NaCl-KCl熔盐体系中AlF3氟化Tb4O7电化学制备Al-Tb合金
2016-11-22王珊珊杨晓光张密林颜永得
韩 伟 季 男 李 梅 王珊珊 杨晓光 张密林 颜永得
(哈尔滨工程大学材料科学与化学工程学院,教育部超轻材料与表面技术重点实验室,哈尔滨150001)
NaCl-KCl熔盐体系中AlF3氟化Tb4O7电化学制备Al-Tb合金
韩伟*季男李梅*王珊珊杨晓光张密林颜永得
(哈尔滨工程大学材料科学与化学工程学院,教育部超轻材料与表面技术重点实验室,哈尔滨150001)
在NaCl-KCl-Tb4O7-AlF3体系中为了制备Al-Tb合金,首先对熔盐中的上清液和沉淀物进行了分析,X射线衍射(XRD)结果确定了Tb4O7能被AlF3氟化生成TbF3。采用一系列的电化学方法对NaCl-KCl-AlF3-Tb4O7体系在Mo电极上的电化学行为进行了研究。循环伏安、方波伏安、计时电位和开路计时电位等电化学方法的研究结果表明Tb(III)在预先沉积的Al电极上发生欠电位沉积。在不同条件下进行恒电流电解制备了Al-Tb合金,并对所得合金样品进行XRD和扫描电镜-能量散射谱(SEM-EDS)表征。结果表明在-2.5A进行恒电流电解得到的Al-Tb合金是由Al和Al3Tb两相组成。采用电感耦合等离子体-原子发射光谱仪(ICP-AES)对实验所得沉积物的组成进行分析,研究了电解条件对合金组成和电流效率的影响。在电流强度为-1.5A进行恒电流电解2 h,电流效率可达76.5%。
电化学行为;Tb4O7;共还原;NaCl-KCl熔盐;Al-Tb合金;电流效率
1 Introduction
Rare earth(RE)metals and their alloys have attracted considerable practical interest due to their functional properties,i.g., high-temperature super-conductors,fluorescent materials,highperformance magnets,and chemical sensors1-3.Meanwhile,rare earth element can be used as additives to improve many properties of Al-based alloys,such as tensile strength,heat resistance,corrosion resistance,vibration resistance,and extrudability4-6.
At present,the Al-RE alloys are produced only by liquid phase mixing and dissolution.However,electrochemical formation using molten salts,as a new preparation method of the rare earth intermetallic compounds,is an effective method,because composition and thickness of the alloys can be controlled by electrochemical parameters7,8.Recently,the preparation of Al-RE alloy compounds was studied by electro-reduction on a reactive Al electrode9-19and electrochemical co-deposition20-24in molten salts. Gibilaro et al.20-22investigated the electrochemical codeposition of Al(III)with RE(III)(RE=Ce,Nd,Sm,Eu)ions on inert W electrode in LiF-CaF2melts.While the electrochemical co-reduction of RE(III)(RE=Sm,Yb)with Al(III)ions was explored in LiCl-KCl melts23,24.Since rare earth chlorides are extremely sensitive to oxygen and easily form their solid oxychlorides and oxides24,25.Therefore,rare earth oxides selected as a raw material with the assistance ofAlCl3were studied to preparedAl-RE(RE= La,Ce,Pr,Sm,Eu,Gd,Tb,Dy)alloys in LiCl-KCl melts26-34. These researchers found that rare earth oxide can be chloridized by AlCl3and RECl3is produced in LiCl-KCl-RE2O3-AlCl3melts as follow reaction:
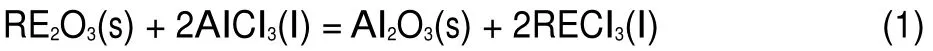
Since the AlCl3(sublimation temperature:452 K)is easily volatile at high temperature,in order to avoid the volatilization of AlCl3,it should be replaced withAlF3to investigate the formation ofAl-RE alloy from RE2O3in NaCl-KCl melts.
So far a few studies concerning the electrochemical behavior of Tb(III)ions were conducted on different electrodes19,33,35-39. Castrillejo et al.35explored the electrochemical behavior of Tb(III) in LiCl-KCl melts.They determined the diffusion coefficient of Tb(III)and apparent standard potential values of the Tb(III)/Tb(0) system in the temperature range of 673-823 K.Using cyclic voltammetry and time-resolved laser-induced fluorescence spectroscopy,Kim et al.36studied electrochemical and spectroscopic properties of Tb(III).The diffusion coefficient of the Tb(III)ions was also calculated at 887 K.Rayaprolu and Chidambaram37investigated the electrochemical deposition of Tb(III)in a molten LiCl-KCl medium.Castrillejo et al.38studied the electrode reaction of Tb(III)/Tb couple on liquid Cd electrode in the eutectic LiCl-KCl.The thermochemical properties of the TbCdxintermetallic compounds were measured by electromotive forces(EMF).Shi et al.33studied the electrochemical extraction Tb from Tb4O7aided by AlCl3and formation of Al-Tb alloy in LiCl-KCl melts.Our group19,39investigated the electrochemical deposition of Tb(III)on Al and Ni electrodes,and producedAl-Tb and Ni-Tb intermetallic compounds in LiCl-KCl melts,respectively.
In order to confirm the feasibility of the formation of Al-Tb alloy from Tb4O7assisted by AlF3in NaCl-KCl melts,the fluorination effect of AlF3on Tb4O7was explored by X-ray diffraction (XRD).The electrochemical behavior of NaCl-KCl-Tb4O7-AlF3system was studied by a series of electrochemical techniques.And then,galvanostatic electrolysis was used to prepare Al-Tb alloys at different conditions.The chemical compositions and morphologies of the Al-Tb alloys were characterized by XRD and scanning electron microscopy and energy dispersive spectrometer (SEM-EDS).The current efficiencies were also calculated by analyzing the compositions of the Al-Tb alloys using inductively coupled plasma-atomic emission spectrometer(ICP-AES).
2 Experimental
2.1Preparation and purification of the molten salt
The mixture of NaCl(99.8%)-KCl(99.9%)(NaCl:KCl mass ratio,44.6:55.4)was dried for 72 h at 473 K to remove residual water and then put it in an alumina crucible placed in a quartz cell inside an electric furnace.The impurities in the molten salts were removed by pre-electrolysis at-2.0 V(vs Ag/AgCl)for 3 h.The powders of Tb4O7(99.9%)and AlF3(99.9%)were added to the mixture of NaCl-KCl contained in an alumina crucible.A nickel chromium-nickel aluminum thermocouple,sheathed by an alumina tube,was employed to measure the temperature of the molten salts.The electrochemical measurement was performed under an argon atmosphere.
2.2Electrodes and electrochemical apparatus
Asilver wire(d=1 mm)dipped into a solution ofAgCl 1%(w, mass fraction)in NaCl-KCl melts contained in a Pyrex tube was used as a reference electrode.In our experiments,all potentials were referred to theAg/AgCl couple.The working electrode was a molybdenum wire(d=1 mm,99.99%),which was polished thoroughly using SiC paper to remove the impurities on the electrode surface,then cleaned with ethanol prior to use.The Mo electrode surface was determined after each experiment by measuring the immersion depth of the electrode in the molten salts. Betweeneachmeasurement,Moelectrode was cleaned byapplying ananodicpolarization.Aspectral puregraphiterod(d=6mm)was employed as the counter electrode.Galvanostatic electrolysis and all electrochemical measurements were performed using an AutolabPGSTAT302N(Metrohm,Ltd.)withNova1.10 software.
2.3Molten salts electrolysis and characterization of deposits
The Al-Tb alloys were prepared by galvanostatic electrolysis at different conditions on Mo electrode.After electrolysis,the alloy samples were washed with distilled water and hexane(99.8%)to remove salts,respectively.These samples were characterized byXRD(X′pert Pro;Philips Co.,Ltd)using Cu Kαradiation at 40 kV and 40 mA.The microstructure and micro-zone chemical analysis of these samples were measured with SEM-EDS(JSM-6480A; JEOL Co.,Ltd.).In order to determine the contents of Tb and Al in the Al-Tb alloys,each sample was dissolved in HCl solution 14%(w),and then was diluted and analyzed by inductively coupled plasma-atomic emission spectrometer(IRIS Intrepid II XSP, Thermo Elemental).
2.4Analysis of the melts
The mixture of AlF3and Tb4O7was added into the NaCl-KCl melt contained in an alumina crucible and stirred for 30 s to make them uniformly mix.Then the alumina crucible was placed in an electric furnace and the temperature was raised to 1073 K.The supernatant salt and the bottom salt containing precipitate were sampled after 2 h without electrolysis.These samples were scraped from the cooled and solidified melts.The bottom salts were the mixture of the precipitate and the solidified melts.In order to identify the supernatant salt and bottom salt,they were analyzed by XRD.
3 Results and discussion
3.1Electrochemical behavior of NaCl-KCl-Tb4O7-AlF3system
3.1.1Cyclic voltammetry
CyclicvoltammetrywasfirstlyperformedtostudytheelectrochemicalbehaviorofTb4O7intheNaCl-KCleutectic.Asshownin Fig.1,the dotted curve obtained in the NaCl-KCl-Tb4O73%(w) meltsisconsistentwiththecurveofblankNaCl-KCleutecticand exhibitsalargeandsharpredoxcoupleA/A′,whichcorrespondsto thedepositionanddissolutionofNametal40-42.Exceptfortheredox couple A/A′,no other obvious electro-signal is observed in the dotted curve,indicating there is no other electroactive species associated with Tb available in the melts to induce any other electrochemicalsignal.Thus,Tb4O7couldnotdissolveinthemoltensalt.
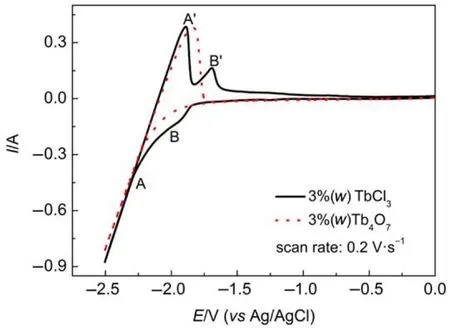
Fig.1 Cyclic voltammograms obtained in NaCl-KCl melts before and after the addition of Tb4O7or TbCl3on Mo electrode(S=0.31 cm2)at 1073 K
The solid curve in Fig.1 shows the cyclic voltemmogram(CV) obtained in NaCl-KCl-TbCl33%(w)on Mo electode.Except for the peaks ofA/A′,a couple of new peaks,B/B′at-1.93 V/-1.70 V(vsAg/AgCl),is observed.According to the previous works19,33,39we can infer the couple of peaks,B/B′,which are ascribed to the formation and subsequent re-oxidation of Tb metal.
The CVs obtained in NaCl-KCl-Tb4O7system after the addition of AlF35.5%(w)were presented in Fig.2.Besides the reduction/ oxidation peaks,A/A′and B/B′,corresponding to the deposition/ subsequent dissolution of Na and Tb metals,two pairs of cathodic/ anodic peaks,C/C′and D/D′,are observed.The cathodic peak D, at-0.93 V,and its corresponding anodic peak D′,at-0.61 V,are associated with the deposition and dissolution of aluminum metal. The signals of C/C′between signals of B/B′and D/D′correspond to the formation of Al-Tb intermetallic compound.The formation mechanism ofAl-Tb alloys could be described as follows:

The underpotential deposition of Tb occurs on the pre-deposited Al electrode because the electrodeposited Tb reacts with Al to form an intermetallic compound,AlxTb,the formation potential of the compound is shifted to the anodic direction.This phenomenon is also called“depolarization effect”.This depolarisation effect is obviously associated with the lowering of the activity of Tb in intermetallic compound,AlxTb:
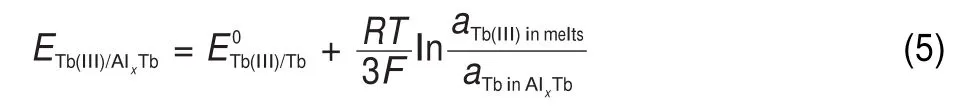
In order to investigate the effect of AlF3on the dissolution of Tb4O7,the supernatant salts and bottom salts were analyzed by XRD(Fig.3).We can observe from Fig.3(a)that NaCl,KCl and Tb2O3exist in the bottom salts before the addition of AlF3in the system.This result indicates that Tb4O7can thermally decompose under a low O2partial pressure44,and the reaction can be described as follows:


Fig.2 Cyclic voltammograms obtained in NaCl-KCl-Tb4O7-AlF3system on a Mo electrode(S=0.31 cm2)
After the addition of AlF3in the system,we can observe fromFig.3(b,c)that the supernatant salts consist of NaCl,KCl,AlF3and TbF3phases,and Al2O3exists in the bottom insoluble salts. Thus,it is considered that the Tb2O3can react with AlF3and form TbF3.The reaction could be described as follow:
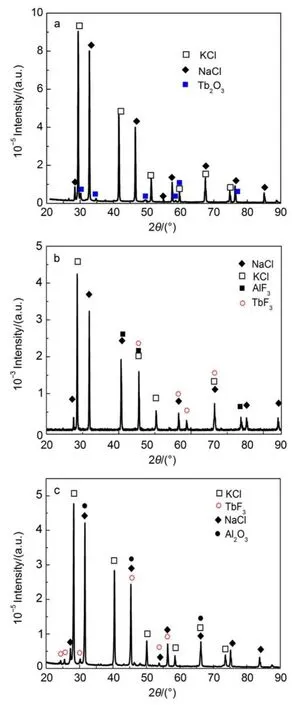
Fig.3 XRD patterns of the bottom salts from NaCl-KCl-Tb4O7melts after heating 2 h at 1073 K(a);the supernatant salts(b)and bottom salts(c)from NaCl-KCl-AlF3-Tb4O7system after heating 2 h at 1073 K

According to the experimental result,the overall process is as follows:
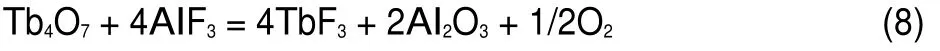
3.1.2Square wave voltammetry
Square wave voltammetry(SWV),as a more sensitive method than cyclic voltammetry,was conducted to further investigate the electrochemical co-reduction of Tb(III)andAl(III)ions in NaCl-KCl-AlF35.5%(w)-Tb4O73%(w)system on a Mo electrode.Fig.4 presents the comparison of the SWVs of the NaCl-KCl-TbCl3melts(curve a),NaCl-KCl-AlF3melts(curve b)and NaCl-KCl-AlF3-Tb4O7system(curve c)at potential step of 1 mV and frequency of 20 Hz.The red curve a shows a large cathodic signal B,at around-1.93 V,corresponding to the formation of Tb metal.A cathodic peak D at-0.93 V,shown in curve b is ascribed to the formation ofAl metal.After the addition of Tb4O7in NaCl-KCl-AlF3melts(curve c),except for the peaks B and D,a new peak C at-1.53 V is observed,which corresponds to the formation of Al-Tb alloy.The result is consistent with the one obtained from cyclic voltammogram(Fig.2).
3.1.3Chronopotentimetry
Fig.5 shows chronopotentiogram obtained at different cathodic current intensities on Mo electrode in the NaCl-KCl-Tb4O73%(w)-AlF35.5%(w)system at 1073 K.Four plateaus can be obviously observed.When the applied cathodic current is more positive than-20 mA,only one plateau(plateau D)is detected,which is related to the deposition of Al in the melts.While the current intensity increases from-70 to-125 mA,the curves show a new plateau C,corresponding to the underpotential deposition of terbium on pre-deposited Al to form Al-Tb intermetallic compound.If the cathodic current from-150 to-155 mA,the third plateau B,belongs to the formation of Tb.In addition,the fourth plateau A at current intensity of-170 mA,belongs to the formation of Na. It should be mentioned that the potential ranges for the deposition of Na,Tb,Al,and Al-Tb alloy are the same as those observed in the CV and SWV.When the cathodic current is more positive than-70 mA,theAl-Tb alloy can be prepared.
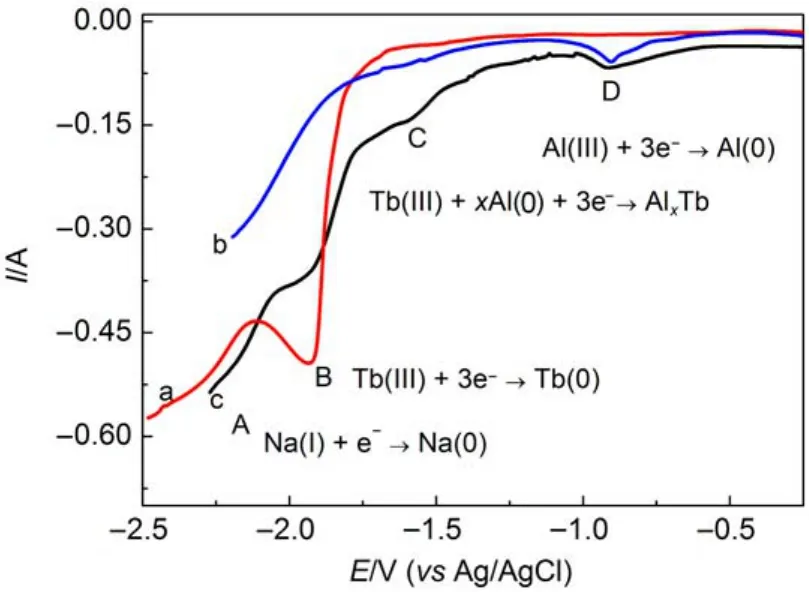
Fig.4 Comparison of square wave voltammograms obtained in the NaCl-KCl-TbCl33%(w)melts(a),NaCl-KCl-AlF35.5%(w) melts(b),and NaCl-KCl-AlF35.5%(w)-Tb4O73%(w)system(c)

Fig.5 Chronopotentiograms obtained at different current intensities on Mo electrode(S=0.31 cm2)in the NaCl-KCl-AlF35.5%(w)-Tb4O73%(w)system at 1073 K
3.1.4Open circuit chronopotentiometry
The electrochemical formation of Al-Tb intermetallic compounds was investigated by open circuit chronopotentiometry.The measurements were conducted as follows:Tb element is deposited on pre-deposited Al electrode at-2.3 V(vs Ag/AgCl)for 35 s at 1073 K.Then the polarization was stopped and the electrode was kept in the molten salt.Meanwhile,current is almost zero,which is considered a“zero current”.During this currentless step,the deposited Tb metal was dissolved fromAl film to the molten salts, cathodic potential was shifted toward the positive direction.When a two-phase equilibrium occurs at the surface of the electrode,the activity of Tb is the same in each phase,a potential plateau could be observed.Fig.6 presents the open circuit chronopotentiogram after potentiostatic electrolysis at-2.3 V(vs Ag/AgCl)for 35 s in the NaCl-KCl-AlF35.5%(w)-Tb4O73%(w)system on a Mo electrode.Four plateaus can be seen in Fig.6.In the beginning,the potential stays at around-2.15 V(plateau A),corresponding to the equilibrium of deposition and dissolution of the Na metal. After that,the plateau B(in inset of Fig.6)is ascribed to the equilibrium of Tb/Tb(III).The plateau C at-1.37 V is related to the two-phase coexisting states of Al-Tb intermetallic compound. The rest plateau D is correlated with theAl/Al(III)system.
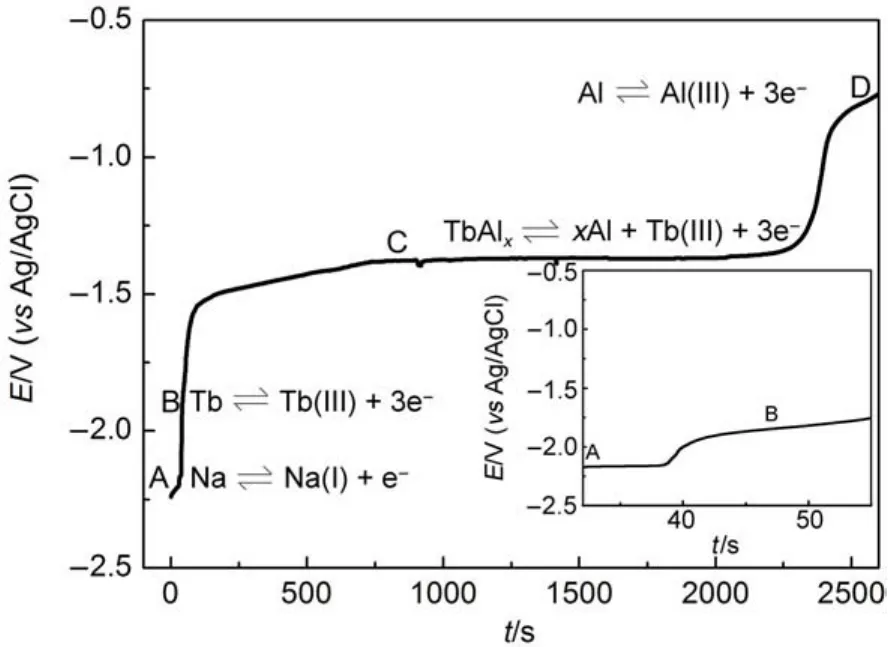
Fig.6 Open circuit chronopotentiogram obtained on Mo electrode(S=0.31 cm2)after potentiostatic electrolysis at-2.3 V (vsAg/AgCl)for 35 s in the NaCl-KCl-AlF35.5%(w)-Tb4O73%(w) system at 1073 K
3.2Electrochemical formation and characterization of Al-Tb alloys
According to the results of cyclic voltammetry,square wave voltammetry,chronopotentiometry and open circuit chronopotentiometry,the Al-Tb alloys were prepared by galvanostatic electrolysis at different current intensities on a Mo electrode in the NaCl-KCl-AlF35.5%(w)-Tb4O73%(w)system at 1073 K.Fig.7 shows the XRDpatterns ofAl-Tb alloys obtained in the NaCl-KCl-AlF3-Tb4O7system by galvanostatic electrolysis at-2.5Afor 2 h. As seen from the pattern,the sample was identified to be Al3Tb phase.Fig.8 shows SEM-EDS analysis images of the Al-Tb alloy obtained by galvanostatic electrolysis at-2.5 A for 2 h.It is noticeable that sample is composed of dark and bright zones.From the mapping analysis of elements,we can find that the element Tb mainly distributes within the bright zones,the element Al in dark matrix.
Fig.7 XRD patterns ofAl-Tb alloy obtained in the NaCl-KCl-AlF35.5%(w)-Tb4O73%(w)system by galvanostatic electrolysis at-2.5Afor 2 h at 1073 K on a Mo electrode(S=0.31 cm2)

Fig.8 SEM-EDS analysis of theAl-Tb alloy obtained by galvanostatic electrolysis at-2.5Afor 2 h in the NaCl-KCl-AlF35.5%(w)-Tb4O73%(w)system at 1073 K on a Mo electrode(S=0.31 cm2)
To examine the distribution of Tb element in the Al-Tb alloy, a mapping analysis is employed.Fig.9 shows the EDS results of the points labeled 001,002,and 003 taken from three represented zones in Fig.8a,which indicate that the deposit is composed of theelements of Tb and Al.The EDS results of the points labeled 002 represented zones in Fig.8a shows few element of Tb in dark phases.The Tb element mainly exists in bright phases.The atom ratio of Tb to Al closes to 1:3,which can further confirm this phase as the formation of theAl3Tb intermetallic compound.
Al samples obtained by galvanostatic electrolysis under different conditions were analyzed by ICP-AES,because of the inaccuracy of the EDS quantification.The alloy composition obtained by ICP,experimental conditions,and current efficiency are presented in Table 1.It can be seen from Table 1,when other conditions are the same,the content of Tb in Al-Tb alloy and current efficiency increase with the increase of temperature. However,the current efficiency decreases when the temperature is higher than 1123 K.The content of Tb in Al-Tb alloy and current efficiency gradually increase with the increase of current intensity,while current intensity reaches to-1.5 A,the current efficiency reaches the maximum value,76.5%,then,the current efficiency decreased with current intensity increase gradually.The reason may be related to the formation of dendritic or powder deposit of Al-Tb which is easy to fall into the electrolyte.The extension of exelectrolysis time is beneficial to increase current efficiency.However,when electrolysis time is more than 2.5 h, current efficiency reduces from 68.1 to 55.8.
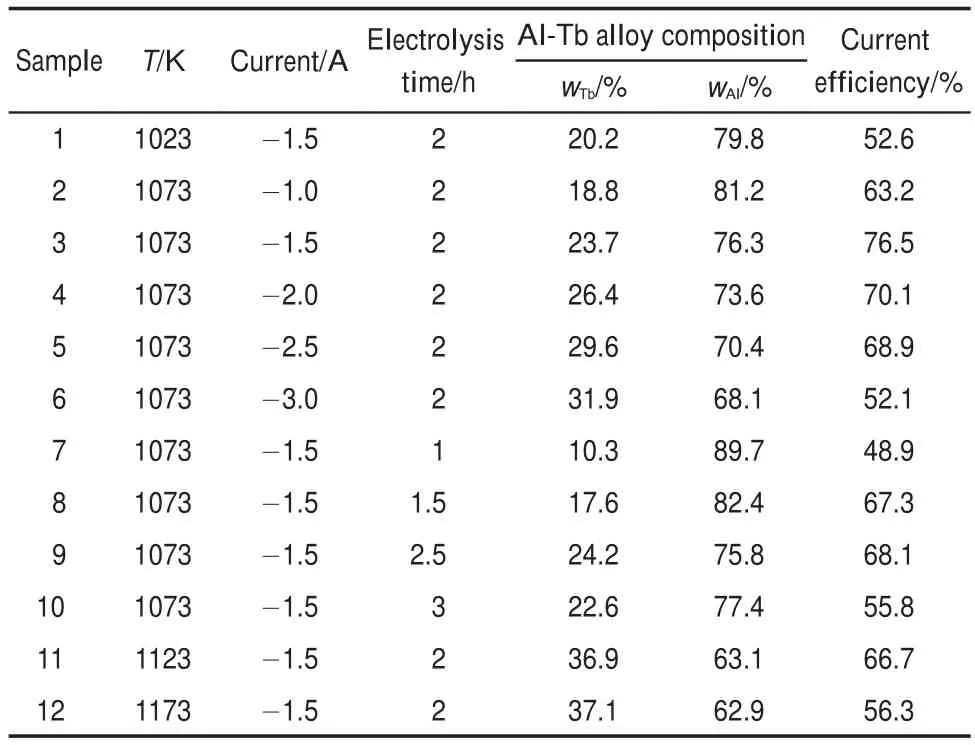
Table 1 ICP-AES analyses of all samples obtained by galvanostatic electrolysis under different conditions on a Mo electrode(S=0.31cm2)in the NaCl-KCl-AlF35.5%(w)-Tb4O73%(w)system
4 Conclusions
Electrochemical formation of Al-Tb intermetallic compound in NaCl-KCl-Tb4O7melts assisted by AlF3was investigated by electrochemical techniques.XRD patterns indicated that Tb4O7could react with AlF3in NaCl-KCl melts.Cyclic voltammetry, square wave voltammetry and open-circuit chronopotentiometry results show that the reduction potential of Tb(III)/Tb on predeposited Al electrode was observed at more positive potential values than those on Mo electrode,because of the formation of Al3Tb intermetallic compound.Then according to the co-reduction conditions studied by different electrochemical methods,theAl-Tb alloys can be directly prepared by galvanostatic electrolysis on a Mo electrode in the NaCl-KCl-AlF35.5%(w)-Tb4O73%(w) system at different conditions.The Al-Tb alloys with different Tb contents were obtained.SEM-EDS analysis and the XRD pattern of the deposits show the Al3Tb intermetallic compound was formed under the condition of-2.5 A for 2 h at 1073 K.The current efficiency could reach 76.5%at-1.5Afor 2 h.
References
(1)Li,Y.M.;Wang,F.L.;Zhang,M.L.;Han,W.;Tian Y.J.Rare Earths 2011,29,378.doi:10.1016/S1002-0721(10)60464-4
(2) Uda,T.;Jacob,K.T.;Hirasawa,M.Science 2000,289,2326. doi:10.1126/science.289.5488.2326
(3) Maestro,P.;Huguenin,D.J.Alloy.Compd.1995,225,520. doi:10.1016/0925-8388(94)07095-4
(4) Lundin,R.;Wilson,J.R.Adv.Mater.Proce.2000,158(1),52.
(5) Ping,D.;Hono,K.;Inoue,A.Metall.Mater.Trans.A 2000,31, 607.doi:10.1007/s11661-000-0004-7
(6) Gschneidner,K.A.;Eyring,L.Handbook on the Physics and Chemistry of Rare Earths;Elsevier:North Holland,1998;Vol. 25,pp 83-99.
(7) Konishi,H.;Nohira,T.;Ito,Y.Electrochim.Acta 2003,48,563. doi:10.1016/S0013-4686(02)00723-5
(8) Iida,T.;Nohira,T.;Ito,Y.Electrochim.Acta 2003,48,1531. doi:10.1016/S0013-4686(03)00031-8
(9) Castrillejo,Y.;Bermejo,R.;Martínez,A.M.;Barrado,E.; DíazArocas,P.J.Nucl.Mater.2007,360,32.doi:10.1016/j. jnucmat.2006.08.011
(10)Yang,X.N.;Yan,Y.D.;Zhang,M.L.;Li,X.;Xue,Y.;Han, W.Acta Phys.-Chim.Sin.2015,31(5),920.[杨晓南,颜永得,张密林,李星,薛云,韩伟.物理化学学报,2015,31(5), 920.]doi:10.3866/PKU.WHXB201503251
(11) Serp,J.;Allibert,M.;Leterrier,A.;Malmbeck,R.;Ougier,M.; Rebizant,J.;Glatz,J.P.J.Electrochem.Soc.2005,152,167. doi:10.1149/1.1859812
(12) Castrillejo,Y.;Fernández,P.;Medina,J.;Hernández,P.; Barrado,E.Electrochim.Acta 2011,56,8638.doi:10.1016/j. electacta.2011.07.059
(13) Bermejo,M.R.;Gómez,J.;Medina,J.;Martínez,A.M.; Castrillejo,Y.J.Electroanal.Chem.2006,588,253. doi:10.1016/j.jelechem.2005.12.031
(14) Castrillejo,Y.;Bermejo,M.R.;Barrado,E.;Martínez,A.M. Electrochim.Acta 2006,51,1941.doi:10.1016/j. jelechem.2005.12.031
(15) Cassayre,L.;Malmbeck,R.;Masset,P.;Rebizant,J.;Serp,J.; Soucek,P.;Glatz,J.P.J.Nucl.Mater.2007,360,49. doi:10.1016/j.jnucmat.2006.08.013
(16) Castrillejo,Y.;Vega,A.;Vega,M.;Hernández,P.;Rodriguez,J. A.;Barrado,E.Electrochim.Acta 2014,118,58.doi:10.1016/j. electacta.2013.11.163
(17) Castrillejo,Y.;Fernndez,P.;Medina,J.;Vega,M.;Barrado,E. Electroanalysis 2011,23,222.doi:10.1002/elan.201000421
(18) Bermejo,M.R.;Barrado,E.;Martínez,A.M.;Castrillejo,Y. J.Electroanal.Chem.2008,617,85.doi:10.1016/j. jelechem.2008.01.017
(19)Li,M.;Gu,Q.Q.;Han,W.;Yan,Y.D.;Zhang,M.L.;Sun,Y.; Shi,W.Q.Electrochim.Acta 2015,167,139.doi:10.1016/j. electacta.2015.03.145
(20) Gibilaro,M.;Massot,L.;Chamelot,P.;Taxil,P.Electrochim. Acta 2009,54,5300.doi:10.1016/j.electacta.2009.01.074
(21) Gibilaro,M.;Massot,L.;Chamelot,P.;Taxil,P.J.Nucl.Mater. 2008,382,39.doi:10.1016/j.jnucmat.2008.09.004
(22) Gibilaro,M.;Massot,L.;Chamelot,P.;Cassayre,L.;Taxil,P. Electrochim.Acta 2009,55,281.doi:10.1016/j. electacta.2009.08.052
(23) Castrillejo,Y.;Fernández,P.;Medina,J.;Hernández,P.; Barrado,E.Electrochim.Acta 2011,56,8638.doi:10.1016/j. electacta.2011.07.059
(24) Castrillejo,Y.;Fernández,P.;Medina,J.;Vega,M.;Barrado,E. Electroanalysis 2011,23,222.doi:10.1002/elan.201000421
(25) Kuznetsova,S.A.;Gaune-Escard,M.J.Nucl.Mater.2009,389, 108.doi:10.1016/j.jnucmat.2009.01.015
(26) Liu,Y.L.;Yuan,L.Y.;Ye,G.A.;Liu,K.;Zhu,L.;Zhang,M. L.;Chai,Z.F.;Shi,W.Q.Electrochim.Acta 2014,147,104. doi:10.1016/j.electacta.2014.08.114
(27)Zhang,M.;Wang,H.Y.;Han,W.;Zhang,M.L.;Li,Y.N.; Wang,Y.L.;Xue,Y.;Ma,F.Q.;Zhang,X.M.Sci.China Chem. 2014,57(11),1477.doi:10.1007/s11426-014-5214-8
(28)Tang,H.;Yan,Y.D.;Zhang,M.L.;Li,X.;Huang,Y.;Xu,Y.L.; Xue,Y.;Han,W.;Zhang,Z.J.Electrochim.Acta 2013,88,457. doi:10.1016/j.electacta.2012.10.045
(29) Liu,K.;Liu,Y.L.;Yuan,L.Y.;He,H.;Yang,Z.Y.;Zhao,X.L.; Chai,Z.F.;Shi,W.Q.Electrochim.Acta 2014,129,401. doi:10.1016/j.electacta.2014.02.136
(30)Yan,Y.D.;Tang,H.;Zhang,M.L.;Xue,Y.;Han,W.;Cao,D. X.;Zhang,Z.J.Electrochim.Acta 2012,59,531.doi:10.1016/j. electacta.2011.11.007
(31)Yan,Y.D.;Li,X.;Zhang,M.L.;Tang,H.;Han,W.;Xue,Y.; Zhang,Z.J.Energy Procedia 2013,39,408.doi:10.1016/j. egypro.2013.07.230
(32) Liu,K.;Liu,Y.L.;Yuan,L.Y.;Zhao,X.L.;Chai,Z.F.;Shi,W. Q.Electrochim.Acta 2013,109,732.doi:10.1016/j. electacta.2013.07.084
(33) Luo,L.X.;Liu,Y.L.;Liu,N.;Liu,K.;Yuan,L.Y.;Chai,Z.F.; Shi,W.Q.RSC Adv.2015,5,69134.doi:10.1039/c5ra11708a
(34) Su,L.L.;Liu,K.;Liu,Y.L.;Wang,L.;Yuan,L.Y.;Wang,L.; Li,Z.J.;Zhao,X.L.;Chai,Z.F.;Shi,W.Q.Electrochim.Acta 2014,147,87.doi:10.1016/j.electacta.2014.09.095
(35) Bermejo,M.R.;Gomez,J.;Martinez,A.M.;Barrado,E.; Castrillejo,Y.Electrochim.Acta 2008,53,5106.doi:10.1016/j. electacta.2008.02.058
(36) Kim,B.Y.;Lee,D.H.;Lee,J.Y.;Yun,J.Electrochem. Commun.2010,12,1005.doi:10.1016/j.elecom.2010.05.009
(37) Rayaprolu,D.;Chidambaram,S.ECS Trans.2014,58,51. doi:10.1149/05845.0051ecst
(38) Castrillejo,Y.;Hernández,P.;Fernández,R.;Barrado,E. Electrochim.Acta 2014,147,743.doi:10.1016/j. electacta.2014.10.005
(39) Han,W.;Sheng,Q.N.;Zhang,M.L.;Li,M.;Sun,T.T.;Liu,Y. C.;Ye,K.;Yan,Y.D.;Wang,Y.C.Metall.Mater.Trans.B 2014, 45,929.doi:10.1007/s11663-013-9984-8
(40) Jia,Y.H.;He,H.;Lin,R.H.;Tang,H.B.;Wang,Y.Q.J.Rad. Nucl.Chem.2015,303,1763.doi:10.1007/s10967-014-3723-8
(41) Kuznetsov,S.A.;Gaune-Escard,M.Electrochim.Acta 2001, 46,1101.doi:10.1016/S0013-4686(00)00708-8
(42) Sahoo,D.K.;Satpati,A.K.;Krishnamurthy,N.RSC Adv.2015, 5,3163.doi:10.1039/c4ra15334k
(43) Kang,Z.C.;Eyring,L.J.Alloy.Compd.1997,249,206. doi:10.1016/S0925-8388(96)02633-3
Electrochemical Formation of Al-Tb Alloys from Tb4O7Fluorinated by AlF3in NaCl-KCl Melts
HAN Wei*JI NanLI Mei*WANG Shan-ShanYANG Xiao-Guang ZHANG Mi-LinYAN Yong-De
(Key Laboratory of Superlight Materials and Surface Technology,Ministry of Education,College of Material Science and Chemical Engineering,Harbin Engineering University,Harbin 150001,P.R.China)
To prepareAl-Tb alloys from Tb4O7assisted byAlF3in NaCl-KCl melts,we initially studied the effect ofAlF3on the dissolution of Tb4O7by analyzing the supernatant and bottom salts.X-ray diffraction(XRD)results revealed that Tb4O7was fluorinated byAlF3to form TbF3.The electrochemical behavior of the NaCl-KCl-AlF3-Tb4O7system was investigated using a Mo electrode at 1073 K.Cyclic voltammetry(CV),square wave voltammetry(SWV),chronopotentiometry(CP)and open circuit chronopotentiometry(OCP)analyses indicated that the under-potential deposition of Tb(III)occurred on pre-depositedAl.The co-deposition ofAl-Tb alloys was investigated by galvanostatic electrolysis under different conditions.These samples were characterized by XRD and scanning electron microscopy and energy dispersive spectrometry(SEM-EDS).The Al-Tb alloy obtained by galvanostatic electrolysis at-2.5Aconsisted ofAl andAl3Tb phases.The effects of the electrolysis conditions on the composition of the alloy and current efficiency were studied by analyzing the compositions of theAl-Tb alloys by inductively coupled plasma-atomic emission spectrometer(ICP-AES).The current efficiency could reach 76.5%under the conditions of galvanostatic electrolysis at-1.5Afor 2 h.
May 19,2016;Revised:July 20,2016;Published online:July 20,2016.
s.HAN Wei,Email:weihan@hrbeu.edu.cn.LI Mei,Email:meili@hrbeu.edu.cn;Tel:+86-451-82569890.
Electrochemical behavior;Tb4O7;Co-reduction;NaCl-KCl melt;Al-Tb alloy; Current efficiency
O646
10.3866/PKU.WHXB201607202
The project was supported by the National Natural Science Foundation of China(21271054,11575047,21173060),Major Research Plan of the National Natural Science Foundation of China(91326113,91226201),and Fundamental Research Funds for the Central Universities,China
(HEUCF2016012).
国家自然科学基金(21271054,11575047,21173060),国家自然科学基金重大研究计划(91326113,91226201)和中央高校基本科研业务经费(HEUCF2016012)资助项目©Editorial office ofActa Physico-Chimica Sinica
