纳米球形炭的无催化化学气相沉积制备及其机理研究
2016-11-22罗瑞盈商海东
张 铀, 杨 威, 罗瑞盈, 商海东
(北京航空航天大学 物理科学与核能工程学院,北京100191)
纳米球形炭的无催化化学气相沉积制备及其机理研究
张 铀, 杨 威, 罗瑞盈, 商海东
(北京航空航天大学 物理科学与核能工程学院,北京100191)
以天然气为碳源,氢气为载气,通过无催化化学气相沉积的方法,合成不同粒径的球形炭。研究了沉积温度、压力和气体比例对化学气相沉积球形炭的影响。用X射线衍射、扫描电子显微镜和透射电子显微镜对实验结果进行表征。研究结果表明制备纳米球形炭(50~100 nm)的优化工艺为沉积温度1 150 ℃、沉积压力5 kPa和天然气/氢气(气体流量比)1∶4。本研究对球形炭的气相沉积机理进行了详细的研究。沉积温度对球形炭的尺寸和微结构影响较小,沉积压力对炭核的碰撞几率影响较大。另外,氢在炭形核过程中起来很重要的作用,氢的存在有效的较少了炭核的碰撞几率。
球形炭; 化学气相沉积; 天然气; 氢气; 形核机理
1 Introduction
Carbon materials have a variety of structures and properties owing to the chemical nature of carbon atoms with various hybridizations (sp, sp2, and sp3) depending on precursors and reaction conditions[1]. Until now, various routes have been used to produce a wide range of carbon materials, such as fullerenes[2], carbon nanotubes[3,4], pyrolytic carbon[5], and carbon spheres[6-10].
Since carbon spheres have potential applications as adsorbents[11], catalyst carriers[12], and anodes in secondary lithium ion batteries[13, 14], their synthetic routes are receiving much attentions in recent years. Typical approaches such as self-assembly template processes[15], pyrolyses[16], reductions[17], and hydrothermal methods[18], have been used to prepare hollow or solid carbon spheres with different sizes and yields. Chemical vapor deposition (CVD), is most popular in terms of economy and versatility and can be divided into three types: catalytic[19], non-catalytic[20], and template methods[21]. To obtain pure carbon spheres from catalytic or template CVD, removal of the catalyst or template from the resultant products is an essential step, which is cost ineffective and complicated. Consequently, research into high yield, one-step, and low cost synthetic routes has been attracted much interest. At present, non-catalytic CVD appears to be the best choice considering all factors of operation and costs. Some researchers[22-24]have synthesized carbon spheres by CVD from liquid hydrocarbons such as styrene, toluene and hexane. But carbon sphere fabrication by the natural gas/hydrogen mixture with the non-catalytic method has not been reported in former studies to our knowledge.
This paper reports preparation of carbon spheres with CVD from a natural gas/hydrogen mixture in the absence of a catalyst. The influences of deposition temperature, pressure and the natural gas/hydrogen ratio on the formation of carbon spheres were investigated using scanning electron microscopy (SEM), transmission electron microscopy (TEM), and X-ray diffraction (XRD) to characterize the sphere morphology, size and structure. Based on the these results, a model is proposed for the formation mechanism of carbon spheres.
2 Experimental
2.1 Preparation of carbon spheres
Preparation of carbon spheres was performed in a typical CVD furnace (Fig. 1). At ambient temperature, air in the CVD furnace was evacuated and flushed with highly pure nitrogen for 10 min. Temperature was measured using a Pt thermocouple, pressure was controlled by an electromagnetic valve connected to an absolute pressure transformer and the flow rate of reactant gas was adjusted by a mass flow controller. When deposition temperature was reached, natural gas and hydrogen (purity>99.999%) were continuously introduced into the reaction chamber for 2 h and the reaction products were collected after the chamber was cooled to room temperature. The natural gas was consisted of methane (98%), propane (0.3%), butane (0.4%), nitrogen (1%), and other hydrocarbons (0.3%). Details of process parameters are listed in Table 1.
2.2 Characterization
The XRD pattern of the resultant products was measured with a Siemens D5000 X-ray diffractometer (CuKα=0.154 18 nm) and the morphology and microstructure were studied by SEM (LEO-5000, 15 kV) and TEM (H7100, 100 kV). Samples for TEM were dispersed into ethanol with ultrasonication for 0.5 h, transferred to a Cu grid, and air-dried.
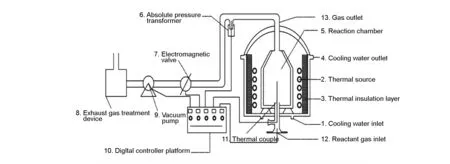
Fig. 1 A schematic illustration of experimental setup of chemical vapor deposition.

SeriesNo.Temperature(℃)Pressure(kPa)Flowrateofnaturalgas(sccm)Flowrateofhydrogen(sccm)T110705500500T211005500500T311505500500T412005500500P111503500500P2115010500500P3115015500500R1115055000R2115055001000R3115055002000
3 Results
3.1 Influence of deposition temperature
The influence of deposition temperature on growth and morphology of carbon spheres was studied over a deposition temperature range of 1 050-1 200 ℃. This range was selected because soot was obtained below 1 050 ℃ and conglomeration of carbon spheres increased above 1 200 ℃ under the other conditions employed here. A SEM image of carbon spheres deposited at 1 070 ℃ showed the diameters of carbon spheres to be 1-2 μm and the spheres were not perfectly spherical, but somewhat elliptical or egg-shaped (Fig. 2a, white arrows). When the deposition temperature was increased to 1 100 ℃, carbon spheres with diameters of 500 nm-1 μm were obtained and the spheres were observed to be brittle, showing many broken carbon spheres, and hollow ones (Fig. 2b, white arrows). With further increase in the deposition temperature, monodispersed carbon spheres were difficult to observe and conglomeration of carbon spheres became apparent (Fig. 2c, 2d). There was discernable difference in the conglomeration of carbon spheres deposited at 1 150 ℃ and 1 200 ℃, with small spheres linked to large ones observed at 1 150 ℃, a phenomenon confirmed in TEM micrographs of these spheres (Fig. 3). In contrast, at 1 200 ℃, more carbon spheres of uniform size were linked into clusters, and the sphere surfaces were much rough.
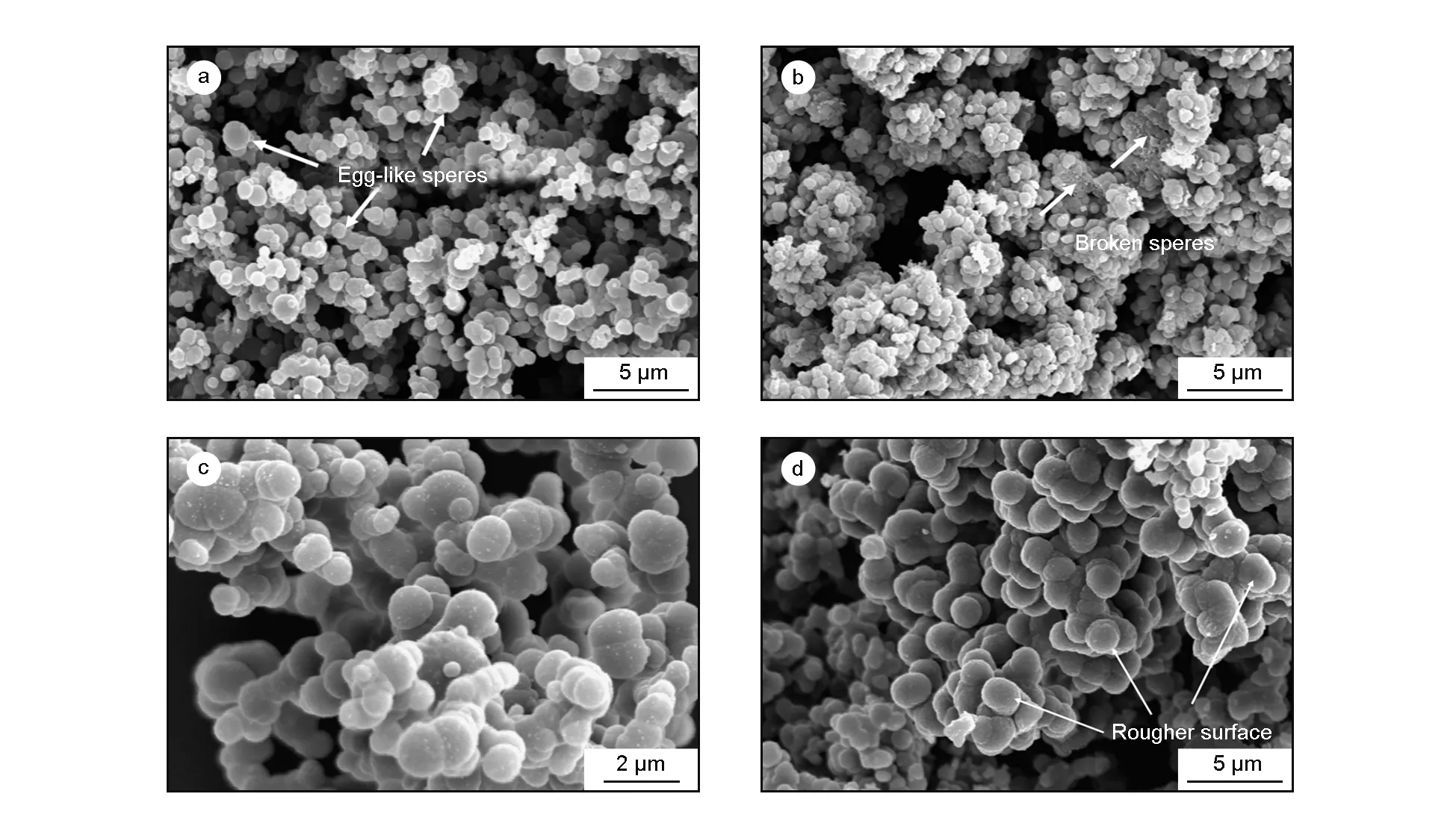
Fig. 2 SEM images of carbon spheres obtained at the deposition pressure of 5 kPa, the natural gas/H2 flow ratio of 1∶1 and at different deposition temperatures: (a) 1 070 ℃, (b) 1 100 ℃, (c) 1 150 ℃ and (d) 1 200 ℃.

Fig. 3 Typical TEM images of carbon spheres obtained at the deposition pressure of 5 kPa, the natural gas/H2 flow ratio of 1∶1 and at different deposition temperatures: (a) 1 150 ℃ and (b) 1 200 ℃.
XRD patterns of carbon spheres obtained under T1, T2, T3, and T4 conditions showed only two broaden peaks at 25.4°- 25.8° and 42.7°- 43.8°, which were characteristic of typical graphite (002) and (100) planes (Fig. 4). However, the interlayer spacingd002value of carbon spheres obtained ranged from 0.344 37 nm to 0.350 51 nm with increasing the deposition temperature from T1 to T2. They all were much higher than thed002value (0.335 41 nm) of graphitic crystal, even higher than thed002value (0.344 0 nm) of turbostratic carbon, indicating a low degree of graphitization and the possible presence of turbostratic carbon. Moreover, with the increase of the deposition temperature, thed002value of carbon sphere obtained increased. This may be attributed to the fact that the rate of carbon formation is higher than rearrangement of carbon layer, resulting in graphitic crystal at high temperature.
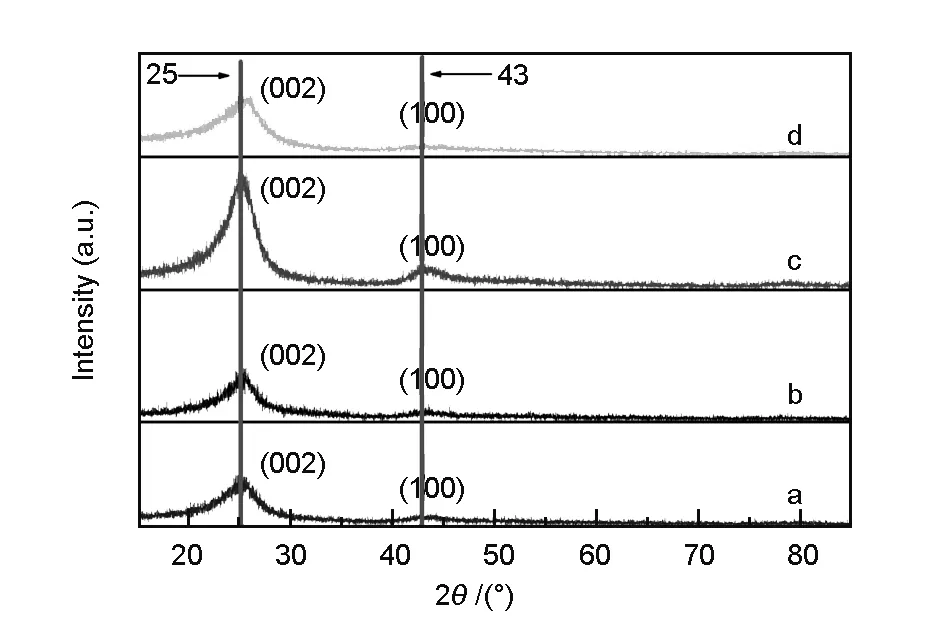
Fig. 4 XRD patterns of carbon spheres obtained at the deposition pressure of 5 kPa, the natural gas/H2 flow ratio of 1∶1 and at different deposition temperatures:(a) 1 070 ℃, (b) 1 100 ℃, (c) 1 150 ℃ and (d) 1 200 ℃.
3.2 Influence of the deposition pressure
The influence of the deposition pressure on the morphologies of carbon spheres prepared under various conditions showed that, with increasing pressure, the size of the carbon spheres increased (Table 2) and the conglomeration of carbon spheres became more prevalent (Fig. 5). At 3 kPa, the sphere size distribution was 500 nm- 2 μm, estimated from TEM images (Fig. 6a). In addition, many carbon nanoparticles were observed on the sphere surfaces (Fig. 6a). At 10 kPa, conglomeration of the spheres increased (Fig. 5b and 6b) and, at 15 kPa, no single carbon sphere was observed in TEM micrographs (Fig. 6c) and hollow spheres were also observed (Fig. 5c, white arrows).
3.3 Influence of the natural gas/hydrogen flow ratio
The natural gas/hydrogen flow ratio had different effects on the morphology of carbon spheres compared with the deposition temperature and pressure. Under the present conditions, it appeared that carbon spheres could not be obtained without hydrogen, yielding products that looked like carbon black neither spherical nor semi-spherical in shape (Fig. 7a). When the natural gas/hydrogen ratio was 1/2 carbon spheres produced had a uniform diameter (Fig. 7b). In addition, spheres were aligned like tree branches, not conglomerated into clusters as described previously, and their diameters were small (~100 nm) (Fig. 8a). When the gas ratio was adjusted to 1/4, sphere conglomeration appeared to cease, favoring contact with adjacent spheres rather than forming clusters (Fig. 7c). The size of the spheres became even smaller (50- 100 nm, Fig. 8b) than those deposited under the 1/2 ratio.

Fig. 5 SEM images of carbon spheres obtained at the deposition temperature of 1 150 ℃,the natural gas/H2 flow ratio of 1∶1 and at different deposition pressures:(a) 3 kPa, (b)10 kPa and (c)15 kPa.

Depositiontemperature(℃)Depositionpressure(kPa)Naturalgasflowrate(sccm)Hydrogenflowrate(sccm)Carbonspheresizedistribution11503500500500nm⁃2μm11505500500800nm⁃1.5μm1150105005001.5⁃2μm1150155005003⁃5μm
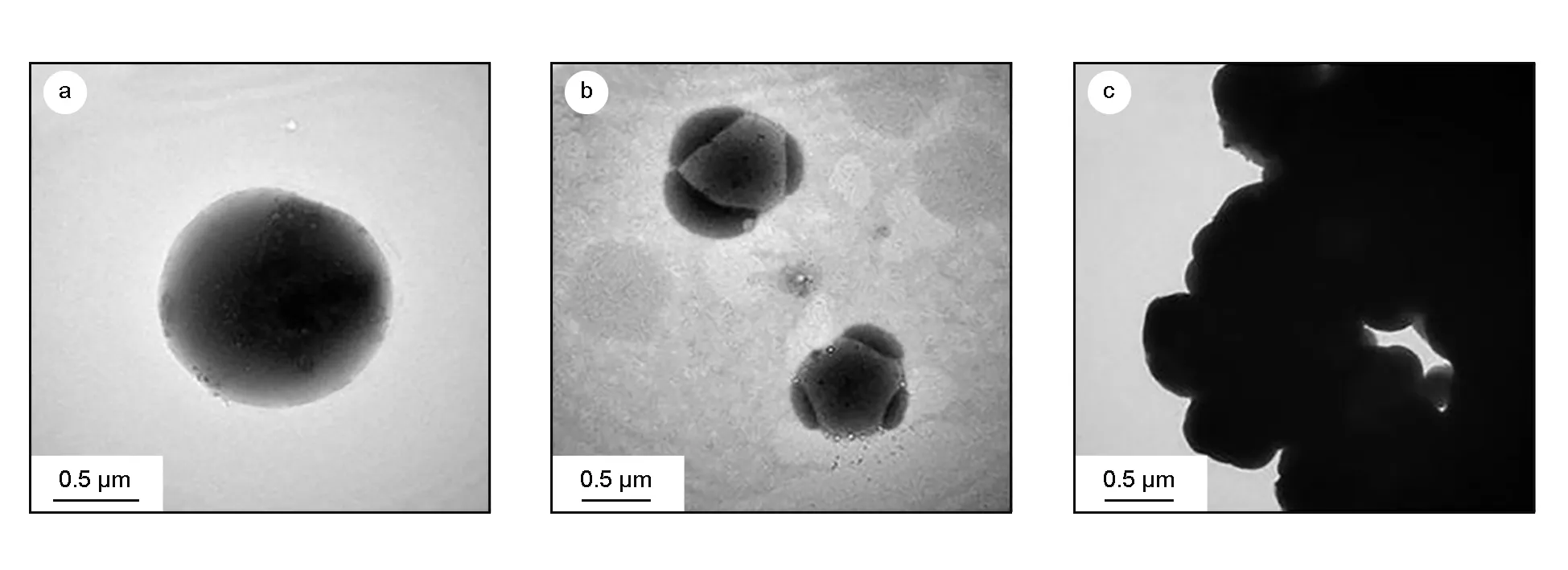
Fig. 6 TEM micrographs of carbon spheres obtained at the deposition temperature of 1 150 ℃, the natural gas/H2 flow ratio of 1∶1 and at different deposition pressures: (a) 3 kPa, (b) 10 kPa and (c) 15 kPa.

Fig. 7 SEM images of carbon spheres obtained at the deposition temperature of 1 150 ℃, the deposition pressure of 5 kPa, and at different natural gas/H2 flow ratios: (a) 1∶0, (b) 1∶2 and (c) 1∶4.

Fig. 8 TEM micrographs of carbon spheres obtained at the deposition temperature of 1 150 ℃, the deposition pressure of 5 kPa, and at different natural gas/H2 flow ratios: (a) 1∶2 and (b) 1∶4.
4 Discussion
The influence of deposition temperature, pressure, and the natural gas/ hydrogen flow ratio on growth and morphology of carbon spheres was investigated with the main goal to optimize the process parameters required to produce monodispersed carbon spheres and to further understand the mechanism of sphere formation under non-catalytic CVD conditions.
Deposition temperature showed no significant effect on the sphere size and structure (Fig. 2- 4), which was more or less consistent with the observations by Liu et al., who reported that temperature hardly affected the sizes of as-synthesized carbon spheres by CVD using Kaolin-supported transition metal catalysts[17]. It was indicated that sphere size was independent of the pyrolysis rate of natural gas. The temperature used here was high enough to form carbon sphere nuclei, which were small disordered carbon particles that are formed upon chemical vapor deposition of hydrocarbon and transformed to condensed carbon spheres with specific size. With the increase of the deposition temperature, the pyrolysis rate of the natural gas increased. This could be confirmed by the fact that the quantities of carbon spheres increased sharply. The reason that the deposition temperature did not have significant effect on size of carbon spheres was that the accumulation of spheres was favored than the coalescence of spheres into lager ones within the deposition temperature range from 1 070 to 1 200 ℃. This may be attributed to the fact that the numbers of nuclei increased with increasing temperature and, in a fixed volume, the probability of nuclei collisions was higher at higher temperatures. Concurrently, a reduction of the surface tension energy occurred as the growth of droplets of the natural gas pyrolysis product into larger spheres. Thus an increase of temperature effectively accelerated the pyrolysis rate, promoted the growth of spherical droplets and the formation of more droplets, which were eventually transformed to carbon spheres (Fig. 2d).
A comprehensive understanding of the growth mechanism of spherical shaped carbon was elaborated by Inagaki[25, 26]. Based on the present results, it was suggested that there was a competitive relationship between the sphere formation and sphere growth, such that, when carbon spheres nuclei were set to form carbon spheres, the inosculation or accretion of spheres took place at the same time, resulting in chain-like spheres or spheres of larger diameter. This may explain the size distribution of carbon spheres although they were prepared under the same conditions, as well as the prevalent accretion of carbon nuclei at high deposition temperature.
The present explanation for carbon sphere formation was somewhat similar to that proposed by Pol et al.[27], who suggested that the phenomena of producing carbon spheres with constant diameter resulted from an equilibrium between the nucleation of carbon spheres and the growth of carbon spheres at 700 ℃. In other words, the ratio between the rate of these two processes was constant. When the mixture was cooled down, the carbon sphere nuclei adhered to the surface of the carbon spheres and, since the number of carbon spheres did not change upon cooling, their growth resulted in the consumption of all the carbon nuclei. It is noteworthy that, in Pol et al.’s experiments, when the reaction system was rapidly cooled, the carbon sphere nuclei served as an adhesive material, filling the interspaces between the spheres, forming continuous, ‘sausage-like’ structures, but not producing monodispersed spheres, as was obtained at relatively slow cooling rates. Notably, inosculation of carbon sphere nuclei took place in both experiments here. However, in the present investigation, inosculation or accretion of carbon spheres was more likely to happen at higher temperature or pressure and had not to do with the rapid cooling or slow cooling. This difference may be attributed to the two different processes for production of carbon spheres. Carbon sphere formation occurred in a closed vessel cell, it was a static condition. Moreover, the carbon spheres were surrounded by a fluid phase. However, in our chemical vapor deposition experiments, reaction took place under a continuous condition. Undoubtedly, the pyrolysis rate of natural gas would be faster at higher temperature under other conditions unchanged. As a consequence, the formation rate of carbon sphere nuclei was faster as well, and the number of carbon spheres nuclei was higher at higher temperature, however, the growth rate of carbon spheres was not changed. In a fixed volume, this resulted in more possibility for collision of carbon sphere nuclei before they transformed to completed solid carbon spheres. Therefore, it was shown that the inosculation or conglomeration of carbon spheres occurred in the investigated conditions. With the increase of the deposition pressure under the other parameters unchanged, it was obvious to increase the chance of collision of carbon spheres nuclei, and as a result, several smaller carbon sphere nuclei would inosculate into larger ones, forming carbon spheres with larger sizes but not with a perfect spherical shape. On the other hand, several carbon sphere nuclei collided with each other, but did not grow into a larger one before solidification. Therefore, the presence of conglomerated carbon spheres existed. This explanation was affirmed by Fig. 2, Fig. 6 and Table 2.
Analysis of TEM and SEM images of carbon spheres revealed some interesting phenomena concerning the effects of the natural gas/hydrogen ratio. In the absence of hydrogen, irregular carbon particles were produced instead of spheres. With increasing the proportions of hydrogen, the sphere diameters decreased to 50-100 nm, the accretion rate of spheres decreased sharply (Fig. 7c, 8a), and the size distribution of spheres was narrowed. This observation was in agreement with the observations made by Pol et al.[28]. Pol had observed that the pyrolysis of hydrocarbon at high C/H ratios resulted in the formation of disordered flakes as well as carbon spheres. In his opinion, sphere formation involved hydrocarbon decomposition, resulting in the growth of clusters composed of aromatic rings. In the current study, it was assumed that, during the evolution of carbon spheres, hydrogen was likely to adsorb to the surface of carbon sphere nuclei and block or reduce the probability of carbon spheres nuclei collisions, resulting in an accretion of larger diameter carbon spheres. This assumption was, to some extent, supported by experiments results (Fig. 7 and 8.) in absent of hydrogen, the obtained products were more like carbon black, not in a spherical shape. It may be caused by the collision of carbon sphere nuclei to reduce the surface energy and then to form solid carbon. As a result, the morphology of obtained products were in other shape but not in a spherical shape. With the increase of hydrogen/natural gas ratio to 2, hydrogen can be adsorbed on the surface of carbon sphere nuclei, and reduce the extent of the collision of carbon spheres. To minimize the surface energy, the carbon sphere nuclei should shrink to form a spherical shape. Therefore, solid carbon spheres were obtained as shown in Fig. 7b, 8a. With a further increase of hydrogen/natural gas ratio up to 4, hydrogen was enough to adsorb on the entire surface of carbon sphere nuclei and this could prevent the collision of nuclei and control the size of nuclei. Therefore, monodispersed carbon spheres with small size were favorably formed under this condition as shown in Fig. 7c, 8b. It should be noted that this assumption did not present a clear understanding of the influence of hydrogen on the growth of carbon spheres. More research is required to further elucidate the mechanism involved.
5 Conclusions
Carbon nanospheres with different sizes were prepared successfully by non-catalytic chemical vapor deposition of natural gas/hydrogen mixtures. In the deposition temperatures ranging from 1 070 to 1 200 ℃, the deposition temperature showed no significant effect on the sphere size and structure. With the increase of deposition pressure, the chance of collision of carbon sphere nuclei was obviously increased. Hydrogen was likely to adsorb to the surface of carbon sphere nuclei and reduce the collision probability of carbon sphere nuclei. It was revealed that carbon nanospheres (50-100 nm) could be produced at 1 150 ℃ and 5 kPa with a gas mixture of natural gas/H2=1/4. A possible mechanism for carbon sphere formation was proposed and discussed.
[1] S H Chen, J F Wu, R H Zhou, et al. Porous carbon apheres doped with Fe3C as an anode for high-rate lithium-ion batteries[J]. Electrochimica Acta, 2015, 180: 78-85.
[2] W Kräschmer, L D Lamb, Fostriropoulos D R Huffman. Progress towards a quantitative understanding of antarctic ozone depletion[J]. Nature, 1990, 347: 347-354.
[3] E F Kukovitsky, S G L’vov, N A Sainov. Correlation between metal catalyst particle size and carbon nanotube growth[J]. Chem Phys Lett, 2000, 317: 97-102.
[4] A K Sinha, D W Hwang, L P Hwang. A novel approach to bulk synthesis of carbon nanotubes filled with metal by a catalytic chemical vapor deposition method[J]. Chem Phys Lett. 2000, 332: 455-460.
[5] W Benzinger, K J Huttinger. Chemistry and kinetics of chemical vapor infiltration of pyrocarbon-IV. Investigation of methane/hydrogen mixtures[J]. Carbon, 1999, 37: 931-940.
[6] M Sharon, K Mukhopadhyay, K Yase, S Ijima. Spongy carbon nanobeads-A new material[J]. Carbon, 1998, 36: 507-511.
[7] Z C Kang, Z L Wang. Pairing of pentagonal and heptagonal carbon rings in the growth of nanosize carbon spheres synthesized by a mixed-valent oxide-catalytic carbonization process[J]. J Phys Chem B, 1996, 100: 5163-5165.
[8] Z C Kang, Z L Wang. Mixed-valent oxide-catalytic carbonization for synthesis of monodispersed nano sized carbon spheres[J]. Philos Mag B, 1996, 73: 905-929.
[9] Y H Yi, G H Zhu, H Sun, et al. Nitrogen-doped hollow carbon spheres rapped with graphene nanostructure for highly sensitive electrochemical sensing of parachlorophenol[J]. Biosens Bioelectron, 2016, 86: 62-67.
[10] J Dou, S M Yin, J Chong, et al. Carbon spheres anchored Co3O4nanoclusters as an efficient catalyst for dye degradation[J]. Appl Catal A, 2016, 513: 106-115.
[11] V Vignal, A W Morawski, H Konno, et al. Effects of carbonization atmosphere and subsequent oxidation on pore structure of carbon spheres observed by scanning tunneling microscopy[J]. J Mater Res, 1999, 14: 1102-1112.
[12] E Auer, A Freund, J Pietsch, T Tacke. Carbons as supports for industrial precious metal catalysts[J]. Appl Catal, 1998, 173: 259-271.
[13] M Sharon, K Mukhopadhyay, K Yase, et al.Spongy carbon nanobeads-A new material[J]. Carbon, 1998, 36: 507-511.
[14] S Flandrois, B Simon. Electrode characteristics of pitch-based carbon fiber as an anode in lithium rechargeable battery[J]. Carbon, 1992, 37: 165-180.
[15] G Hu, D Ma, M J Cheng, et al. Direct synthesis of uniform hollow carbon spheres by a self-asembly template approach[J]. Chem Commun, 2002, 17: 1948-1949.
[16] H Hou, A K Schaper, F Weller. Carbon nanotubes and spheres produced by modified ferrocene pyrolysis[J]. Cheminform, 2002, 14: 3990-3994.
[17] J W Liu, M W Shao, Q Tang, et al. A medial-reduction route to hollow carbon spheres[J]. Carbon, 2003, 41: 1682-1685.
[18] X Yang, C Li, W Wang, et al. Chemical route from PTFE to amorphous carbon nanospheres in supercritical water[J]. Chem Commun, 2004, 3: 342-343.
[19] J Y Miao, D W Hwang, K V Narasimhulu, et al. Synthesis and properties of carbon nanospheres grown by CVD using Kaolin supported transition metal catalysts[J]. Carbon, 2004, 42: 813-822.
[20] M Inagaki. Carbon materials structure, texture and intercalation[J]. Solid State Ionics, 1996, 86: 833-839.
[21] M Inagaki. Room temprature exfoliation of graphite microgravity[J]. Carbon, 1993, 8: 711-713.
[22] A Huang, X Zhu, S Wang, et al. Synthesis of bimodal mesoporous carbon spheres from biomass starch and CBZ release[J]. Mater Lett, 2016, 169: 54-57.
[23] X Guo, H Liu, Y Shen, et al. Theoretical and experimental studies on silica-coated carbon spheres composites[J]. Appl Surf Sci, 2013, 283: 215-221.
[24] X Zhao, W Li, S X Liu. Coupled soft-template/hydrothermal process synthesis of mesoporous carbon spheres from liquefied larch sawdust[J]. Mater Lett, 2013, 107: 5-8.
[25] A Govindaraj, R Sen, B V Nagaraju, et al. Carbon nanospheres and tubules obtained by the pyrolysis of hydrocarbons[J]. Philos Mag Lett, 1997, 76: 363-367.
[26] V G Pol, M Motiei, A Gedanken, et al. Carbon spherules: Synthesis, properties and mechanistic elucidation[J]. Carbon, 2004, 42: 111-116.
[27] H S Qian, F M Han, B Zhang, et al. Non-catalytic CVD preparation of carbon spheres with a specific size[J]. Carbon, 2004, 42: 761-766.
[28] V G Pol, V S Pol, J M-C Moreno, et al. High yield one-step synthesis of carbon spheres produced by dissociating individual hydrocarbons at their autogenic pressure at low temperatures[J]. Carbon, 2006, 44: 3285-3292.
Preparation of carbon nanospheres by non-catalytic chemical vapor deposition and their formation mechanism
ZHANG You, YANG Wei, LUO Rui-ying, SHANG Hai-dong
(SchoolofPhysicsandNuclearEnergyEngineering,BeihangUniversity,Beijing100191,China)
Carbon spheres with different sizes were prepared by non-catalytic chemical vapor deposition fromgas mixtures of CH4and H2. The influence of the deposition temperature, pressure, and gas ratio on the formation and growth mechanism of the spheres was investigated. The carbon spheres obtained were characterized by XRD, SEM and TEM. Results indicated that carbon nanospheres (50-100 nm) could be obtained at 1 150 ℃ and 5 kPa from a gas mixture with a CH4/H2volume ratio of 0.25. The deposition temperature had no obvious effect on the sphere size and structure. The sphere size increased with the deposition pressure and the gas ratio. A model was proposed to elucidate their formation and growth.
Carbon spheres; CVD; Natural gas; Hydrogen; Nucleation mechanism.
National Defense Basic Scientific Research Program of China(A2120132005).
LUO Rui-ying. E-mail: ryluo@buaa.edu.cn
国防基础科研计划重点项目(A2120132005).
罗瑞盈. E-mail: ryluo@buaa.edu.cn
1007-8827(2016)05-0467-08
TQ127.1+1
A
10.1016/S1872-5805(16)60025-2
Receiveddate: 2016-07-28;Reviseddate: 2016-10-03
English edition available online ScienceDirect (http:www.sciencedirect.comsciencejournal18725805).
