe0/ACP复合物),并采用XRD、EDAX和FTIR对产物进行了表征。同时通过SEM和TEM分析可知所合成材料的粒径为300 nm左右。磁滞回线表明在磁场中可以将该复合物从非磁性材料中分离出来。氮气吸附脱附曲线表明所合成材料具有吸附性能。此外所合成Fe0/ACP复合物被用来吸附CdTe量子点,并采用二级动力学方程对吸附过程进行了分析。吸附产物采用XRD、FTIR、荧光和磁滞回线进行了表征,结果表明该吸附产物不仅具有磁性,而且具有很好的荧光性质。
无定形磷酸钙;零价铁;沉淀法;CdTe量子点
Amorphous calcium phosphate(in short,ACP) stored in natural bone is an important form of calcium phosphate[1],which consists of non-crystalline calcium phosphate with initial Ca/P atomic ratio of 1.5 [Ca9(PO4)6][2].ACP with amorphous and porous structure has widely applied as effective absorber[3-4],catalyst and packing agent[5],or component in hydraulic bone cements[6].Also owing to its excellent bioactivity and high biodegradation[7]ACP has also widely used as drug loading and controlled release[8-11],or nucleic acid into living cells[12].
ACP is first recognized by Posner in the synthesis of hydroxyapatite and recognized as intermediate material[13].The amorphous phase of ACP can keep stable in the organism or in dryness and dehydration condition,but it transforms into hydroxyapatite in wet condition[14].The lifetime of ACP in aqueous solution is usually determined by a variety of factors such as the additive,temperature,pH value,and ionic strength[15]. Many attempts have been explored to synthesize the amorphous phase of ACP in aqueous solution, including adding inorganic substance(such as Mg2+, P2O74-,CO32-and F-,ZrOCl2and Si)[16],polyethylene glycol[17],adenosine triphosphate(ATP)[18],magnesium ions[19],and poly(ethylene glycol)-block-polylactide (PEG-PLA)[20].Especially,a large number of existing literatures have proven that ACP plays a crucial role in the precipitation of calcium phosphate in a neutral solution with biomolecules,such as proteins,peptides, enzyme or amino acids[21].These biomolecules-nanocomposites have been engineered to form nanoparticles in the particles size distribution of 20~300 nm[22-23].
Furthermore the investigation on magnetically targeted drug-delivery systems has become a hot research topic.Magnetic iron oxides(MIOs),such as Fe3O4and γ-Fe2O3,modified by polymers[24-27],silica[28-29], calcium silicate[30-31],and calcium phosphate[32-34]have been investigated to apply in targeted drug delivery. Note,liu[35]has prepared HMIOs/drug/ACP drugdelivery system,which exhibited superparamagnetism and pH-responsive drug-release behavior.However, the development of zero-valent iron(in short,Fe0) modified by amorphous calcium phosphate(ACP) using precipitation method is still scarce.
In this article,a co-precipitation method was proposed to synthesis zero-valent iron/amorphous calcium phosphate composites(in short,Fe0/ACP composites).Here sodium borohydride was added as a bifunctional agent:both as the precursor of the precipitation agent to make the formation of the ACP only from the calcium chloride and calcium dihydrogen phosphate,and as the reducing agent to make the formation of Fe0from the ferrous chloride (excessive sodium borohydride was added to ensure that divalent iron were completely transferred into zero valent iron)[36].Interestingly,we found that asprepared Fe0/ACP composites could adsorb 3-Mercaptopropyl acid(MPA)-stabilized CdTe quantum dots on its surface(designated as Fe0/ACP/CdTe composites,in short,FAC)(Scheme 1).The FAC with tuning spectrum could be obtained using different emission wavelength of CdTe quantum dots.FAC was also a magnetic material that could be separated from the nonmagnetic materials in a magnetic field.These magnetic-fluorescent composites are the integration of fluorophores and magnetic particles in amorphous calcium phosphate,which can be detected/imaged using multiple modalities,such as fluorescence,MRI, X-ray and ultrasound.It has all of the characteristics of the CdTe quantum dots,Fe0and ACP embedded, and also integrated most of their applications.The combination of magnetic and fluorescent properties is a new powerful tool,allowing manipulation by magnetic fields and visualization/detection by fluorescence,which could affect protein and DNAseparation and detection,bio-imaging and sorting in vitro and in vivo,drug delivery and therapy tools for cancer treatment.Thus,the combination of magnetic and fluorescent properties in amorphous calcium phosphate would open up great prospects both in nano-and bio-technology.And there is great demand for developing high-resolution,target-specific particles with real-time multimodal-imaging capabilities[37].Also, FAC with the outermost amorphous calcium phosphate have the properties of porous structure,excellent bioactivity and high biodegradation,and could be used as drug loading and controlled release.It is expected that the as-prepared Fe0/ACP composites and adsorption product(FAC)are promising for the applications in various fractionation-adsorption and imaging fields.
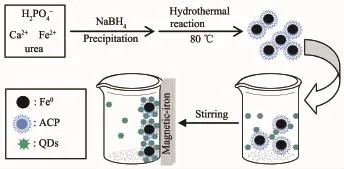
Scheme 1 Schematic illustration of the preparation of Fe0/ACP composites and its absorption of CdTe quantum dots(QDs)
1 Materials and general methods
1.1Reagents and materials
All chemicals used were as purchased without purification.3-Mercaptopropyl acid(HOOCCH2CH2SH, MPA,99+%),tellurium powder(Te,~200 mesh, 99.8%),CdCl2(99+%),NaBH4(99%)were from Aldrich Chemical Co.Water used throughout was doubly distilled water(>18 MΩ·cm).
1.2Instrumentation and spectrometry
Powder X-ray diffraction(PXRD)patterns were collected on a DX-2700 X-ray diffractometer with graphite monochromatized Cu Kα radiation(λ=0.154 nm,U=35 kV,I=30 mA)and 2θ ranging from 10°to 90°with an increment of 0.02°and a scanning rate of 5°·min-1.Brunauer-Emmett-Teller(BET)specific surface area and pore size distribution were measured with TriStar II 3020 surface area and pore size analyzer.Scanning electron microscopy(SEM) micrographs were obtained with a ZEISS fieldemission scanning electron microscope(10 kV).The transmission electron micrographs were recorded with a Tecnai G220 electron microscope.EDAX were recorded with a JSM-6490LV electron microscope. The FTIR spectra were recorded using KBr pellets in the range 4 000~400 cm-1on a NICOLET6700 spectrometer.Fluorescence spectra were measured on a F-280 spectrofluorophotometer equipped with a xenon lamp and quartz carrier at room temperature with excitation and emission slit at 2.0 nm.The fluorescence photograph were recorded using Nikon D7000. Hysteresis loops of the products were measured at room temperature by a Lake Shore 7410 vibrating sample magnetometer.
1.3Synthesis of aqueous-compatible CdTe quantum dots
CdTe presoma was prepared as described in previous papers[38-39].In brief,freshly prepared NaHTe solution,produced by reaction of NaBH4solution with Te powder at a molar ratio of 2∶1,was added to nitrogen-saturated 1.25×10-3mol·L-1CdCl2aqueous solution at pH=11.4 in the presence of MPA as a stabilizing agent.The solutions were stirred for another 20 min and stored at 0~4℃.The resulting mixture was then subjected to refluxing to control the size of the CdTe nanocrystals.Finally,QDs of different sized were synthesiszed under different refluxing conditions separately.
1.4Ppreparation of Fe0/ACP composites
0.5001 g FeCl2,0.399 8 g CaCl2·2H2O,0.607 1 g Na2HPO4·12H2O,and 1 g[CO(NH2)2]were dissolved in 60 mL deaerated water,the pH value was adjusted to about 3.1 using 1 mol·L-1HNO3(aq).The solution was purged with nitrogen to prevent the oxidation of zero valent iron,after 30 min of purging,0.6 g NaBH4(dissolved in 20 mL water)was added into the solution drop by drop to initiate the reaction.Upon delivering all of the NaBH4solution,the suspended precipitation was stirred for another 0.5 h.The resulting mixture was then transferred to a teflonlined stainless steel autoclave and heated at 80℃for 3 h. The gray product was collected with a magnet,washed with deionized water several times,and dried at 60℃.
2 Results and discussion
2.1Characterization of composites
As mention above,ACP as a transient phase can be stabilized in aqueous solution by using appropriate additive molecules and ions to prevent thetransformation from ACP to HAP.In this paper,coprecipitation method was used to obtain Fe0/ACP composites,whose results indicated that ACP can be stabilized in aqueous solution by using Fe0molecules. The co-precipitation process can be briefly described as below,with the addition of sodium hydroxide,a large number of Ca3(PO4)2and Fe3(PO4)2white precipitate were firstly observed,then most of Fe3(PO4)2changed to zero valent iron as excessive sodium borohydride was added,and the color of solution changes from white to black.Due to the existence of large number quantity of PO43-,FePO remain in the surface of zero valent iron,then both zero valent iron and FePO were covered by ACP under suitable condition,these were certified by XPS,as shown in Fig.1.When compared with XPS analysis of FePO and Fe0in literature[40],the binding energy of Fe2p corresponded to that of FePO and Fe0were shifted to higher field direction.This means that the corresponding electron cloud density decreases,and interaction between ACP and FePO(FePO and Fe0) occurs.Also XPS analysis of P2p shows similar result.
When compared with the standard data(JCPDS No.09-0432),the characteristic diffraction peaks of Fe0/ACP composites could be indexed to ACP with diffraction peaks at 2θ=30°(Fig.2a),and no other phases could be detected,this due to the doping structure of Fe0/ACP composites and the larger proportion of ACP in the surface of the final products. Fe0synthesized without ACP on the same experimental condition was used as a control.The diffraction peaks of Fe0at about 2θ=45°is detected obviously,which suggest the formation of zero-valent iron resulted from the reduction of iron ions by sodium borohydride. Furthermore,calcium phosphate precipitates synthesized without Fe0on the same experimental condition was also used as a control,whose diffraction peaks are at 2θ=25.8°,31.7°,39.6°,46.7°,49.6°and 53.2°, assigned to poor crystalline hydroxyapatite(JCPDS 9-432).This implied that doped Fe0can effectively hinder the movement and collision of calcium/ phosphate ions,and slow the crystallization rate of calcium phosphate,resulting in the formation of ACP. Free calcium ions and phosphate ions can collide witheach other rapidly without doped Fe0,and result in the formation of hydroxyapatite.These results verified the previous assumption above.EDXA of the Fe0/ACP composites indicated the existence of Ca,P,O and Fe,confirming the detectable levels of dopant ions within the product(Fig.2b).Chemical bonding in the synthesized composites was analyzed by FTIR spectra (Fig.2c).The band at 3 417 cm-1of O-H stret-ching vibration,at 1 398 cm-1of H2O still appears after drying indicates that is not adsorbed on the surface, but constitutional water.There are two obviously broad absorption peaks,which are at 1 040 cm-1of the triply degenerate asymmetric stretching mode vibration(ν3)of phosphate groups,and at 571 cm-1of bending mode(ν4)of the P-O bond(a single peak manner indicates the out-layer is amorphous phase). These non-split peaks result from the isotropic structure of amorphous materials and are the characteristics of ACP[17].

Fig.1 Narrow XPS spectra scan of Fe2p(a)and P2p(b)of the Fe0/ACP composites

Fig.2 XRD patterns of Fe0/ACP composites,Fe0and HA(a),EDAX(b)and FTIR spectra(c)of the Fe0/ACP composites
As the basic structural unit of ACP is a roughly spherical cluster of ions,and there is no preferential growth direction,the resulting Fe0/ACP composites morphology is spherical,which are consistent with conclusion of SEM and TEM images of Fe0/ACP composites,as shown in Fig.3.And all of Fe0magnetic composites are well coated by the ACP,the Fe0/ACP composites′shells cluster together during drying,and the core-shell structure of Fe0/ACP composites with an average size of 300 nm.The thickness of ACP shell in the doped structure is about 14 nm and can be increased by increasing the concentrations of calcium chloride and sodium dihydrogen phosphate used during the synthesis.The HRTEM image of zero-valent iron(Fig.3c)shows a crystalline lattice plane with an interplaner distance of 0.202 nm which corresponds to the(110)diffraction plane of iron,this crystalline lattice plane is also observed in zero-valent iron.

Fig.3 SEM(a)and TEM(b,c)images of Fe0/ACP composites

Fig.4 Hysteresis loops(a)and Nitrogen adsorption-desorption isotherm(b)of the Fe0/ACP composites
The Fe0/ACP composites were characterized by hysteresis loops and nitrogen adsorption-desorption isotherm,respectively,as shown in Fig.4.The magnetic property at room temperature was studied using hysteresis loops(Fig.4a).It can be seen that these composites exhibit negligible coercivity(Hc)andremanence at 300 K in the range from-20 to 20 kOe. The saturation magnetization of 17.2 emu·g-1indicated that Fe0/ACP composites could be separated from the nonmagnetic materials in a magnetic field.Fig.4b shows the nitrogen adsorption-desorption isotherm and the corresponding Barett-Joyner-Halenda(BJH) pore size distribution curve of Fe0/ACP composites. The Brunauer-Emmett-Teller(BET)specific surface area of the as-prepared ACP porous composites is about 20 m2·g-1.While the BJH desorption cumulative pore volume between 1.7 nm and 300 nm diameter is 0.05 cm3·g-1,and BJH desorption average pore diameter(d=4V/A)is 5.6 nm,which all indicate that as-prepared product has the porous and hollow surface.Although there was more works about synthesis mechanism shall be done in future,we do provide a simple method for obtain Fe0doped ACP materials.
2.2Characterization of adsorption product
In addition,the absorption ability of as-prepared Fe0/ACP composites was studied using MPA-modified CdTe quantum dots,and the absorption product was designated as Fe0/ACP/CdTe composites(in short, FAC).The XRD,FTIR and hysteresis loops were used to characterize FAC,respectively,as shown in Fig.5. XRD pattern(Fig.5a)shows that the peaks of CdTe quantum dots in the pattern are much stronger than those of the ACP phase,due to the larger proportion of CdTe on the surface of FAC.which was consistent with FTIR spectrum(Fig.5b).The peaks around 2 925 and 2 849 cm-1attribute to asymmetric and symmetric vibrations of C-H in-CH2-,and the peaks at 1 226 cm-1refer to the stretching and flexural vibration of -COOH,which all arise from the MPA,indicated that the MPA stabilized CdTe quantum dots had been successfully adsorbed on the ACP.As shown in Fig. 5c,compared with Fe0/ACP composites,the saturation magnetization value of FAC did change,but not much, which indicated that the absorption of CdTe quantum dots had little effect on the magnetic property of product.
Interestingly,these adsorption products not only maintained the magnetic properties,but also exhibited good fluorescence emission ability.Both fluorescence emission and excitation spectra of CdTe quantum dots solution(The concentration was 3.1×10-4mol·L-1CdCl2)and FAC(50 mL 3.1×10-4mol·L-1CdTe quantum dots was absorbed by 0.5 g Fe0/ACP composites)solid powder are shown in Fig.6.After CdTe quantum dots was absorbed onto the surface of the Fe0/ACP composites,both fluorescence emission and excitation spectra of CdTe quantum dots red shifted further to longer wavelength and becomes broader (Fig.6a insert).In comparison with unabsorbed CdTe quantum dots,such red shifts are also observed in refs.[41].It is probably because of the aggregation of the CdTe quantum dots during the absorption process. Above all,FAC have magnetic-fluorescent properties and could be applied in future.

Fig.5 XRD of FAC,CdTe quantum dots and Fe0/ACP composites(a),FTIR spectrum of FAC(KBr pellets in the range of 4 000~400 cm-1)(b),Hysteresis loops of FAC and Fe0/ACP composites(c)
The magnetic separation process of FAC under normal light and 365 nm excitation were also studied (Fig.7).In the absence of an external magnetic field, the solution of FAC is well dispersed in the aqueous solution under both normal light and UV irradiation(c and d).When a magnetic field is placed near the solution,the products are attracted and accumulatedtoward the magnet,and the bulk solution becomes a clear phase,indicating that magnetic separation occurs(e and f).After the magnet was removed and followed by vigorous stirring,the FAC could quickly redispersed in water(like c and d).These results suggested that our FAC could be applied in magnetic guiding and separation.Meanwhile,the color of FAC is gray under natural light and a dark green color when the excitation wavelength is at 365 nm(UV light).Notably a single light source could excite multicolor emission spectrum of FAC absorbed with different emission wavelength of CdTe quantum dots (Fig.7g,h),which clearly made them ideal candidates for simultaneous multicolor imaging in biological and medical applications.

Fig.6 Fluorescence emission spectra(a)and excitation spectra(b)of CdTe quantum dots solution and FAC solid power (Concentration of CdTe quantum dots solution was 3.1×10-4mol·L-1CdCl2,pH=9,the emission and excitation slit width were 5 nm);Insert was normalized fluorescence spectrum of CdTe quantum dots solution and FAC solid power
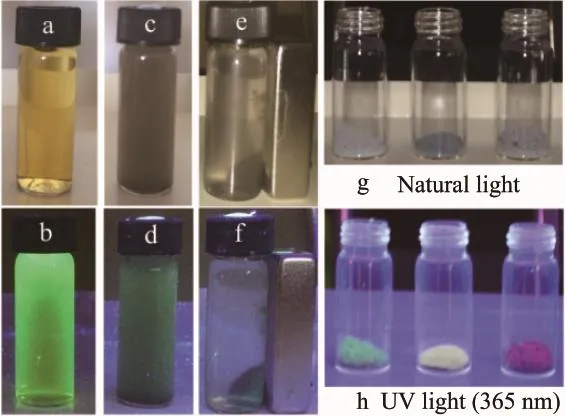
Fig.7 Photographs of aqueous solutions of QDs(a and b)and the as-synthesized FAC without applying a magnetic field(c,d), with a magnetic field(e,f),under normal light(a,c,e,g)and 365 nm excitation(b,d,f,h)
2.3Mechanism of the adsorption
In addition,pseudo-second-order rate equation was used to study this adsorption process,which canbe represented in the following linear form:

Where,qeand qtare the amounts of CdTe quantum dots adsorbed on Fe0/ACP composites at equilibrium and at any time t,respectively;and k2is the secondorder rate constant,and h0is the initial adsorption rate.The rate of CdTe quantum dots adsorbed on Fe0/ ACP composites was determined by measuring the fluorescence emission intensity of residual CdTe quantum dots in solution.The regression data indicated that this adsorption system fitted the pseudo-secondorder rate equation,as shown in Fig.8.By calculation, qeis 16.10 mg·g-1,k2is 0.006 6 g·mg-1·min-1,h0is 1.72 mg·g-1·min-1and the correlation coefficients R2is 0.995 2.These results suggest that the rate-limiting step of chemisorption controls the adsorption process.

Fig.8 Adsorption kinetics of CdTe quantum dots on Fe0/ACP composites,and the inset shows linear plot of t/qtvs time for Fe0/ACP composites absorption system
3 Conclusions
Amorphous calcium phosphate modified zerovalent iron(Fe0/ACP composites)has been successfully synthesized by precipitation method.The resulting product with spherical morphology exhibits excellent magnetic properties and high adsorption capacity.Therefore with the help of an applied magnetic field the Fe0/ACP composites can be used as powerful adsorbent for different applications.Therefore,we used as-prepared Fe0/ACP composites to absorb MPA-modified CdTe quantum dots directly, whose absorption process was analyzed by the pseudosecond-order rate equation.Notably these adsorption products not only maintained the magnetic properties, but also exhibited good fluorescence emission ability, which clearly made them ideal candidates for simultaneous multi-mode imaging in biological and medical applications.
Acknowledgments:We gratefully thank the National Natural Science Foundation of China(Grants No.21405058 and 21375046),and Jilin Institute of Chemical Technology,china (Grants No.201341).We gratefully thank Dr.Dayong Lu for the XRD analysis.
References:
[1]Dorozhkin S V.Materials,2009,2:399-498
[2]Makowski G S,Ramsby M L.Inflammation,2001,25(5):319-329
[3]Chen F,Yang B,Qi C,et al.RSC Adv.,2015,5(122):100682-100688
[4]Ding G J,Zhu Y J,Qi C,et al.RSC Adv.,2015,5(50):40154 -40162
[5]Narasaraju T S B,Phebe D E.J.Mater.Sci.,1996,31:1-21
[6]Dorozhkin S V.J.Mater.Sci.,2008,43:3028-3057
[7]Tadic D,Peters F,Epple M.Biomaterials,2002,23:2553-2559
[8]Ratner B D,Biomaterials Science:an Introduction to Materials in Medicine.Amsterdam:Elsevier,2004:151-162
[9]Qi C,Zhu Y J,Chen F,et al.J.Mater.Chem.B,2015,3(39): 7775-7786
[10]Qi C,Zhu Y J,Zhang Y G,et al.J.Mater.Chem.B,2015,3 (37):7347-7354
[11]Ding G J,Zhu Y J,Qi C,et al.J.Mater.Chem.B,2015,3 (9):1823-1830
[12]Sokolova V,Kovtun A,Prymak O,et al.J.Mater.Chem., 2007,17:721-727
[13]Eanes E D,Gillesse I H,Gosner A S,et al.Nature,1965, 208:365-367
[14]Posner A S,Betts F.Acc.Chem.Res.,1975,8:273-281
[15]Termine J D,Peckauskas R A,Posner A S.Arch.Biochem. Biophys.,1970,140:318-325
[16]Blumenthal N C,Betts F,Posner A S.Calcif.Tissue Res., 1977,23:245-250
[17]Li Y B,Weng W J.J.Mater.Sci:Mater.Med.,2007,18:2303 -2308
[18]Qi C,Zhu Y J,Zhao X Y,et al.Chem.Eur.J.,2013,19(3): 981-987
[19]Lu B,Zhu Y,Chen F,etal.Chem.Asian J.,2014,9:2908-2914
[20]Tang Q L,Zhu Y J,Duan Y R,et al.Dalton Trans.,2010, 39:4435-4439
[21]Hwang E T,Tatavarty R,Chung J Y.ACS Appl.Mater. Interfaces,2013,5:532-537
[22]Klesing J,Chernousova S,Kovtun A,et al.Mater.Chem., 2010,20:6144-6148
[23]Sokolova V,Epple M.Angew.Chem.Int.Ed.,2008,47:1382 -1395
[24]Ma W F,Wu K Y,Tang J,et al.J.Mater.Chem.,2012,22: 15206-15214
[25]Schleich N,Sibret P,Danhier P,et al.Int.J.Pharm.,2013, 447:94-101
[26]Lu B Q,Zhu Y J,Zhao X Y,et al.Mater.Res.Bull.,2013, 48,895-900
[27]Cao S W,Zhu Y J,Ma M Y,et al.J.Phys.Chem.C,2008, 112:1851-1856
[28]Liu J,Qiao S Z,Hu Q H,et al.Small,2011,7:425-443
[29]Zhao W R,Gu J L,Zhang L X,et al.J.Am.Chem.Soc., 2005,127:8916-8917
[30]Lu B Q,Zhu Y J,Ao H Y.ACS Appl.Mater.Interfaces, 2012,4:6969-6974
[31]Lu B Q,Zhu Y J,Cheng G F,et al.Mater.Lett.,2013,104: 53-56
[32]Li W M,Chen S Y,Liu D M,et al.Acta Biomater.,2013,9: 5360-5368
[33]Chen F,Li C,Zhu Y J,et al.Biomater.Sci.,2013,1:1074-1081
[34]Cao H Q,Zhang L,Zheng H,et al.J.Phys.Chem.C,2010, 114:18352-18357
[35]Cha E J,Sun I C,Lee S C,et al.Macromol.Res.,2012,20: 319-326
[36]Hwanga Y H,Kimb D G,Shina H S.Appl.Catal.B:Environ., 2011,105:144-150
[37]Wang G,Su X.Analyst,2011,136(9):1783-98
[38]Correa-Duarte M A,Giersig M,Liz-Marzán L M.Chem. Phys.Lett.,1998,286:497-501
[39]Doat A,Pelle F,Gardant N.J.Solid State Chem.,2004,177: 1179-1187
[40]YANG Jing-He(杨敬贺),ZHAO Bo(赵博),ZHAO Hua-Bo (赵华博),et al.Acta Chim.Sinica(化学学报),2013,71(10): 1365-1368
[41]Jin L,Yu D D,Liu Y,et al.Talanta,2008,76(5):1053-1057
Synthesis and Adsorption Properties for CdTe Quantum Dots of Zero-Valent Iron/Amorphous Calcium Phosphate Composites
JIN Li1NA Li-Hua2LIU Fang-Tong1GONG Li-Ying1LOU Da-Wei1ZHANG Jian-Po*,1
(1School of Chemical and Pharmaceutical Engineering,Jilin Institute of Chemical Technology,Jilin,Jilin 132022,China)
(2Key Laboratory of Synthetic Rubber,Chinese Academy of Sciences,Changchun Institute of Applied Chemistry,Changchun 130022,China)
A zero-valent iron amorphous calcium phosphate composite(Fe0/ACP composites)was prepared via coprecipitation method under a nitrogen atmosphere.Powder X-ray diffraction patterns,EDAX and FTIR all illustrated the formation of the Fe0/ACP composites.Meanwhile SEM and TEM analysis showed the formation of particles with size of 300 nm.Hysteresis loops indicated that Fe0/ACP composites could be separated from the nonmagnetic materials in a magnetic field.Nitrogen adsorption-desorption isotherm showed the adsorption capacity for the Fe0/ACP composites.Additionally,as-prepared product was used to absorb CdTe quantum dots, whose absorption process was analyzed by the pseudo-second-order rate equation.The XRD,FTIR,fluorescence spectrum and hysteresis loops were also used to characterize the absorption product,which indicated that these adsorption products not only maintained the magnetic properties,but also exhibited good fluorescence emission ability.
amorphous calcium phosphate;zero-valent iron;precipitation method;CdTe quantum dots
O611.4
A
1001-4861(2016)11-2025-09
10.11862/CJIC.2016.251
2016-03-06。收修改稿日期:2016-08-26。
国家自然科学基金(No.21405058,21403087)资助项目。
*通信联系人。E-mail:zhangjp725@126.com;会员登记号:S06N8530M1004。
