芳香烃受体在体外高糖环境诱导心肌肥大过程中的表达*
2016-11-24唐雪娇雷建明郭静文
唐雪娇, 肖 骅, 张 磊, 魏 潇, 雷建明, 郭静文
(重庆医科大学附属第一医院心血管内科, 重庆 400016)
芳香烃受体在体外高糖环境诱导心肌肥大过程中的表达*
唐雪娇, 肖 骅△, 张 磊, 魏 潇, 雷建明, 郭静文
(重庆医科大学附属第一医院心血管内科, 重庆 400016)
目的: 观察高糖环境诱导心肌细胞肥大过程中芳香烃受体(AhR)的表达情况,并探讨其可能的作用机制。方法: 以体外培养的大鼠心肌细胞H9c2为研究对象,实验分为正常糖浓度组、高糖组、DMSO组和白藜芦醇(AhR拮抗剂)组。免疫荧光染色观察AhR的表达情况,罗丹明标记的鬼笔环肽染色细胞骨架并计算细胞表面积,DCFH-DA法检测细胞内活性氧簇(ROS)的生成水平,实时荧光定量PCR及Western blot法检测AhR、CYP1A1、心钠素(ANP)和脑钠素(BNP)的表达情况。结果: 正常糖浓度环境下,AhR的表达主要定位于细胞质,高糖刺激时转入细胞核内。高糖刺激可促使心肌细胞肥大、心肌细胞内ROS生成增加,白藜芦醇阻滞AhR后,心肌肥大得到明显改善,同时ROS生成水平明显减少。与正常糖浓度组及白藜芦醇组相比,高糖组的AhR、CYP1A1、ANP和BNP mRNA及蛋白表达水平明显升高,上述指标高糖组与DMSO组相比差异无统计学显著性,而白藜芦醇组明显低于DMSO组。结论: 在高糖诱导的心肌肥大过程,心肌细胞的AhR表达增加可能参与维持正常糖代谢过程;高糖环境可激活AhR转入细胞核内,上调CYP1A1的表达,并促进ROS的生成,这可能是高糖诱导心肌肥大的重要机制之一。
高糖; 心肌肥大; 芳香烃受体
糖尿病是全球发病率较高的慢性疾病之一,其中多数为2型糖尿病;2型糖尿病主要在成人中发病,但近年在青少年儿童中发病率持续上升,已占青少年儿童糖尿病发病率的50%[1]。糖尿病与心力衰竭、心绞痛、心肌梗塞的发病密切相关,且心血管疾病是糖尿病患者最常见的死因之一[2-3]。高血糖是糖尿病患者最突出的表现,能够直接损伤心肌细胞,引起心肌肥大[4]。目前糖尿病引起心肌肥大的发病机制尚未完全清楚。芳香烃受体(aryl hydrocarbon receptor,AhR)是一种配体激活的转录因子,可调节细胞色素酶P450(cytochrome P450,CYP)的表达,与胰岛素抵抗和糖耐量异常发生密切相关[5-6]。研究表明,AhR/CYP1A1通路在外界毒物刺激引起心肌肥大的病理生理过程中发挥重要作用[7]。但在心肌细胞中,关于AhR在高糖环境诱导心肌肥大中的表达研究,尚未见报道。本研究观察高糖环境对体外培养的H9c2细胞内AhR的影响,为防治糖尿病心肌病提供理论依据。
材 料 和 方 法
1 材料
大鼠心肌细胞H9c2细胞株购自中国科学院上海生命科学研究院。低糖DMEM培养基和高糖DMEM培养基(Corning);胎牛血清(PAN Biotech);AhR阻滞剂白藜芦醇(resveratrol, RES; Sigma);细胞总RNA提取试剂盒、RNA逆转录试剂盒、实时荧光定量PCR试剂盒以及心钠素(atrial natriuretic peptide,ANP)和脑钠素(brain natriuretic peptide,BNP)的引物(TaKaRa);GAPDH、AhR和CYP1A1的引物(上海生物工程有限公司);DCFH-DA试剂盒(上海碧云天生物技术研究所);罗丹明标记的鬼笔环肽(上海翊圣生物科技有限公司);抗AhR抗体、CYP1A1抗体、ANP抗体(Abcam);抗BNP抗体(万类生物科技有限公司);抗GAPDH抗体(CST)。
2 细胞培养及分组
H9C2细胞于10%胎牛血清的低糖DMEM(5.5 mmol/L葡萄糖)中培养,细胞生长至75%~85%培养面积后用0.25%胰蛋白酶(含0.02% ETDA)消化,1∶2传代,8~9代细胞用于实验。取对数生长、密度为1×108/L的细胞培养24 h,待细胞成融合状态换用无血清低糖DMEM培养24 h,使细胞同步化,再干预细胞。实验分组:正常糖浓度(normal glucose,NG)组用低糖DMEM培养细胞;高糖(high glucose,HG)组用高糖DMEM(25 mmol/L葡萄糖)干预细胞48 h[4];DMSO组用0.02% DMSO和高糖DMEM培养细胞48 h;白藜芦醇组用含20 μmol/L白藜芦醇和高糖DMEM处理细胞48 h。
3 主要方法
3.1 细胞内AhR的表达情况 通过荧光染色观察AhR表达情况。细胞爬片,4%多聚甲醛固定细胞,0.1% Triton X-100破膜,山羊血清封闭,AhR I 抗(1∶50)4 ℃过夜,DyLight 649荧光标记的IgG(1∶400)37 ℃孵育2 h,DAPI染核5 min,荧光显微镜下观察。
3.2 细胞表面积检测 罗丹明标记的鬼笔环肽标记细胞骨架并计算细胞表面积。细胞爬片,4%多聚甲醛固定细胞,0.1% Triton X-100透化,山羊血清封闭,鬼笔环肽(1∶200)室温孵育1 h,DAPI染核5 min,荧光显微镜观察并摄片。每视野取10~15个细胞,Image-Pro Plus软件计算细胞平均面积。
3.3 细胞内活性氧簇(reactive oxygen species,ROS)的检测 DCFH-DA法检测ROS含量。1∶1 000稀释的 DCFH-DA于37 ℃孵育细胞30 min,荧光显微镜观察并摄片。Image-Pro Plus软件进行分析,平均荧光强度表示ROS的相对含量。
3.4 AhR、CYP1A1及心肌肥大标志物ANP、BNP的mRNA检测 荧光定量PCR检测mRNA表达情况。参照实时荧光定量PCR试剂盒操作说明进行。AhR的上游引物序列为5’-CGCTAACGGATGAAGAAGGA-3’,下游引物序列为5’-GGAGAGAAAGGGCTGGAGAT-3’;CYP1A1的上游引物序列为5’-TGAGACAGTATTGTGTAGTCCAAGT-3’,下游引物序列为5’-GAGACCAAGAGCTGGTGTAGC-3’;ANP的上游引物序列为5’-CTGGGGAAGTCAACCCGTCT-3’,下游引物序列为5’-TCTGGGCTCCAATCCTGTCA-3’;BNP的上游引物序列为5’-AGCCAGTCTCCAGAACAATCCA-3’,下游引物序列为5’-TGTGCCATCTTGGAATTTCGA-3’。以GAPDH为内参照。反应体系10 μL,PCR扩增条件为95 ℃ 30 s;95 ℃ 5 s、60 ℃ (AhR、CYP1A1、BNP)/62 ℃ (ANP)30 s、40个循环;65 ℃ 缓慢升高至95 ℃,每5 s增加0.5 ℃,分析熔解曲线。扩增得到的Ct值使用2-ΔΔCt法计算目的mRNA的相对表达量。
3.5 AhR、CYP1A1及心肌肥大标志物ANP、BNP的蛋白表达水平检测 采用Western blot法检测蛋白表达情况。0.25%胰蛋白酶消化,离心收集细胞。RIPA蛋白裂解液裂解细胞收集蛋白,BCA法测定样品蛋白浓度。SDS-PAGE分离蛋白后电转至PVDF膜,5%脱脂奶粉室温封闭1 h,I抗(AhR 1∶2 000,CYP1A1 1∶100,ANP 1∶5 000,BNP 1∶1 000)4 ℃孵育过夜,IgG II抗(1∶7 500)室温孵育1 h,ECL法显色。以GAPDH作为内参照。电泳条带使用Quantity One系统进行分析,以目的条带灰度值与GAPDH条带灰度值之比表示目的蛋白的相对表达量。
4 统计学处理
采用SPSS 20.0统计软件分析数据。计量资料采用均数±标准差(mean±SD)表示,多组间比较采用单因素方差分析(one-way ANOVA),方差齐则使用Bonferroni法检验,若方差不齐则采用Dunnett’s T3法检验。以P<0.05为差异有统计学意义。
结 果
1 AhR在心肌细胞内的表达情况
如图1所示,在NG组,AhR主要分布在细胞质,少量表达于细胞核内;在HG组,AhR在细胞核和细胞质均有表达。这提示高糖环境刺激心肌细胞,可使AhR从细胞质转入细胞核内,呈激活状态。

Figure 1.High glucose induced AhR nuclear localization (×400). AhR was visualized by DyLight 649 (red). The nuclei of the cardiomyocytes were visualized by DAPI staining (blue).
图1 高糖诱导AhR转入细胞核内
2 各组细胞表面积比较
罗丹明标记的鬼笔环肽染细胞骨架荧光结果显示,HG组的细胞表面积较NG组和RES组显著增大(P<0.01),而与DMSO组比较差异无统计学显著性;RES组与DMSO组相比,细胞表面积明显下降(P<0.01),见图2。这表明白藜芦醇阻滞AhR可改善高糖环境引起的心肌肥大,AhR可能参与高糖导致的心肌肥大过程。
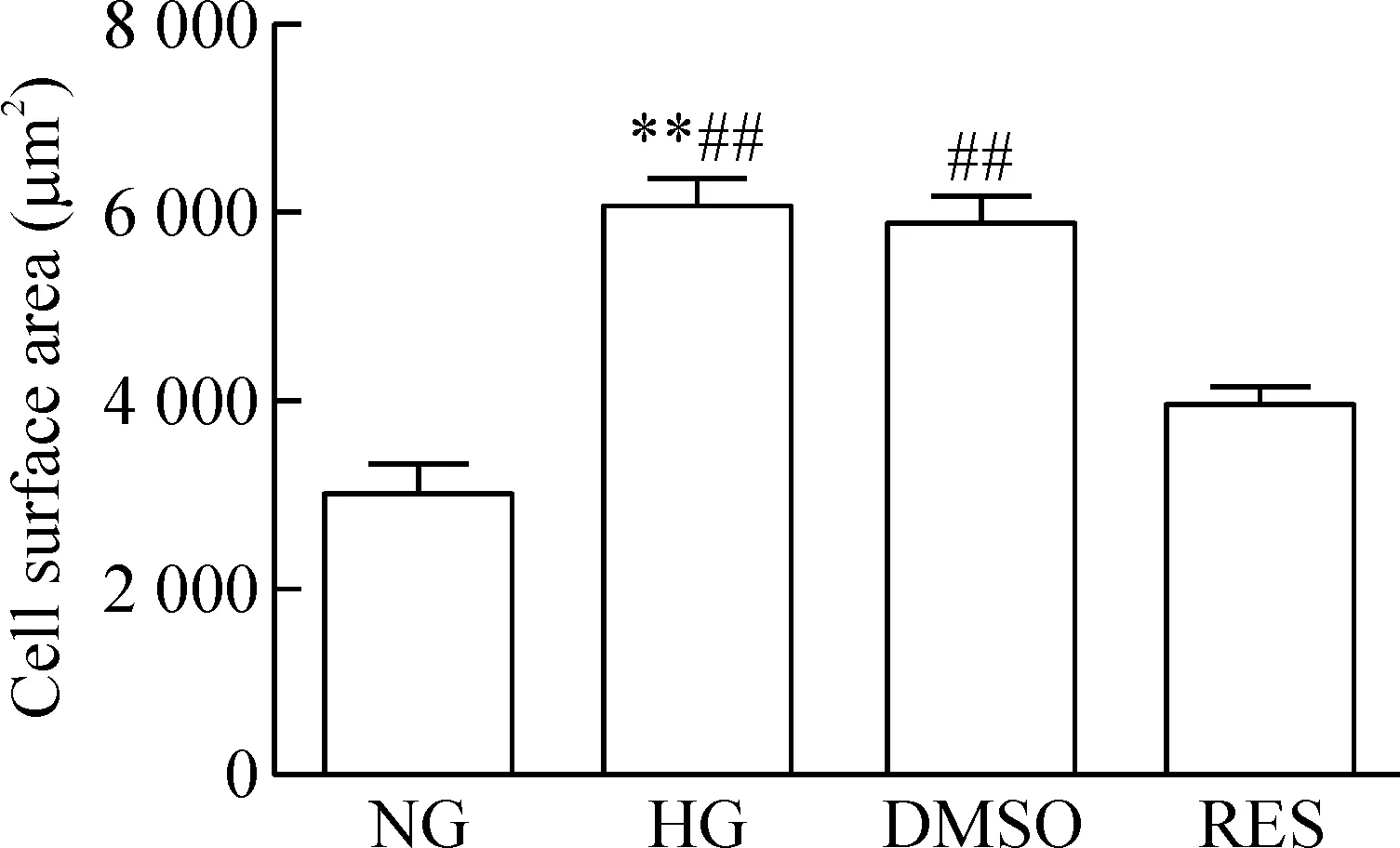
Figure 2.Comparison of the cell surface area among groups. Mean±SD.n=3.**P<0.01vsNG;##P<0.01vsRES.
图2 各组细胞表面积比较
3 各组细胞内ROS生成水平比较
心肌细胞内的ROS生成水平在HG组明显上升,与NG组和RES组对比差异有统计学显著性(P<0.05);与DMSO组相比,RES组的ROS生成水平明显下降(P<0.05),而HG组的ROS生成水平与DMSO组相比差异无统计学显著性,见图3。这些结果提示,高糖环境下,细胞内ROS表达增加,加入白藜芦醇阻滞AhR后,ROS生成水平下降,表明高糖环境下ROS生成可能受AhR调控。

Figure 3.Comparison of the ROS generation among groups. The cellular ROS production was assessed by 2′,7′-dichlorofluorescin diacetate (DCFH-DA) staining. Mean±SD.n=3.*P<0.05vsNG;#P<0.05vsRES.
图3 各组ROS生成水平比较
4 各组细胞AhR、CYP1A1、ANP和BNP的mRNA相对表达水平比较
细胞分组干预后,HG组AhR和CYP1A1的mRNA表达较NG组和RES组均明显增多(P<0.01),而与DMSO组比较差异无统计学显著性;与DMSO组对比,RES组AhR和CYP1A1的mRNA表达明显减少(P<0.01)。心肌肥大标志物ANP和BNP的mRNA在HG组表达水平明显增高,与NG组及RES组相比差异有统计学显著性(P<0.01);在RES组ANP和BNP的mRNA表达水平与DMSO组比较明显下降(P<0.01);但在DMSO组与HG组之间ANP和BNP的mRNA的表达差异无统计学显著性,见图4。上述结果证实高糖环境可促使心肌肥大,而白藜芦醇阻滞AhR可保护心肌细胞,抑制心肌细胞肥大,提示高糖诱导心肌肥大过程可能通过激活AhR调控CYP1A1,促使心肌肥大标志物表达。
5 各组细胞AhR、CYP1A1、ANP和BNP蛋白表达水平比较
与NG组和RES组相比,HG组的AhR和CYP1A1蛋白表达水平明显升高(P<0.05);上述指标与DMSO组、HG组比较差异无统计学显著性,但RES组的表达水平明显低于DMSO组(P<0.01)。HG组心肌肥大标志物ANP和BNP的蛋白表达水平较NG组、RES组明显增高(P<0.05),但与DMSO组比较差异无统计学显著性;RES组的ANP和BNP蛋白表达水平明显低于DMSO组(P<0.05),见图5。这进一步证实,高糖可激活AhR,诱导CYP1A1表达上升,调控心肌肥大标志物ANP和BNP的表达,参与心肌肥大过程。
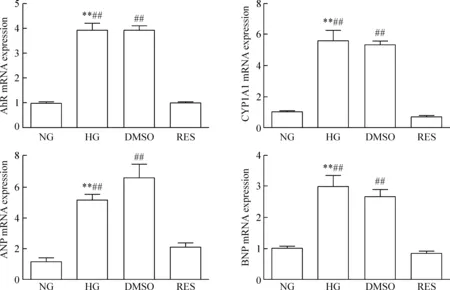
Figure 4.Comparison of the relative mRNA expression of AhR, CYP1A1, ANP and BNP among groups. Mean±SD.n=8~9.**P<0.01vsNG;##P<0.01vsRES.
图4 各组AhR、CYP1A1、ANP和BNP 的mRNA相对表达水平比较
讨 论
糖尿病是一种全球流行性慢性疾病,发病率逐年增高[8]。尽管糖尿病的治疗手段一直在进步,但糖尿病引起的心血管疾病发病率和死亡率仍然较高[2]。高血糖是糖尿病的主要特征,可损伤心肌细胞,影响心肌功能,诱发糖尿病心肌病,最终导致心力衰竭、心律失常和心肌梗塞[9]。心肌肥大是糖尿病心肌病的主要表现之一,发病机制较为复杂,目前尚无特效治疗药物。
新近研究显示,血清中AhR配体的活性与2型糖尿病发病密切相关,可能是2型糖尿病发病的独立危险因素,其潜在的机制是胰岛素抵抗[5]。多项研究[6,10-11]发现,不管AhR基因缺失还是AhR被激活,都将导致糖脂代谢失衡,提示AhR在机体调节糖脂代谢过程中起关键作用。还有研究[7,12]表明,AhR在外界毒物刺激心肌细胞时介导心肌肥大的病理生理过程,在机体适应和感受环境刺激方面发挥重要作用。本研究观察到,高糖环境可激活心肌细胞内的AhR表达,促使心肌肥大。因此,AhR既可能是糖尿病发病的关键环节,又可能是糖尿病导致心肌肥厚的重要介导者。本研究结果显示,AhR一般在细胞质丰富表达;当高糖环境激活AhR时,AhR从细胞质转移到细胞核内,发生位置改变。这与高糖刺激人血管内皮细胞的研究结果一致[13];Terashima等[14]在低糖刺激常规高糖培养的人肝癌细胞HepG2同样观察到这一现象。这提示AhR可能在心肌细胞调节糖代谢方面起重要作用。
CYP酶在体内主要参与生物体内的甾醇类激素合成等过程。AhR/CYP被认为是心肌肥大的重要信号通路,AhR介导心肌肥大主要与CYP1A1的改变有关[7]。许多研究表明,心肌肥大时ANP和BNP表达明显增高,ANP和BNP是评价心肌肥大的重要标志物[15-16]。我们发现,激活的AhR转入细胞核内,上调CYP1A1表达,诱发心肌细胞表面积增大伴心肌肥大标志物ANP、BNP表达水平升高;而白藜芦醇可阻滞AhR,下调CYP1A1表达,从而改善心肌肥大[17]。Maayah等[12]在心肌肥大过程中发现,CYP1A1表达增加的时间早于ANP、心肌营养素-1(CT-1)等心肌肥大标志物,且心肌肥大标志物ANP、CT-1等的表达强度与CYP1A1的表达水平呈正相关并呈浓度和时间依赖性,表明CYP1A1可能是心肌肥大标志物产生的重要因素。这提示CYP1A1可能是AhR下游调控心肌肥大的重要环节,可能成为糖尿病心肌肥厚治疗的关键靶点。
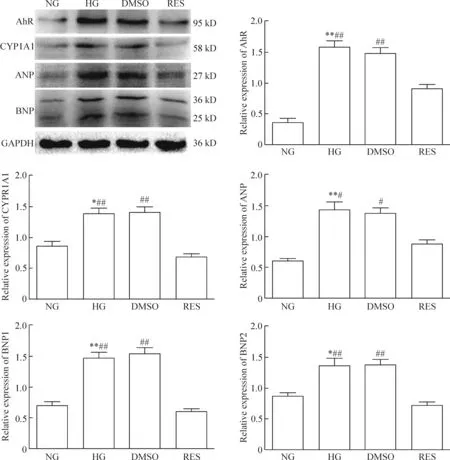
Figure 5.Comparison of the relative protein expression of AhR, CYP1A1, ANP and BNP among groups. BNP underwent 2 cleavage events, one within the cell (BNP1, 36 kD) and the other after secretion into the blood (BNP2, 25 kD). Mean±SD.n=3.*P<0.05,**P<0.01vsNG;#P<0.05,##P<0.01vsRES.
图5 各组AhR、CYP1A1、ANP和BNP蛋白相对表达水平比较
高糖环境下心肌细胞内氧化应激反应增强,ROS生成明显增多,而过多的ROS可促进心肌肥大过程[18-19]。我们发现,细胞内ROS生成水平与AhR、CYP1A1表达水平呈正相关。相关研究结果显示,AhR基因缺失导致的心肌肥大与心肌细胞内ROS生成增多密切相关[20]。另有研究证明,下调AhR表达时,CYP1A1表达水平及ROS生成水平均下降;当阻断CYP1A1表达时,ROS生成减少,提示ROS生成水平呈AhR依赖及受CYP1A1调控[21]。CYP1A1诱导ROS生成水平升高,可能通过激活核内Nrf2等信号通路,促使心肌肥大[22-23]。这些研究提示CYP1A1诱导心肌细胞内ROS生成水平增加,激活核内其它信号通路,可能是心肌肥大的重要机制。
综上所述,高糖环境激活心肌细胞内AhR从细胞质转入细胞核内,诱导CYP1A1的表达,进而上调ROS生成水平,这可能是AhR参与糖尿病导致心肌肥厚的重要作用机制之一。本研究既丰富了糖尿病心肌病的发病机理,又为探索糖尿病心肌病治疗药物奠定理论基础。
[1] Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2015 update: a report from the American Heart Association [J]. Circulation,2015,131(4):e29-e322.
[2] Fox CS, Golden SH, Anderson C, et al. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association [J]. Circulation,2015,132(8):691-718.
[3] Shah AD, Langenberg C, Rapsomaniki E, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people [J]. Lancet Diabetes Endocrinol,2015,3(2):105-113.
[4] Bugyei-Twum A, Advani A, Advani SL, et al. High glucose induces Smad activation via the transcriptional coregulator p300 and contributes to cardiac fibrosis and hypertrophy [J]. Cardiovasc Diabetol,2014,13:89.
[5] Roh E, Kwak SH, Jung HS, et al. Serum aryl hydrocarbon receptor ligand activity is associated with insulin resistance and resulting type 2 diabetes [J]. Acta Diabetol,2015,52(3):489-495.
[6] Zhang L, Hatzakis E, Nichols RG, et al. Metabolomics reveals that aryl hydrocarbon receptor activation by environmental chemicals induces systemic metabolic dysfunction in mice [J]. Environ Sci Technol,2015,49(13):8067-8077.
[7] Zordoky BN, El-Kadi AO. 2,3,7,8-Tetrachlorodibenzo-p-dioxin and beta-naphthoflavone induce cellular hypertrophy in H9c2 cells by an aryl hydrocarbon receptor-dependant mechanism [J]. Toxicol In Vitro,2010,24(3):863-871.
[8] Whiting DR, Guariguata L, Weil C, et al. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030 [J]. Diabetes Res Clin Pract,2011,94(3):311-321.
[9] Liu F, Song R, Feng Y, et al. Upregulation of MG53 induces diabetic cardiomyopathy through transcriptional activation of peroxisome proliferation-activated receptor alpha [J]. Circulation,2015,131(9):795-804.
[10]Baker NA, Shoemaker R, English V, et al. Effects of adipocyte aryl hydrocarbon receptor deficiency on PCB-induced disruption of glucose homeostasis in lean and obese mice [J]. Environ Health Perspect,2015,123(10):944-950.
[11]Biljes D, Hammerschmidt-Kamper C, Kadow S, et al. Impaired glucose and lipid metabolism in ageing aryl hydrocarbon receptor deficient mice [J]. EXCLI J,2015,14:1153-1163.
[12]Maayah ZH, Ansari MA, El Gendy MA, et al. Development of cardiac hypertrophy by sunitinibinvivoandinvitrorat cardiomyocytes is influenced by the aryl hydrocarbon receptor signaling pathway [J]. Arch Toxicol,2014,88(3):725-738.
[13]Dabir P, Marinic TE, Krukovets I, et al. Aryl hydrocarbon receptor is activated by glucose and regulates the thrombospondin-1 gene promoter in endothelial cells [J]. Circ Res,2008,102(12):1558-1565.
[14]Terashima J, Habano W, Gamou T, et al. Induction of CYP1 family members under low-glucose conditions requires AhR expression and occurs through the nuclear translocation of AhR [J]. Drug Metab Pharmacokinet,2011,26(6):577-583.
[15]Liu CJ, Cheng YC, Lee KW, et al. Lipopolysaccharide induces cellular hypertrophy through calcineurin/NFAT-3 signaling pathway in H9c2 myocardiac cells [J]. Mol Cell Biochem,2008,313(1-2):167-178.
[16]Zordoky BN, Aboutabl ME, El-Kadi AO. Modulation of cytochrome P450 gene expression and arachidonic acid metabolism during isoproterenol-induced cardiac hypertrophy in rats [J]. Drug Metab Dispos,2008,36(11):2277-2286.
[17]Ciolino HP, Yeh GC. Inhibition of aryl hydrocarbon-induced cytochrome P-450 1A1 enzyme activity and CYP1A1 expression by resveratrol [J]. Mol Pharmacol,1999,56(4):760-767.
[18]Fiordaliso F, Bianchi R, Staszewsky L, et al. Antioxidant treatment attenuates hyperglycemia-induced cardiomyocyte death in rats [J]. J Mol Cell Cardiol,2004,37(5):959-968.
[19]刁雪红, 申 鍔, 张跃力,等. 抑制Rac1通过降低磷酸化p38MAPK提高1型糖尿病小鼠的心脏功能 [J]. 中国病理生理杂志,2010,26(7):1285-1289.
[20]Lund AK, Peterson SL, Timmins GS, et al. Endothelin-1-mediated increase in reactive oxygen species and NADPH oxidase activity in hearts of aryl hydrocarbon receptor (AhR) null mice [J]. Toxicol Sci,2005,88(1):265-273.
[21]Kopf PG, Walker MK. 2,3,7,8-Tetrachlorodibenzo-p-dioxin increases reactive oxygen species production in human endothelial cells via induction of cytochrome P4501A1 [J]. Toxicol Appl Pharmacol,2010,245(1):91-99.
[22]Tsuji G, Takahara M, Uchi H, et al. Identification of ketoconazole as an AhR-Nrf2 activator in cultured human keratinocytes: the basis of its anti-inflammatory effect [J]. J Invest Dermatol,2012,132(1):59-68.
[23]Li H, Yao W, Irwin MG, et al. Adiponectin ameliorates hyperglycemia-induced cardiac hypertrophy and dysfunction by concomitantly activating Nrf2 and Brg1 [J]. Free Radic Biol Med,2015,84:311-321.
(责任编辑: 林白霜, 罗 森)
Changes of aryl hydrocarbon receptor in cardiac hypertrophy induced by high glucose in vitro
TANG Xue-jiao, XIAO Hua, ZHANG Lei, WEI Xiao, LEI Jian-ming, GUO Jing-wen
(DepartmentofCardiology,TheFirstAffiliatedHospitalofChongqingMedicalUniversity,Chongqing400016,China.E-mail:xiaohua197408@163.com)
AIM: To investigate the changes of aryl hydrocarbon receptor (AhR) in the process of cardiomyocyte hypertrophy induced by high glucose, and to explore its potential mechanisms. METHODS: The rat cardiomyocytes (H9c2 cells) were divided into normal glucose group, high glucose group, DMSO group and resveratrol (an AhR antagonist) group. The content and distribution of AhR were observed with immunofluorescence staining. The myocardial cells were stained with rhodamine-labeled phalloidin to visualize cytoskeleton, and the cell surface area were determined after imaging by fluorescence microscopy. The generation of reactive oxygen species (ROS) in the cardiomyocytes was measured using a fluorescent probe DCFH-DA. The mRNA expression of AhR, CYP1A1, atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) were evaluated by real-time quantitative PCR (RT-qPCR). The protein levels of AhR, CYP1A1, ANP and BNP were assessed by Western blot. RESULTS: AhR was constitutively presented in the cytosol under normal-glucose condition and was translocated to the nuclei under high-glucose condition. High glucose induced cardiac hypertrophy, and increased ROS generation. Significant reductions in the cell size and ROS generation were observed after treated with resveratrol. The expression of AhR, CYP1A1, ANP and BNP at mRNA and protein levels in high glucose group was increased as compared with normal glucose group and resveratrol group, and the above-mentioned indexes significantly decreased in resveratrol group as compared with DMSO group. CONCLUSION: High glucose-induced cardiac hypertrophy increases AhR expression, which may be involved in the maintenance of glucose homeostasis in the cardiomyocytes. AhR translocation to the nucleus induced by high glucose results in the increases in CYP1A1 expression and ROS generation, which may be an important mechanism of high glucose-induced cardiomyocyte hypertrophy.
High glucose; Cardiac hypertrophy; Aryl hydrocarbon receptor
1000- 4718(2016)10- 1744- 06
2016- 05- 24
2016- 07- 14
国家自然科学基金资助项目(No. 81300140)
△通讯作者 Tel: 023-89011565; E-mail: xiaohua197408@163.com
R541.8; R363
A
10.3969/j.issn.1000- 4718.2016.10.003
杂志网址: http://www.cjpp.net
