Absent MicroRNAs in Different Tissues of Patients with Acquired Cardiomyopathy
2016-11-17ChristineSiegismundMariaRohdeUweKuhlFelicitasEscherHeinzPeterSchultheissDirkLassner
Christine S.SiegismundMaria RohdeUwe Ku¨hlFelicitas EscherHeinz Peter SchultheissDirk Lassner*f
1Institute for Cardiac Diagnostics and Therapy(IKDT),12203 Berlin,Germany
2Department of Cardiology,Campus Virchow,Charite´-University Hospital Berlin,13353 Berlin,Germany
ORIGINAL RESEARCH
Absent MicroRNAs in Different Tissues of Patients with Acquired Cardiomyopathy
Christine S.Siegismund1,a,Maria Rohde1,b,Uwe Ku¨hl1,2,c,Felicitas Escher1,2,d,Heinz Peter Schultheiss1,e,Dirk Lassner1,*,f
1Institute for Cardiac Diagnostics and Therapy(IKDT),12203 Berlin,Germany
2Department of Cardiology,Campus Virchow,Charite´-University Hospital Berlin,13353 Berlin,Germany
Received 28 November 2015;revised 29 March 2016;accepted 18 April 2016 Available online 28 July 2016
Handled by Andreas Keller
Cardiomyopathy;
Heart muscle biopsy;
Absent miRNAs;
Peripheral blood mononuclear cell;
Serum
MicroRNAs(miRNAs)can be found in a wide range oftissues and body fluids,and their specific signatures can be used to determine diseases or predictclinicalcourses.The miRNAprofiles in biological samples(tissue,serum,peripheral blood mononuclear cells or other body fluids)differ significantly even in the same patient and therefore have their own specificity for the presented condition.Complex profiles of deregulated miRNAs are of high interest,whereas the importance of non-expressed miRNAs was ignored.Since miRNAs regulate gene expression rather negatively,absent miRNAs could indicate genes with unaltered expression that therefore are normally expressed in specific compartments or under specific disease situations.For the first time,non-detectable miRNAs in different tissues and body fluids from patients with different diseases(cardiomyopathies,Alzheimer’s disease,bladder cancer,and ocular cancer)were analyzed and compared in this study.miRNA expression data were generated by microarray or TaqMan PCR-based platforms.Lists of absent miRNAs of primarily cardiac patients(myocardium,blood cells,and serum)were clustered and analyzed for potentially involved pathways using two prediction platforms,i.e.,miRNA enrichment analysis and annotation tool(miEAA)and DIANA miRPath. Extensive search in biomedicalpublication databases for the relevance of non-expressed miRNAs in predicted pathways revealed no evidence for their involvement in heart-related pathways as indicated by software tools,confirming proposed approach.
Introduction
Cardiovascular diseases as life-threatening diseases are the most common cause of death in Western European countries[1].Myocarditis and non-ischemic dilated cardiomyopathy(DCM)are acute or chronic disorders of heart muscle which arises mainly from myocardial inflammation or infections by cardiotropic viruses[1-6].More than 12 million patients in Europe and 15 million patients in the United States(US)are suffering from heart failure including four million with DCM,according to an estimation of the European Society of Cardiology(ESC)[3].The traditional clinical diagnosis based on individual patient’s clinical symptoms,medical and family history,laboratory and imaging evaluations should be expanded by endomyocardial biopsy(EMB)diagnostics(virology,histology,and immunohistochemistry)to confirm myocardial disease for following treatment decisions[3,7,8].
Improvements in human genetic studies and the continuously-expanding field of biomarker discovery revealed the potential of physiological biomarkers such as microRNAs(miRNAs)or gene expression profiles for diagnosis of complex diseases such as cardiomyopathies and for applications in personalized medicine[9-14].miRNA profiling can serve as a new exciting tool in modern diagnostics,which is comparable to gene expression analysis but with less amount of analytes.In addition,approximately 2500 human mature miRNAs have been discovered so far,which seems to be relatively small in number compared to the enormous number of genes discovered[15-21].
miRNAs are 20-22 nucleotides in length and highly conserved non-coding RNAs.They have been demonstrated to play multiple roles in negative or positive regulation of gene expression including transcript degradation,translational suppression,or transcriptional and translational activation.miRNAs are present in a wide range of tissues[10,15,18-20,22-26]. In body fluids such as serum,plasma or spinal fluid,miRNAs are protected from endogenous RNase activity by inclusion in exosomes or protein complexes[19,22,24,25].Due to their high biostability,circulating miRNAs can be used as reliable blood-based markers to identify cardiovascular or other human disorders[11,13,14,16-19].
Up to now,about 800 expressed miRNAs have been experimentally detected in EMBs[21].As shown for DCM,hypertrophic and inflammatory cardiomyopathy,the expression of miRNAs is characteristically altered in heart tissue[17].Differential miRNA patterns allow the identification of different heart disorders or disease situations[17,21].The role of these human miRNAs in pathogenesis[18]highlights their value as potential molecular biomarkers for complex diseases such as cardiomyopathies[16,27,28].The discriminating power of single miRNAs for diagnosis of complex diseases can be increased by its integration in a larger panelpresenting a specific miRNA signature.The application of myocardial miRNA profiling allows the differentiation of distinct phases of viral infections and the prediction of the clinical course of virally-induced disease at the time point of primary diagnostic biopsy[16,11,28,12].In the same individual,miRNA signatures in tissue,serum,peripheral blood mononuclear cells(PBMCs),or other body fluids show specific features for the current condition.Therefore these disease-specific biomarkers are of increasing interest for personalized medicine[12,29,30]. Non-expressed miRNAs in their entirety were ignored and corresponding data were rarely presented[25].Due to rather negative regulation of miRNAs in general,absent miRNAs would indicate genes which are not altered in terms of expression and therefore normally expressed in specific compartments.Occurrence of previously absent miRNAs could be an easy predictor for changes in functional activity in analyzed biological sample or in the disease situation under examination.
Analyses of expression data by bioinformatic software(miEAA and DIANA[31])are currently based on two strategies:(1)presentation of published data of deregulated miRNAs and their association with affected pathways or diseases and(2)prediction of involved miRNAs extrapolated from data of differentially expressed genes in corresponding disease situation as presented in the Kyoto Encyclopedia of Genes and Genomes(KEGG)schemata.Comprehensive expression data of indicated pathways or associated disorders are limited by availability of larger patient cohorts and comparability of analytical methods.
In this article,we focused on the non-detectable miRNAs measured on different platforms in myocardial tissue,blood cells,and serum in a large cohort of cardiac patients suffering from different forms of inflammatory or virally-induced heart muscle diseases[1-5].The underlying disease was diagnosed by routine EMB[3,6,32,33].The bioinformatic analyses of generated data using two current freely-available prediction tools revealed no evidence for their involvement in heart-related pathways.Experimental findings for cardiac patients were confirmed by comparisons of absent miRNAs in large cohorts of patients with different diseases[22,24,25]measured on the same analytical platforms.
Results
We performed miRNA expression studies with three analytical platforms,the Geniom Biochips(Febit,Heidelberg,Germany)and two TaqMan PCR-based high-throughput systems including low density array(LDA)and OpenArray(Thermo Fisher Scientific,Waltham,MA,USA).Based on the analysis of deregulated miRNAs,we presented lists and pathways of non-detectable miRNAs in different tissues of primarily cardiac patients.All data were generated in the same laboratory to facilitate comparative data analysis.
Comparison of absent miRNAs in EMBs,serum,and PBMCs of cardiac patients
miRNA preparations were obtained for patients with inflammatory or virally-induced cardiomyopathies from EMBs(n=284),PBMCs(n=67),or serum(n=287)including corresponding controls(Table 1).miRNAs in EMBs and serum were measured using two different platforms,which cover different sets of miRNAs(Table 2).Therefore,an additive list for EMBs and serum of absent miRNAs of each system was generated and used for all following calculations.A list of absent miRNAs was generated to indicate common or unique tissues in which miRNAs are not detectable(Table S1).Furthermore,a Venn diagram analysis was performed to reveal overlapping absent miRNAs in EMBs,serum,and PBMCs and miRNAs exclusively absent in particular tissues.As shown in Figure 1,we detected 1107 miRNAsin total absent in 1-3 sample groups.179 miRNAs were found to be absent in all three sample sources from cardiac patients. The miRNA Enrichment Analysis and Annotation Tool(miEAA)analysis showed that these miRNAs are involved in 685 pathways,implying possibly unaltered genes in these pathways.7 out of 685(1.0%)pathways were indicated to be heart-related.In addition,there are 2(0.3%)pathways described for viral myocarditis and DCM.Six miRNAs seem to be associated with these 2 pathways,which include hsamiR-19b-1-5p,hsa-miR-1295a,hsa-let-7a-5p,hsa-miR-99b-3p,hsa-miR-16-1-3p,and hsa-miR-34b-3p.
On the other hand,some miRNAs are absent only in one sample group.These include 3 miRNAs exclusively absent in EMBs,6 absent in PBMCs,and 650 absent in serum.For miRNAs absent in EMB or PBMC samples,miEAA revealed 8 pathways but none were heart-related pathways,whereas DIANA miRPath prediction indicated 3 heartrelated KEGG pathways for EMBs(57 others)and one for PBMCs(50 others),respectively.For the 650 miRNAs exclusively absent in serum samples,miEAA analysis revealed 14 pathways other than heart-relates ones.Since these patients suffer from cardiac diseases,the missing heart-related pathways are in concordance with the absence of these 650 miRNAs in serum.DIANA miRPath analysis for these miRNAs could not be performed due to limited miRNA input possibility.

Table1 Number of analyzed samples sorted by diagnosis and sample type of cardiac patients
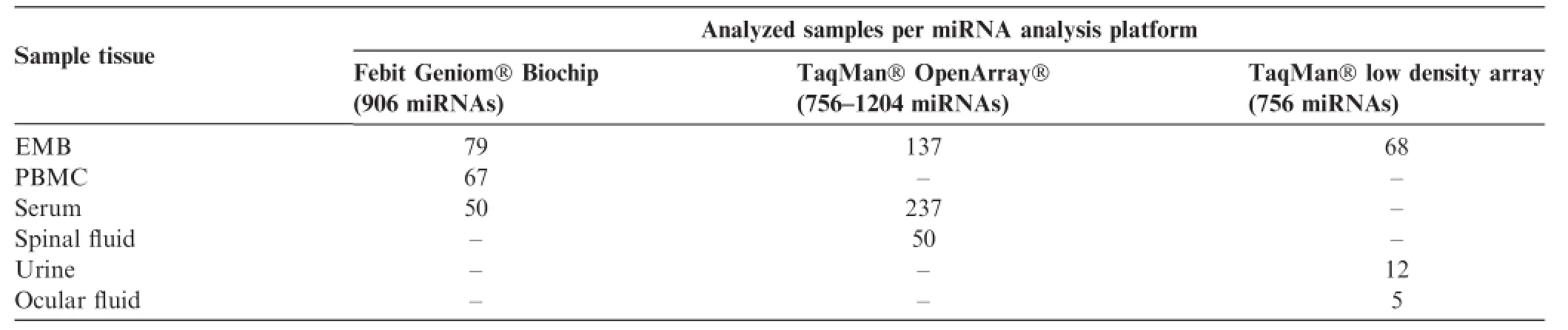
Table2 Number of analyzed samples sorted by platform and sample type
Comparison of absent miRNAs in cardiac patients to those in patients with other diseases
To validate experimental findings for cardiac patients and minimize methodological bias,panels of absent RNAs were evaluated with data from large cohorts of patients with different diseases[22,24,25]measured on the same analyticalplatforms.
We compared the aforementioned 1107 miRNAs absent in any one or more sample groups of EMBs,serum,and PBMCs taken from cardiac patients to those absent in spinal fluid(Alzheimer’s disease patients),urine(bladder cancer patients)or ocular fluid(ocular cancer patients)samples.There are totally 432,217,and 187 miRNAs absent in spinal fluid,ocular fluid,and urine samples,respectively.Venn diagram showed that 24 absent miRNAs were found to be common among all different tissue types tested.These 24 absent miRNAs were listed in Table 3[34-49].On the other hand,some miRNAs are only absent in one particular group.We found 13,9,and 31 miRNAs specifically absent in spinal fluid,urine and ocular fluid samples,respectively(Figure 2).
Pathway comparison using different prediction tools
Next,different pathway prediction tools were employed to analyze the pathways involving the 24 absent miRNAs sharedby all samples examined(Table 3).miEAA analysis revealed that these 24 absent miRNAs were involved in only one pathway and in regulation of 10 genes(Table 4)and one disease related to the analyzed miRNAs(Table 5),whereas more than 80 KEGG pathways were predicted with DIANA tool Tar-Base(Table 6)or microT(Table 7).As shown in Tables 4-7,the number of predicted pathways varied greatly depending on selected prediction algorithm.In addition,the predicted pathways based on the same 24 miRNAs showed associations with completely different diseases or organs using the two software tools.These data raise the question about plausibility and authenticity of the used pathway analysis tools.

Figure1 Venn diagram of absent miRNAs in different sample types from cardiac patients
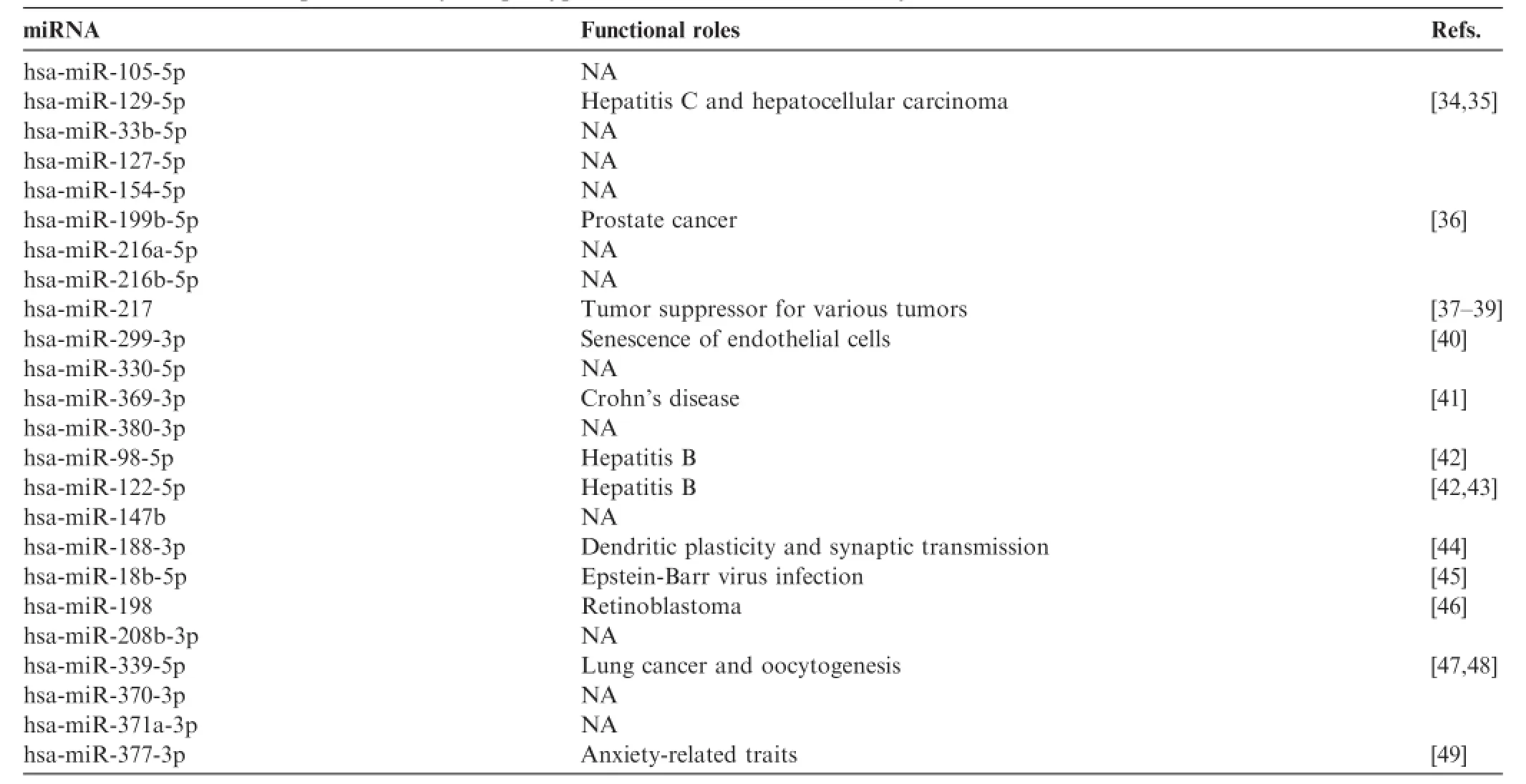
Table3 miRNAs not expressed in any sample type examined in the current study
Discussion
The importance of differentially-expressed miRNAs for characterization of various disease situations has been shown impressively[19,22-25,28,30].miRNAs are mainly negative regulators of gene expression.Therefore absentmiRNAs could indicate genes which are not affected for the disease situation examined or in the corresponding sample material.The different pattern of non-expressed miRNAs in separate tissues or organs could be explained by their biological functions.
The current study described,for the first time,the set of non-expressed miRNAs of the largest published cohort of patients(more than 200,including controls)with inflammatory or virally-induced cardiomyopathies that were diagnosed using EMBs[3,4,6,32].Absent miRNAs were revealed with different analytical platforms and compared to data from other diseased patients(Alzheimer’s disease,ocular cancer,bladder cancer)measured with identical assays in the same laboratory.The demonstration of differentially regulated miRNAs was not the aim of this study,corresponding data for the differentially-regulated miRNAs were shown previously[16,22,24,25,28].
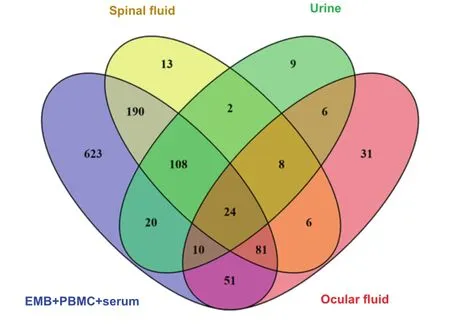
Figure2 Venn diagram of absent miRNAs in EMBs,PBMCs,and other body fluids

Table4 Overrepresented pathways and genes generated for the 24 commonly-absent miRNAs using miEAA ORA with FDR adjustment

Table5 Predicted diseases by enriched pathways generated for the 24 commonly-absent miRNAs using miEAA G(SEA)with FDR adjustment
Bioinformatic evaluation of identified absent miRNAs was performed by application of two freely-available pathwayprediction tools(miEAA and DIANA miRPath)to confirm experimental findings.For cardiac patients,6 heart-related pathways were recovered using miEAA.For the 6 miRNAs commonly not expressed in EMBs,serum,and PBMCs of cardiac patients,the software predicted association with myocarditis and DCM.Intensive search of biomedical publication databases provided no hint for their involvement in heart muscle diseases.Instead,hsa-miR-16-1-3p is related to chronic lymphocytic leukemia[50]and age-related cataract[51],whereas hsa-miR-34b-3p is related to spermatogenesis[52]. Similarly,hsa-let-7a-5p seems to be related to infectious mononucleosis but not cardiac diseases[45].Moreover,there lacks proof in literature or through in silico prediction tools for the involvement of the remaining 3 miRNAs in any disease or pathway.
DIANA analysis revealed one DCM-related pathway based on the 24 common miRNAs that are never detected in any of EMB,serum,PBMC,spinal fluid,ocular fluid or urine samples.Literature screening in PubMed retrieved no publications related to DCMor other cardiomyopathies for all 24 common absent miRNAs,therefore no experimental proof as well(Tables 3-7).
Both examples of detailed search(6 miRNAs and 24 miRNAs)for the relevance of miRNAs in distinct pathways revealed no evidence for their involvement in heart-related pathways as stated in DIANA tool.Pathway prediction tools could generate a broad amount and variety of potential networks which might only exist in theory but not in reality. In addition,these prediction tools have their limits in terms of amount of miRNAs that can be uploaded for analysis(especially DIANA tool),literature evidence of theirs predicted pathways,and comparability between different prediction tools.The best way to overcome this deficiency in pathway prediction is the evaluation of larger sample cohorts or multiple data sources.The involvement of sets of nonexpressed miRNAs for more diseases,as presented in this study,will sharpen the predictive power of bioinformatic analyses.These data are easily available but often not requested for publication.In future,predicted pathways should be double checked against list of absent miRNAs. The theoretical output of prediction tools shows high divergence from experimental validation,at least for our study. Therefore,users of prediction tools should take caution and assess the output critically.
The spectrum of non-expressed miRNAs in body fluids for defined diseases such as serum of patients suffering from cardiomyopathies is of keen interest.Today circulating miRNAs have the most important scientific and diagnostic impact[19,22,25,26,29,30].In this article,we described for the first time a panel of absent miRNAs in serum,PBMCs,EMBs,spinal fluid,urine,and ocular fluid of diseased patients including corresponding healthy controls.Implementing this spectrum in comparison to miRNA studies in different disorders,disease-specific miRNAs can be identified expeditiously.
Further studies have to confirm especially which of these absent serum miRNAs in cardiomyopathies are not versatile. Circulating miRNAs will be the novel diagnostic biomarkers,also for heart muscle diseases[14,15,21,24,26].Some of these serum miRNAs are present in other disorders not corresponding to cardiomyopathies,which could be of scientific interest for understanding of specific pathomechanisms or finally as therapeutic targets for miRNA modulation to deal with discrete disease situations.
There are some limitations in the current study.Three analytical platforms were used in generating data for overlapping sample sets to infer miRNAs absent alone or in different combinations.EMBs and PBMCs were measured with microarraybased technology for former sets of available miRNAs(miRBase v14),whereas Taqman PCR-based analysis were performed later and used to measure miRNAs in serum(OpenArrays,miRBase v16 and higher),EMBs(LDA and OpenArrays)[15],spinal fluid(OpenArray),urine(LDA),and ocular fluid(LDA).In addition,only two freelyavailable software tools were used for pathway prediction.
The bioinformatic and translational perspective of presented approach is manifold.This first preliminary study on non-detectable miRNAs should sensitize scientific community to present not only data of deregulated candidates,but also data of completely absent miRNAs[25]as a valuable dataset for improvement of commonly used software tools.Nondetectable miRNAs should be excluded from further prediction of corresponding pathways.Otherwise the collection of these data for all tissues,cells,or body fluids would be an important reservoir for future research or also pharmaceutical studies,and thus should be propagated by bioinformatics.The unexpected finding of previously-described non-expressed miRNAs in an experiment or clinical study will facilitate the identification of newly involved pathways or functional dysregulations in an observed setup.
Material and methods
Samples
EMB,PBMC,and serum samples were obtained from healthy controls and patients suffering from inflammatory or virally induced myocarditis as shown in Table 1[9,10,15,16,11,53,54].The study was performed within the Transregional Collaborative Research Centre(Inflammatory Cardiomyopathy-Molecular Pathogenesis and Therapy)[Sfb/Tr19].The study protocol was approved by the local ethics committees of the participating clinical centers,as well as by the committees of the respective federal states.An informed written consent was obtained from each participant.
Spinal fluid samples were received from healthy controls and patients suffering from Alzheimer’s disease,with the ethical statement described previously[25].Urine samples were acquired from healthy controls and patients harboring bladder cancer,with the ethical statement described previously[22,24]. In addition,we analyzed pooled ocular fluid from random patients.
miRNA isolation
miRNAs were obtained from patients,using mirVanaTMmiRNA Isolation Kit(Thermo Fisher Scientific,Waltham,MA,USA)resp.mirVanaTMPARISTMRNAand Native Protein Purification Kit(Thermo Fisher Scientific,Waltham,MA,USA)for low content samples such as serum,urine,ocular fluid,and spinal fluid according to manufacturer’s instructions.All presented expression studies were performed in the same laboratory.
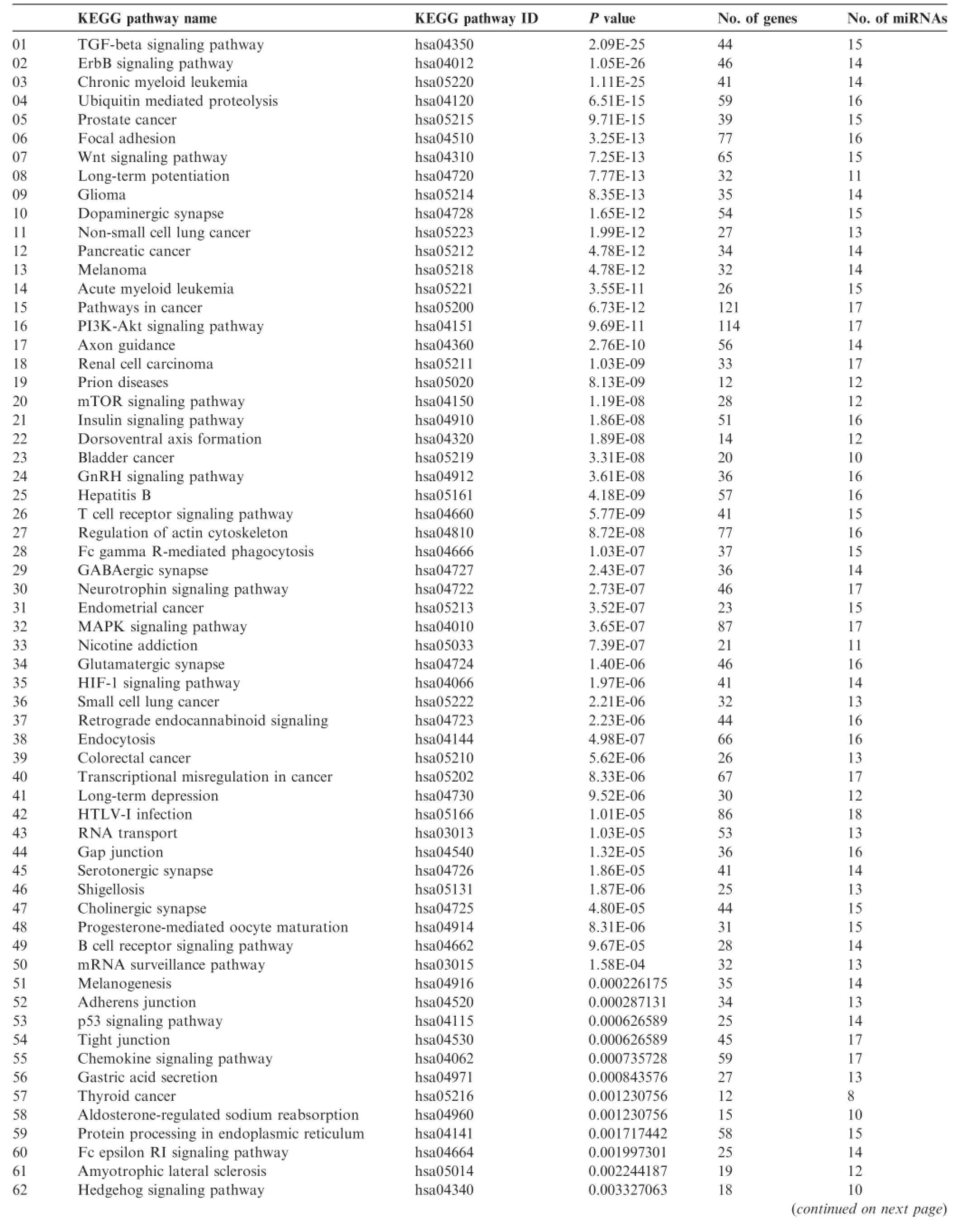
Table6 Pathways generated for the 24 commonly-absent miRNAs using DIANA TarBase

Table6 (continued)
miRNA reverse transcription,pre-amplification and expression analysis using TaqMan real-time PCR
Total RNA including miRNA fraction was reversely transcribed to cDNA using Megaplex stem-loop RT primer(Thermo Fisher Scientific,Waltham,MA,USA)for Human Pool A and B in combination with the TaqMan MicroRNA Reverse Transcription Kit(Thermo Fisher Scientific,Waltham,MA,USA).This allowed simultaneous cDNA synthesis of 377 unique miRNAs for Pool A and B each.Except for biopsy materials,a pre-amplification protocol was performed for all low content samples to increase the detection rate. The entire procedure for quantification using TaqMan® OpenArray®[25]and TaqMan®LDA[28]is described elsewhere.miRNAs which were not detectable or above cycle threshold 28(OpenArrays)resp.32(LDA)were considered to be absent in the sample.
miRNA labeling and expression analysis using Febit Geniom® Biochip
The expression analysis of all 906 miRNA and miRNA*sequences as annotated in Sanger miRBase version 14.0 was performed with the Geniom Real Time Analyzer(Febit,Heidelberg)and the Geniom biochip MPEA hsapiens V14. Sample labeling with biotin was carried out by using the ULS labeling Kit from Kreatech(Amsterdam,The Netherlands).All essential steps such as hybridization,washing,as wellas signalamplification and measurement,were done automatically by Geniom Real Time Analyzer.The resulting detection images were evaluated using the Geniom Wizard Software for background correction and normalization of generated data.miRNA expression analyses were carried out using the normalized and background-subtracted intensity values.
Bioinformatic algorithms and miRNA target identification
miRNAs not detectable in all samples of corresponding biological material were regarded as absent for this material and disease.All following bioinformatics analyses by pathway prediction tools were based on the list of these candidates. Venn diagrams of intersecting sets of miRNAs between different tissues and platforms are generated using Venny v2.0(http://bioinfogp.cnnb.csic.es/tools/venny/index.html).miEAA(http://www.ccb.uni saarland.de/mieaa_tool)and DIANA miRPath v.2.0[31]were used for miRNA target prediction and pathway analysis.Allgiven lists of miRNAs are translated and annotated according to miRBase v14 nomenclature.
Authors’contributions
CS conducted the bioinformatic algorithms and miRNAtarget identification,and drafted the manuscript.CS and MR carried out miRNA expression studies.DL conceived the study,and participated in study design and coordination.UK,FE,and HPS had primary responsibility for patient characterization and management.All authors discussed the results,read,and approved the final manuscript.
Competing interests
The authors declare no competing financial interests or relationships relevant to the content of this paper to disclose.
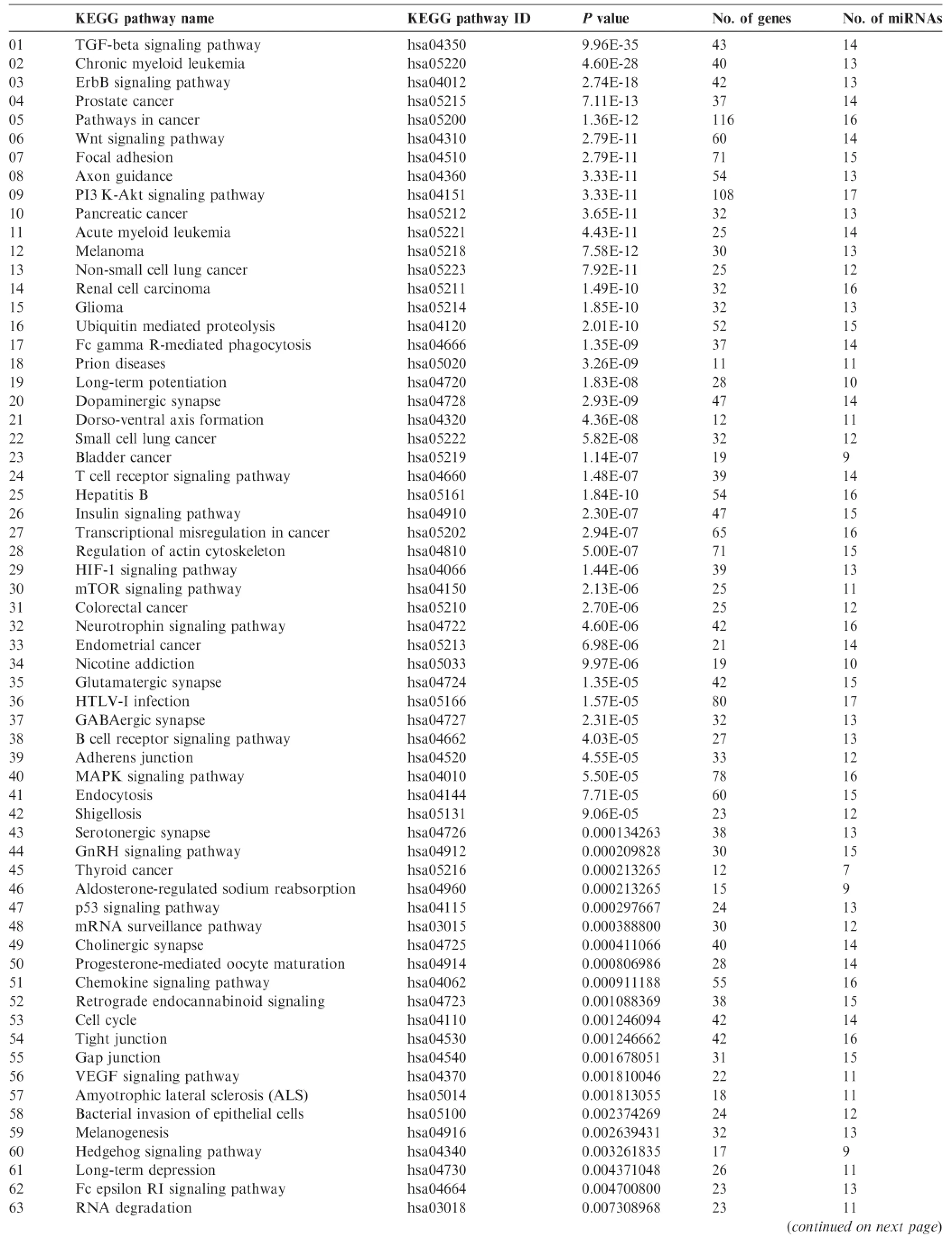
Table7 Pathways generated for the 24 commonly-absent miRNAs using DIANA microT
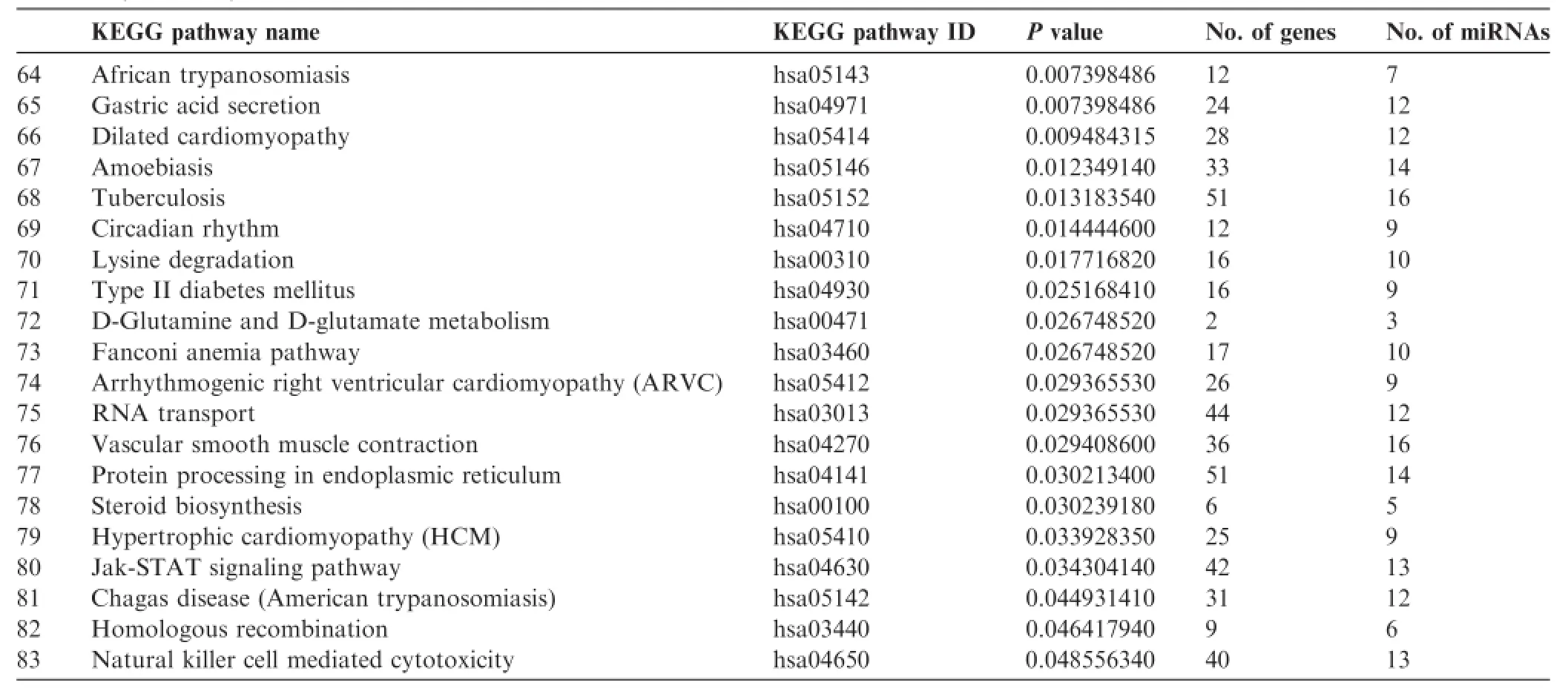
Table7 (continued)
Acknowledgments
This work was supported by grants from the German Research Foundation,the Transregional Collaborative Research Centre(Inflammatory Cardiomyopathy-Molecular Pathogenesis and Therapy)[SFB/TR19],and the Federal Ministry of Education and Research for the Small and Medium-sized Enterprises Innovative Program(Grant No.616 0315296)of Germany. We would like to thank Drs.Holger Jahn,Angelika To¨lle,and Enken Grundlach for permission to use the lists of absent miRNAs in their investigated specimens.We thank Mrs.Kitty Winter,Susanne Ochmann,and Claudia Seifert for their excellent technical assistance.
Supplementary material
Supplementary material associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j. gpb.2016.04.005.
[1]Schultheiss HP,Ku¨hl U,Cooper LT.The management of myocarditis.Eur Heart J 2011;32:2616-25.
[2]Ku¨hl U,Schultheiss HP.Viral myocarditis:diagnosis,aetiology and management.Drugs 2009;69:1287-302.
[3]Caforio ALP,Pankuweit S,Arbustini E,Basso C,Gimeno-Blanes J,Felix SB,et al.Current state of knowledge on aetiology,diagnosis,management,and therapy of myocarditis:a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases.Eur Heart J 2013;34:2636-48,2648a-d.
[4]Ku¨hl U,Pauschinger M,Seeberg B,Lassner D,Noutsias M,Poller W,et al.Viral persistence in the myocardium is associated with progressive cardiac dysfunction.Circulation 2005;112: 1965-70.
[5]Kieninger B,Eriksson M,Kandolf R,Schnabel PA,Scho¨nland S,Kristen AV,et al.Amyloid in endomyocardial biopsies.Virchows Arch 2010;456:523-32.
[6]Chimenti C,Frustaci A.Contribution and risks of left ventricular endomyocardial biopsy in patients with cardiomyopathies:a retrospective study over a 28-year period.Circulation 2013;128:1531-41.
[7]Towbin JA,Lowe AM,Colan SD,Sleeper LA,Orav EJ,Clunie S,et al.Incidence,causes,and outcomes of dilated cardiomyopathy in children.JAMA 2006;296:1867-76.
[8]Codd MB,Sugrue DD,Gersh BJ,Melton LJ.Epidemiology of idiopathic dilated and hypertrophic cardiomyopathy.A population-based study in Olmsted County,Minnesota,1975-1984. Circulation 1989;80:564-72.
[9]Lassner D,Ku¨hl U,Siegismund CS,Rohde M,Elezkurtaj S,Escher F,et al.Improved diagnosis of idiopathic giant cell myocarditis and cardiac sarcoidosis by myocardial gene expression profiling.Eur Heart J 2014;35:2186-95.
[10]Kuhl U,Lassner D,Dorner A,Rohde M,Escher F,Seeberg B,et al.A distinct subgroup of cardiomyopathy patients characterized by transcriptionally active cardiotropic erythrovirus and altered cardiac gene expression.Basic Res Cardiol 2013;108:372.
[11]Wittchen F,Suckau L,Witt H,Skurk C,Lassner D,Fechner H,et al.Genomic expression profiling of human inflammatory cardiomyopathy(DCMi)suggests novel therapeutic targets.J Mol Med(Berl)2007;85:257-71.
[12]Lassner D,Siegismund CS,Stehr J,Rohde M,Escher F,Tscho¨pe C,et al.Recent advances in molecular diagnostics and treatment of heart muscle diseases.J Anal Sci Method Instrum 2013;3:98-109.
[13]Heidecker B,Kittleson MM,Kasper EK,Wittstein IS,Champion HC,Russell SD,et al.Transcriptomic biomarkers for the accurate diagnosis of myocarditis.Circulation 2011;123:1174-84.
[14]Heidecker B,Kasper EK,Wittstein IS,Champion HC,Breton E,Russell SD,et al.Transcriptomic biomarkers for individual risk assessment in new-onset heart failure.Circulation 2008;118: 238-46.
[15]Siegismund CS,Rohde M,Ku¨hl U,Lassner D.Multiparametric diagnostics of cardiomyopathies by microRNA signatures. Microchim Acta 2014;181:1647-53.
[16]Ku¨hl U,Rohde M,Lassner D,Gross UM,Escher F,Schultheiss H-P.miRNA as activity markers in Parvo B19 associated heart disease.Herz 2012;37:637-43.
[17]Ikeda S,Kong SW,Lu J,Bisping E,Zhang H,Allen PD,et al. Altered microRNA expression in human heart disease.Physiol Genomics 2007;31:367-73.
[18]Thum T,CatalucciD,Bauersachs J.MicroRNAs:novelregulators in cardiac development and disease.Cardiovasc Res 2008;79:562-70.
[19]Jaguszewski M,Osipova J,Ghadri JR,Napp LC,Widera C,Franke J,et al.A signature of circulating microRNAs differentiates takotsubo cardiomyopathy from acute myocardial infarction.Eur Heart J 2014;35:999-1006.
[20]Zampetaki A,Mayr M.MicroRNAs in vascular and metabolic disease.Circ Res 2012;110:508-22.
[21]Leptidis S,El Azzouzi H,Lok SI,de Weger R,Olieslagers S,Olieslagers S,et al.A deep sequencing approach to uncover the miRNOME in the human heart.PLoS One 2013;8:e57800.
[22]To¨lle A,Jung M,Rabenhorst S,Kilic E,Jung K,Weikert S. Identification of microRNAs in blood and urine as tumour markers for the detection of urinary bladder cancer.Oncol Rep 2013;30:1949-56.
[23]Chung SH,Gillies M,Sugiyama Y,Zhu L,Lee SR,Shen W. Profiling of microRNAs involved in retinal degeneration caused by selective Mu¨ller cell ablation.PLoS One 2015;10:e0118949.
[24]To¨lle A,Ratert N,Jung K.MiRNA panels as biomarkers for bladder cancer.Biomark Med 2014;8:733-46.
[25]Denk J,Boelmans K,Siegismund C,Lassner D,Arlt S,Jahn H. MicroRNA profiling of CSF reveals potential biomarkers to detect Alzheimer’s disease.PLoS One 2015;10:e0126423.
[26]Chen X,Ba Y,Ma L,Cai X,Yin Y,Wang K,et al.Characterization of microRNAs in serum:a novel class of biomarkers for diagnosis of cancer and other diseases.CellRes 2008;18:997-1006.
[27]Baek D,Ville´n J,Shin C,Camargo FD,Gygi SP,Bartel DP.The impact of microRNAs on protein output.Nature 2008;455:64-71.
[28]KuehlU,Lassner D,Gast M,Stroux A,Rohde M,Siegismund C,et al.Differential cardiac microRNA expression predicts the clinicalcourse in human enterovirus cardiomyopathy.Circ Heart Fail 2015;8:605-18.
[29]Burgos KL,Javaherian A,Bomprezzi R,Ghaffari L,Rhodes S,Courtright A,et al.Identification of extracellular miRNA in human cerebrospinal fluid by next-generation sequencing.RNA 2013;19:712-22.
[30]Torres A,Torres K,Pesci A,Ceccaroni M,Paszkowski T,Cassandrini P,et al.Diagnostic and prognostic significance of miRNA signatures in tissues and plasma of endometrioid endometrial carcinoma patients.Int J Cancer 2013;132:1633-45.
[31]Vlachos IS,Kostoulas N,Vergoulis N,Georgakilas T,Reczko G,Maragkakis M.DIANA miRPath v.2.0:investigating the combinatorial effect of microRNAs in pathways.Nucleic Acids Res 2012;40:W498-504.
[32]Holzmann M,Nicko A,Ku¨hl U,Noutsias M,Poller W,Hoffmann W,et al.Complication rate of right ventricular endomyocardial biopsy via the femoral approach:a retrospective and prospective study analyzing 3048 diagnostic procedures over an 11-year period.Circulation 2008;118:1722-8.
[33]Cooper LT.Myocarditis.N Engl J Med 2009;360:1526-38.
[34]Song R,Liu Q,Liu T,Li J.Connecting rules from paired miRNA and mRNA expression data sets of HCV patients to detect both inverse and positive regulatory relationships.BMC Genomics 2015;16:S11.
[35]Chen L,Ma H,Hu H,Gao L,Wang X,Ma J,et al.Specialrole of Foxp3 for the specifically altered microRNAs in regulatory T cells of HCC patients.BMC Cancer 2014;14:489.
[36]Medina-Villaamil V,Martı´nez-Breijo S,Portela-Pereira P,Quindo´s-Varela M,Santamarina-Caı´nzos I,Anto´n-Aparicio LM,et al. Circulating MicroRNAs in blood of patients with prostate cancer. Actas Urol Espan˜olas 2014;38:633-9.
[37]Sun B,Yang M,Li M,Wang F.The microRNA-217 functions as a tumor suppressor and is frequently downregulated in human osteosarcoma.Biomed Pharmacother 2015;71:58-63.
[38]Su J,Wang Q,Liu Y,Zhong M.MiR-217 inhibits invasion of hepatocellular carcinoma cells through direct suppression of E2F3.Mol Cell Biochem 2014;392:289-96.
[39]Deng S,Zhu S,Wang B,Li X,Liu Y,Qin Q,et al.Chronic pancreatitis and pancreatic cancer demonstrate active epithelialmesenchymal transition profile,regulated by miR-217-SIRT1 pathway.Cancer Lett 2014;355:184-91.
[40]Jong HL,Mustafa MR,Vanhoutte PM,AbuBakar S,Wong PF. MicroRNA 299-3p modulates replicative senescence in endothelial cells.Physiol Genomics 2013;45:256-67.
[41]Jensen MD,Andersen RF,Christensen H,Nathan T,Kjeldsen J,Madsen JS.Circulating microRNAs as biomarkers of adult Crohn’s disease.Eur J Gastroenterol Hepatol 2015;27:1038-44.
[42]Tan Y,Ge G,Pan T,Wen D,Gan J.Serum miRNA panel as potential biomarkers for chronic hepatitis B with persistently normal alanine aminotransferase.Clin Chim Acta 2015;451:232-9.
[43]Ward J,Kanchagar C,Veksler-Lublinsky I,Lee RC,McGill MR,Jaeschke H,et al.Circulating microRNA profiles in human patients with acetaminophen hepatotoxicity or ischemic hepatitis. Proc Natl Acad Sci U S A 2014;111:12169-74.
[44]Lee K,Kim J-H,Kwon O-B,An K,Ryu J,Cho K,et al.An activity-regulated microRNA,miR-188,controls dendritic plasticity and synaptic transmission by downregulating neuropilin-2.J Neurosci 2012;32:5678-87.
[45]Gao L,Ai J,Xie Z,Zhou C,Liu C,Zhang H,et al.Dynamic expression of viraland cellular microRNAs in infectious mononucleosis caused by primary Epstein-Barr virus infection in children. Virol J 2015;12:208.
[46]Zhao JJ,Yang J,Lin J,Yao N,Zhu Y,Zheng J,et al. Identification of miRNAs associated with tumorigenesis of retinoblastoma by miRNA microarray analysis.Childs Nerv Syst 2009;25:13-20.
[47]Guo WG,Zhang Y,Ge D,Zhang YX,Lu CL,Wang Q,et al. Bioinformatics analyses combined microarray identify the desregulated microRNAs in lung cancer.Eur Rev Med Pharmacol Sci 2013;17:1509-16.
[48]Xu YW,Wang B,Ding CH,Li T,Gu F,Zhou C.Differentially expressed micoRNAs in human oocytes.J Assist Reprod Genet 2011;28:559-66.
[49]Hommers L,Raab A,Bohl A,Weber H,Scholz C-J,Erhardt A,et al.MicroRNA hsa-miR-4717-5p regulates RGS2 and may be a risk factor for anxiety-related traits.Am J Med Genet B Neuropsychiatr Genet 2015;168B:296-306.
[50]Rossi M,Fuligni F,Ciccone M,Agostinelli C,Righi S,Luciani M,et al.Hsa-miR-15a and hsa-miR-16-1 expression is not related to proliferation centers abundance and other prognostic factors in chronic lymphocytic leukemia.Biomed Res Int 2013;2013:715391.
[51]Li Y,Liu S,Zhang F,Jiang P,Wu X,Liang Y.Expression of the microRNAs hsa-miR-15a and hsa-miR-16-1 in lens epithelial cells of patients with age-related cataract.Int J Clin Exp Med 2015;8:2405-10.
[52]Salas-Huetos A,Blanco J,Vidal F,Godo A,Grossmann M,Pons MC,et al.Spermatozoa from patients with seminal alterations exhibit a differential micro-ribonucleic acid profile.Fertil Steril 2015;104:591-601.
[53]Lassner D,Rohde M,Gross UM,Escher F,Schultheiss HP,Linke R-P,et al.Classification of four chemically different amyloid types in routine endomyocardial biopsies by advanced immunohistochemistry.Amyloid 2011;18:76-8.
[54]Noutsias M,Pauschinger M,Gross U,Lassner D,Schultheiss HP,Ku¨hl U.Giant-cell myocarditis in a patient presenting with dilated cardiomyopathy and ventricular tachycardias treated by immunosuppression:a case report.Int J Cardiol 2008;128:e58-9.
*Corresponding author.
E-mail:info@ikdt.de(Lassner D).
aORCID:0000-0001-7909-1694.
bORCID:0000-0002-2046-8757.
cORCID:0000-0002-2476-0050.
dORCID:0000-0003-0678-5681.
eORCID:0000-0002-2185-3710.
fORCID:0000-0003-0815-7013.
Peer review under responsibility of Beijing Institute of Genomics,Chinese Academy of Sciences and Genetics Society of China.
http://dx.doi.org/10.1016/j.gpb.2016.04.005
1672-0229©2016 The Authors.Production and hosting by Elsevier B.V.on behalf of Beijing Institute of Genomics,Chinese Academy of Sciences and Genetics Society of China.
This is an open access article under the CC BY license(http://creativecommons.org/licenses/by/4.0/).
杂志排行
Genomics,Proteomics & Bioinformatics的其它文章
- Personalized Computer Simulation of Diastolic Function in Heart Failure
- Comparison of Cox Model Methods in A Low-dimensional Setting with Few Events
- Profiling and Validation of the Circular RNA Repertoire in Adult Murine Hearts
- Comparative Gene Expression Analysis of Mouse and Human Cardiac Maturation
- The Role of Quality Control in Targeted Next-generation Sequencing Library Preparation
- Long non-coding RNA Databases in Cardiovascular Research
