New frontiers in biomaterials research for tissue repair and regeneration
2016-11-16HuilingLiuHaoranLiuAaronClaskyHuilinYangLeiYang
Huiling Liu, Haoran Liu, Aaron Clasky, Huilin Yang, Lei Yang(✉)
1Institute of Orthopedics and Department of Orthopedics, the First Affiliated Hospital, Soochow University, Suzhou 215006, China
2International Research Center for Translational Orthopedics (IRCTO), Soochow University, Suzhou 215006, China
3Nanotechnology Engineering Program, Faculty of Engineering, University of Waterloo, Ontario N2L 3G1, Canada
§These authors contributed equally to this work.
New frontiers in biomaterials research for tissue repair and regeneration
Huiling Liu1,2,§, Haoran Liu1,2,§, Aaron Clasky2,3, Huilin Yang1,2, Lei Yang1,2(✉)
1Institute of Orthopedics and Department of Orthopedics, the First Affiliated Hospital, Soochow University, Suzhou 215006, China
2International Research Center for Translational Orthopedics (IRCTO), Soochow University, Suzhou 215006, China
3Nanotechnology Engineering Program, Faculty of Engineering, University of Waterloo, Ontario N2L 3G1, Canada
§These authors contributed equally to this work.
ARTICLE INFO
Received: 20 May 2016
Revised: 30 May 2016
Accepted: 31 May 2016
© The authors 2016. This article
is published with open access
at www.TNCjournal.com
biomaterials;
tissue engineering;
hydrogel;
metamaterials;
synthetic biology
The field of biomaterials has recently emerged to augment or replace lost or damaged tissues and organs due to the human body's limited ability to self-heal large defects. Historically, metallic components, polymers, ceramics, and composite materials were utilized as synthetic materials along with natural materials to assist in therapy. Various novel biomaterials were developed to respond to a significant amount of new medical challenges in the past decade. Therefore, there is a need to review these newly developed biomaterials and their potential to improve tissue repair and regeneration in a variety of applications. Here, we briefly review the different strategies and attempts to use novel biomaterials, including self-assembled and macromolecular biomaterials, hydrogels, metamaterials, decellularized tissues, and biomaterials obtained via synthetic biology, used either for tissue repair and regeneration or for therapeutic use by exploiting other mechanisms of healing. All these methods aim to create functional materials, devices, systems, and/or organisms with novel and useful functions on the basis of catalogued and standardized biological building blocks. This review details the various methods and introduces the applications of these biomaterials in tissue repair and regeneration, especially for bone, nerve, and skin applications.
1 Introduction
The human body is capable of repairing or regenerating small defects caused by diseases or accidents. However, large defects are difficult or impossible for the injured tissues to heal on their own due to congenital limitations[1]. Therefore, the use of biomaterials has emerged as a promising approach for regenerative applications. In a broad sense, biomaterials include any matter, surface, or construct that interacts with living issues. They are developed and applied to augment or replace natural tissues and organs that have been lost or damaged as a result of injury, disease, or aging.
Biomaterials can be derived either from nature or by utilizing metallic components, polymers, ceramics, or composite materials. The medical use of metallic materials can be traced back to the 19th century and was primarily driven by demands for novel approaches to bone repair[2]. Metallic materials play a major role in orthopedic treatments and have recently been studied in the context of reconstruction of hard tissues and novel alloys for bone repair and regeneration. On the contrary, polymers attract more attention in repairing and regenerating soft tissues. There has been an increasing interest in blends of synthetic and natural polymers which can form new materials with improved mechanical properties and biocompatibility[3]. Before the 1960s, different types of glasses and ceramics were used to bind to living bone. Nowadays, ceramics are widely used in clinical treatments as dental and bone implants[4]. Apart from being used as implants due to their physico-chemical properties, some ceramics also have excellent resistance, which makes them suitable as replacements for malfunctioning joints. Furthermore, to meet some special needs required in medical applications, two or more constituent materials can be combined into a composite to obtain significantly different physical or chemical properties compared with those of single components.
As time has advanced, medical demands such as replacement of injured organs and repair of damaged tissues have become more urgent. Thanks to biotechnological advancements and innovations, various novel biomaterials have been developed to meet these demands. Hence, there is a need to review new biomaterials that have been developed over the past decade. This article will explore some new frontiers of biomaterials research, such as self-assembled and macromolecular biomaterials, biomaterials synthesized using synthetic biology, novel hydrogels, metamaterials, and decellularized tissues. The bioactive self-assembly technique, by reversibly modifying structures of peptides, enables a variety of self-assemblies including layered and lamellar structures. It also shows good potential for designing novel scaffolds for neuronal engineering applications. Synthetic biology is an emerging research area that will provide new opportunities not only for development of novel drugs and “green” fuels but also for targeted therapies and biomaterials for tissue repair and regeneration. Hydrogels are popular in tissue engineering, implantable devices, and biosensors due to their high water content and superior tribological properties[5]. Metamaterials have been studied for their possible application as biomaterials because of their unique mechanical, optical, and electrical properties. Additionally, significant efforts have been made to develop decellularized tissues, which are prepared by isolating the extracellular matrix (ECM) of a tissue from its inhabiting cells, due to their excellent immunoreaction avoidance. Although many applications are still in the experimental phase, these materials may have a substantial impact on future therapeutic strategies. This article will attempt to identify trends in biomaterials research with respect to tissue repair and regeneration, especially for nerve and bone tissues.
2 Self-assembled and macromolecular biomaterials
Molecular self-assembly is a process by which non- covalent, weak interactions formed between molecules drive their assembly and organization, creating supramolecular structures that define the material. Most self-assembling molecules are amphiphilic, containing both hydrophobic and hydrophilic domains, such as lipids, peptides, and proteins. The concept of using amphiphilicity to drive molecular assembly is grounded in nature, where amphiphilic molecules commonly serve as building blocks for the construction of functional assemblies, such as cellular membranes, cytoskeleton, and extracellular matrix[6]. Supramolecular self-assembly can combine molecular signals, control the concentration of signals in a specific structure, and even mediate interactions among the structures in order to create more complex objects.
Common self-assembling monomers include lipids, block copolymers, peptides, and proteins. Intermolecular interactions that define and drive self-assembly include formation of polar interactions and hydrophobic association, respectively. The use of peptides to build self-assembling monomers is logical because it allows incorporation of known biological signals recognized by receptors and other intracellular proteins. The self-assembly of peptide molecules as building blocks for bottom-up fabrication of biomaterials is ofincreasing importance due to the inherent biological specificity of peptides. The biomaterials discussed below are organized according to the tissues for which they are used.
2.1 Nerve
At least 2 million people worldwide suffer from peripheral nerve injuries. Autografting, the current gold standard for treatment of peripheral nerve injuries, possesses a number of disadvantages, such as limited availability, possible loss of sensation at the donor site, and the requirement for two surgeries[7]. Molecular self-assembly has recently emerged as a new approach for engineering artificial scaffolding materials that emulate natural ECM both structurally and functionally, especially for peripheral nerve repair. Self-assembling not only incorporates specific biological components of the ECM but also mimics the process of ECM assembly from the bottom up[8]. Specific polypeptide sequences have the capacity to self-assemble into various structures, ranging from the assembly of β-sheets via hydrogen bonding to cylindrical micelles via hydrophobic interactions.
For example, Zhang et al.[9]developed a scaffolding material that self-assembled from amphiphilic oligopeptides that contained alternating positively and negatively charged residues separated by hydrophobic residues. The scaffold material, RAD16 (AcN-RARADADARARADADA-CNH2; A, alanine; R, arginine; D, aspartic acid) was employed to measure neuronal growth using rat hippocampal neurons. Growth of these cells in culture, accompanied by functional synapse formation, was observed.
In order to comprehensively and extensively understand this field, Aggarwal et al.[10]reviewed the structure, synthesis, and assembly of central nervous system (CNS) myelin. In the CNS, oligodendrocytes synthesize large amounts of cellular membrane to form multiple myelin internodes of highly stable membranes with a specific set of tightly packed lipids and proteins. Myelin is a remarkably stable structure that appears to exist close to its thermodynamic equilibrium state. Interestingly, self-assembly is in a static state of minimum energy due to interaction of the components to form a higher-order unit that itself is in equilibrium.
To further understand the phenomena involved in self-assembly of these biomaterials and to develop effective therapies in neural tissue engineering, glycine spacers[11]have been added to influence the functional motif exposure and the self-assembling propensity of functionalized substrates tailored for neural stem cell cultures[12].
2.2 Bone
Due to their special properties, peptides are also widely used in bone repair and regeneration. The use of peptide amphiphiles (PAs) for biomimetic bone mineralization and bone regeneration is an important research area[13]. PAs can self-assemble from aqueous media into supramolecular nanofibers with high aspect ratios. This can then direct the mineralization of hydroxyapatite (HA) crystals that align themselves with the long axes of collagen fibers. Biomimetic mineralization has been sequentially extended from 2D to 3D by utilizing self-assembling peptide nanostructured gels containing phosphoserine residues[14]. It has been shown that enzyme-mediated harvesting of phosphate ions combined with nanofiber surface nucleation can lead to spatially selective and biomimetic mineralization in an in vitro 3D environment by using functionalized PA. These results also suggest that both spatial and temporal elements are necessary to achieve biomimetic mineralization in synthetic materials for bone repair.
In other related research, conventional controlled release technologies and surface engineering were combined with self-assembly to form biomimetic scaffolds for tissue regeneration. Salem et al.[15]reported a novel porous scaffold that can be delivered by syringe into a tissue or cavity, such as cartilage or bone, as a polymer and cell slurry. It can fill the damaged space within the body and supply signals to cells to trigger their proliferation and differentiation. The slurry can self-assemble immediately into a porous scaffold with the cells uniformly distributed. Self- assembly involves the cross-linking of polymer particles by a molecular-interaction mechanism that does not interfere with cell function. The self-assembled porous scaffolds within the tissue act as prerequisites for tissue remodeling, whereas the basic components of the scaffold are biodegradable microparticles of poly(α-hydroxyacids) or other polymers, as shown in Figure 1.
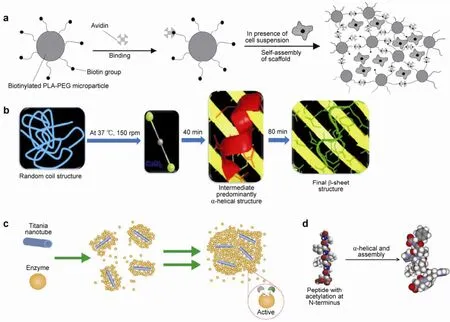
Figure 1 (a) Schematic representation of scaffold self-assembly, reproduced with permission from Ref. [15]. (b) Self-assembly process of fibroin, regulating the conformation of silk biomaterials, reproduced with permission from Ref. [16]. (c) Self-assembly of protein-based biomaterials initiated by titania nanotubes, reproduced with permission from Ref. [17]. (d) Schematic representation of the peptide motif forming helical fibers through α-helical and assemble[18].
Another strategy[19]to form hybrid bone implants was established with bioactive implant surfaces by self- assembly of PA nanofibers within porous titanium (Ti). The PA-Ti hybrid strategy allows for tailoring of the initiation of mineralization and can direct a cellular response from the host tissue into porous implants to form new bone and thereby improve the fixation, osseointegration, and long-term stability of implants.
Natural, ultrasmall, predominantly aliphatic peptide amphiphiles that self-assemble to form strong temperature-resistant helical fibers[18]in scaffolds are suitable for tissue engineering (Figure 1d). The resulting materials are biocompatible and heat resistant up to 90°C, and demonstrate tunable, high mechanical strength. The combination of their physical properties and biocompatibility makes these peptides well suited for regenerative applications in spine regeneration and cartilage replacement.
2.3 Skin
Tissue-engineered skin repair and regeneration represents an innovative therapeutic option for the treatment of burns and skin ulcers. Skin substitutes play a crucial role in both clinical applications and fundamental research. They can provide a mechanical barrier against infection and fluid loss[20]or replace animal models for dermopharmaceutical testing[21]. Skin substitutes produced via the self-assembly approach share many characteristics with normal human skin. Skin substitutes produced via this approach have demonstrated many advantages compared to other skin substitute models, especially with regard to theirhistological characteristics and similarity to normal human skin[22].
Jean et al. reported the self-assembly approach methodology in detail, as well as the use of tissue engineering in the skin engineering field[23]. The self-assembly approach is based on the capacity of fibroblasts to create their own extracellular matrix in vitro, producing cell sheets that are easy to handle. Moreover, various other cell types (e.g., keratinocytes, melanocytes, adipocytes, and endothelial and immunological cells) can be added to this system as required. Therefore, a skin substitute devoid of exogenous extracellular matrix proteins and synthetic material is produced, which demonstrates many histological, physico-chemical, and mechanical characteristics found in normal human skin in vivo.
The self-assembly approach for skin bioengineering can lead to a better understanding of pathological and normal skin mechanisms[21]for developing new pharmaceutical therapies for skin engineering.
2.4 Other applications
2.4.1 Drug delivery system
For block copolymers, self-assembly can be performed in the presence of small molecules. This process is usually spontaneous, producing drugs encapsulated in micelles. For instance, as an anticancer and anti- rheumatoid arthritis agent, methotrexate (MTX), a PEG-PLA block copolymer, can be sequestered in micelles with a loading capacity of 12% by weight and a maximum encapsulation efficiency of 50%[24].
Jeong and Lim[25]developed functional macrocyclic peptide building blocks that formed self-assembled peptide vesicles with molecular recognition capabilities. Macrocyclic peptides are significantly different from conventional amphiphiles, because they can self- assemble into vesicles at very high hydrophilic-to- total-mass ratios. The unique features of small size and robust structural and thermal stability make the peptide vesicles capable of entrapping hydrophilic drugs and releasing the payload over a long period of time. This allows the cells to internalize drugs in a highly efficient manner due to the cell-penetrating and nucleus localization activities of the HIV-1 Rev ARM peptide. It is important to note that such peptide vesicles exhibit molecular recognition capabilities, in that they are able to bind selectively to target RNA through surface displayed peptides. It was demonstrated that self-assembled peptide vesicles can be used as strong, controlled intracellular delivery vehicles that can recognize specific biomacromolecular targets and simultaneously modulate biomacromolecular interactions.
2.4.2 Protein-based self-assemblies
Silk fibroin self-assembly can consist of gradual conformational transition from random coil to β-sheet structure. Dubey et al.[16]elucidated the intermediate secondary conformation in the presence of Ca2+ions during fibroin self-assembly as well as the mechanism behind this interaction through molecular modeling of the N-terminal region of fibroin with Ca2+ions. The modulation of the self-assembly mechanism of fibroin can potentially be utilized to develop silk- based biomaterials consisting of a tunable secondary conformation, which may have a significant implication in the development of tailor-made silk biomaterials for tissue engineering.
The assembled biomaterials must sufficiently retain the near-native structure of proteins and provide
molecular access to biocatalysts and biomaterials in order to stabilize and deliver biopharmaceuticals. Protein-based biomaterials are a promising strategy for creating robust, highly selective biocatalysts. The first non-covalent assembly of enzymes into an insoluble solid was reported by Forstater et al., which contained >99% enzymes by weight and had enhanced catalytic activity enabled by the unique under- coordinated surface chemistry of the TiO2anatase (001) surface. This method could immobilize extensive protein multilayers on a nanomaterial and cause it to emerge as a self-assembled mesophase of protein- nanotube conjugates. These findings also present a nanotechnology-enabled mechanism of biomaterial growth which has proven to be a fruitful strategy for creating novel enzyme immobilization substrates, and may act as an enabling technology for creating protein- based biomaterials or enzyme biocatalysts[17].
The incorporation of nanoparticles during the hierarchical self-assembly of protein-based materials can impart functionality to the resulting composite materials. Through the use of a recombinant enhanced green fluorescent protein-ultrabithorax (EGFP-Ubx)fusion protein in conjunction with luminescent CdSe- ZnS core-shell quantum dots (QDs), Majithia et al. demonstrated that the structure and nanoparticle distribution of composite fibers is sensitive to the method of nanoparticle addition due to physicochemical properties of both the nanoparticle and the protein[26]. QDs introduced at different stages of the EGFP-Ubx hierarchical self-assembly process affect fiber morphology in a controllable manner prior to the initiation of the EGFP-Ubx protein self-assembly process. This represents a unique strategy to induce surface roughness in protein materials generated by bottom-up self-assembly techniques. Moreover, different QD surface functional groups and surface charges affect the self-assembly process and lead to varying nanoparticle distributions within macroscale materials. Similar design motifs can be applied to generate polymeric protein-nanoparticle composites that can serve as optically active, functionalized sensors.
3 Hydrogels
Hydrogels consist of a 3D network formed by polymer chains through physical or chemical bonds with large amounts of water retained within[27](Figure 2). Due to their inherent characteristics, hydrogels have been widely employed in permanent contact with living tissues[28], the main reason for which is their excellent biocompatibility. For instance, the biological activity and function of biomolecules will not be impaired due to prolonged contact with the hydrogel[29].
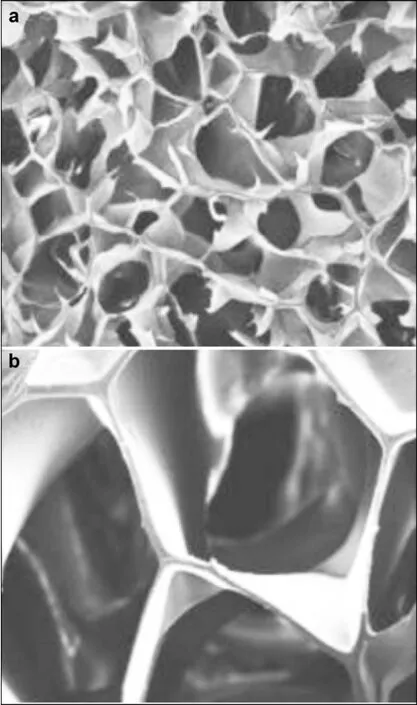
Figure 2 (a) and (b) Scanning electron microscope (SEM) images of hydrogel scaffold, reproduced with permission from[27].
3.1 Nerve
Due to their water content and similarity to soft tissues (tunable elasticity, nutrient permeability, biocompatibility, and low interfacial toughness), hydrogels have caught the attention of researchers for use as ECM mimetic scaffolds. This has led to a large range of hydrogels to be designed for building scaffolds. Furthermore, the properties of these scaffolds, such as degradation rate and mass porosity, make it convenient to tune biochemical cues to better mimic the native microenvironment[30].
Hydrogels are intended to be used as nerve guidance materials that are often shaped as tubes to improve regeneration and functional outcomes. As alternatives to grafts for peripheral nerve repair, poly(2-hydroxyethyl methacrylate-co-methacrylate) (PHEMA-MMA) porous tubes have been synthesized to study the regenerative capacity of peripheral nerves[31]. Hydrogel nerve tubes, each with an inner diameter of 1.3 mm, an outer diameter of 1.8 mm, and a length of 12 mm, were produced by a technique developed by Dalton and Shoichet[32]. The ability to support nerve regeneration was assessed in vivo at 4, 8, and 16 weeks using a series of tests. The results indicated that axonal regeneration in artificial tubes was comparable to that in autografts at 8 and 16 weeks. Likewise, another team reported the in vitro processing and in vivo application of the same hydrogel tubes filled with collagen gel impregnated with acidic fibroblast growth factor (FGF-1)[33]. Nerve regeneration was improved in tubes containing growth factor,compared with empty tubes and those without tubes. The tubes with FGF-1 were shown to be a promising alternative to autografts for regeneration of damaged nerves. A silk fibroin peptide (SF16) was used as a component in the scaffolds and the functional recovery of the nerve was evaluated by walking track analysis[34]. Nerve cells in SF16 demonstrated better performance, including greater axon density, larger average axon diameter, and thicker myelin, compared to that of negative control (physiological saline). The performance of a polyamidoamines hydrogel implant as a scaffold was also reported[35]. PAA hydrogels have not been used as scaffolds for nerve regeneration, which makes them an innovative implantable material for tissue engineering. The data show that PAA has excellent properties, including biocompatibility, and lack of inflammatory reaction, and can lead to functional nerve regeneration. PAA hydrogel tubes as conduits for the regeneration of injured peripheral nerves have shown great potential.
The control of neural cell behavior, including migration, proliferation, and differentiation, is critical to improve the ability of nerves to repair and regenerate themselves. Bai et al. studied the control of neural cell fate by investigating the synergy between topography and mechanical rigidity based on the control of the features of biomaterials[36]. Hydrogels were formed by silk fibroin self-assembled nanofibers with a β-sheet-enriched structure to concurrently utilize topography and mechanical rigidity. Stiffness was tuned by different annealing processes, which also ensured coherence of topography. Compared to neural stem cells on non-annealed nanofibers, cells on annealed nanofibers, with stiffness similar to that of nerve tissue, differentiated into neurons. The mechanical properties of nanofiber hydrogels could modulate migration and differentiation without affecting proliferation. By promoting differentiation of nerve stem cells and inhibiting glial differentiation differentiation was regulated and the neuroinflammation and neurodegeneration that occur when regulating both was inhibited by adding growth factors. Nerve stem cells grown on nanofiber hydrogels showed a tendency to favor neuron differentiation and inhibit glial differentiation without any addition of growth factors. These results demonstrate that the control of nerve cell behavior has the potential to repair injured nerves by combining topographic and mechanical cues.
3.2 Bone
Hydrogels overcome the limitations of autografts as a therapeutic method of bone repair and regeneration. These limitations include donor site morbidity and limited availability. Though the problem of donor site morbidity can be addressed by making use of allografts, the drawbacks of allografts (slow bone formation and disease transmission) cannot be neglected[30]. A solution to this problem is introduction of osteoinductive growth factors into the defect site. However, adverse reactions may be caused by such a high dose, which is why Martínez-Sanz et al. reported developing nonimmunogenic and degradable biomaterials that could retain and release growth factors for a prolonged time to increase efficacy of treatment[37].
Hydrogels have also been used in clinical trials for their excellent combination of properties, including biocompatibility, biodegradability, and especially minimally invasive injectability. Injectable cell and protein delivery carriers[38-40]and extracellular matrix mimetic scaffolds[41, 42]are two major bone repair and regeneration applications of hydrogels.
Serving as delivery carriers, hydrogels have been developed to control osteoinductive growth factor release (such as bone morphogenetic protein-2, BMP-2) and have been used as bone grafts. A functional nanoparticle-hydrogel complex composed of heparin- functionalized nanoparticles and fibrin gel was used as an efficient BMP-2 sustained release system[38]. Heparin-binding BMP-2 was loaded into heparin- functionalized nanoparticles, which were added during fibrin gel formation to achieve the final formulation.
By combining alginate with osteogenic cells and BMP-2 plasmid DNA, Wegman et al. developed an economical, efficient BMP-2 gene delivery method[43]. The possibility of achieving osteogenic differentiation both in vitro and in vivo was investigated by using plasmid DNA-based gene therapy to prolong the presence of BMP-2. The alginate hydrogel serves as a transfection and release system that can release protein for at least 6 weeks. The protein that was released after 6 weeks has been demonstrated to be biologically active and could even induce early osteogenic differentiation.
In addition to being used for delivery, hydrogels can be developed as extracellular matrix mimetic scaffolds. By exploiting the three mechanisms of action that bone regeneration has to undergo when regeneration occurs, viz., osteoinduction, osteoconduction, and osteogenesis, hydrogels can serve as scaffolds to support and encourage cellular ingrowth during bone formation. A method that causes hyaluronic acid hydrogel to degrade at different rates was designed to test the effect of degradation rates on bone formation[42]. The results indicate that the rate of scaffold degradation can modulate bone formation. Moreover, the degradation rate had an effect on bone healing, especially in the organization of the collagen matrix.
To overcome the limitations encountered in a wide range of applications (e.g., poor mechanical strength, adverse immune reactions, and relatively late angiogenesis), Vermonden and Klumperman made significant progress in developing novel hydrogels. They found that large performance changes could be achieved by making relatively minor modifications in hydrogel formulations, e.g., changing the polymer concentration or the method of crosslinking[29].
Techniques that utilize growth factors like BMP are complicated, susceptible to adverse immune reactions, and expensive for clinical applications. As an alternative to these systems, Yeom et al. developed a novel hybrid bone graft consisting of bioactive Mega Gen synthetic bone (MGSB) and natural biopolymers of hyaluronate and gelatin[44]. Experiments showed that proliferation of preosteogenic cells was enhanced with increasing molecular weight of hyaluronate. The MGSB/HA-GEL hydrogels resulted in effective bone regeneration after implantation into critical-sized calvarial bone defects.
The main challenge for constructing hydrogels to be used as temporary skeletons is their poor mechanical strength. One solution to this problem is to introduce covalent bonds into hydrogels, which may result in reduction of water content. This is the logic behind development of self-reinforcing hydrogels based on noncovalent crosslinking, which relies on the supramolecular interactions of cyclodextrin and adamantine[45]. The hydrogel forms quickly due to noncovalent crosslinking and is subsequently reinforced by Diels-Alder chemical crosslinking. Another research team developed a novel hybrid scaffolding system in which an electrospun mat based on biodegradable poly hydroxy butyrate (PHB) and hydroxyapatite (HA) was combined with a protein based hydrogel in a single tri-layered scaffold[46]. While the hydrogel layers provided a suitable environment for cell encapsulation, the incorporated fibers acted as a strong backbone, enhancing the mechanical properties of the scaffold. These results demonstrated that mechanical properties of the novel material were higher than traditional hydrogels.
Despite the importance of osteogenic induction in the process of bone formation, successful bone regeneration requires early angiogenesis, especially when the defect is large and/or tissue engineered cells are used. The main purpose for using hydrogels as agents to promote angiogenesis is to deliver angiogenic factors, as the hydrogel structure is similar to that of ECM[47]. Angiogenic factors such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) encapsulated in hydrogels can be released in a sustained manner with conserved bioactivity[48, 49]. Angiogenic factors have a higher releasing ability in alginate hydrogels compared to direct release in cells, which may be due to their enhanced stability due to the encapsulation process. However, potential side effects from overdoses of VEGF or bFGF, such as the formation of tumors or vascular leakage, do exist and should be taken into account. Therefore, alginate heparinization has been used to prevent the burst release of growth factors during the initial phases so that release from alginate hydrogels is more stable[50]. In addition, Perez et al. proposed a novel scaffold designed to deliver angiogenic and osteogenic factors in a sequential manner in order to synergize bone regeneration[51]. BMP-2 was loaded into the core while the shell incorporated Co ions that are known to serve as angiogenic factors. In vivo rat calvarium tests confirmed the synergistic roles of BMP2/Co in improving bone formation.
Hydrogels provide a controllable scaffold to encapsulate cells[48]. Poly(ethylene glycol) (PEG) hydrogels have been demonstrated to be beneficial for cartilage formation by chondrocytes and mesenchymal stem cells[50]. Salinas et al. demonstrated that gel degradation could be controlled and macroscopic properties could be changed by subtly altering hydrogel chemistry.Secretion and distribution of ECM could also be controlled by minor variations in hydrogel chemistry. Isolated variables of poly(ethylene glycol) could also be changed to enhance degradation, viability, and chondrogenesis[51]. By incorporating enzymatically cleavable peptide sequences into PEG hydrogels, the introduced arginine-lysine-aspartic acid (RGD) maintained high levels of hMSC survival and initiated extensive chondrogenesis. Additionally, a thermosensitive chitosan-pluronic (CP) hydrogel was also designed as a delivery carrier for cartilage regeneration[52]. The CP hydrogel displayed improved mechanical properties, stability, and biocompatibility and presented a sol-gel transition around 25°C. Furthermore, the transition time, which is long enough for injection, indicates that the CP hydrogel can be used for thermosensitive injectable therapy.
3.3 Skin
Skin regeneration is an important application of tissue engineering, especially in the context of burns. It has been reported that a new biomaterial consisting of a bi-layer physical hydrogel has the ability to induce the reconstruction of skin in a pig dorsal deep burn model[53]. By inducing inflammatory cell migration and stimulating blood vessels, the material exhibits superior performance not only in being tolerated, but also in regenerating tissue. In addition, a thermoresponsive chitosan-agarose hydrogel was designed for skin regeneration[54]. The moist and hydrated hydrogel could effectively prevent water loss and wound dehydration. The presence of nutrients and oxygen and carbon dioxide exchange was confirmed by high cell proliferation.
4 Decellularized tissues
Decellularized tissues are increasingly being used as biomaterials for clinical use due to lesser regulatory restrictions compared to new synthetic materials. To avoid immunoreaction, decellularization has been developed to isolate the extracellular matrix (ECM) of a tissue from its inhabiting cells. A natural ECM scaffold is created through decellularization, and adverse immune responses can be eliminated by recellularizing the scaffold with patients' cells. At present, a number of decellularized tissues are commercially available in China, such as Acellular Porcine Skin (Aierfu, China Regenerative Medicine International) and Acellular Corneal Stroma (Acornea, China Regenerative Medicine International). A number of important or new decellularized biomaterials are discussed below.
4.1 Nerve
Allografts offer an effective way to generate scaffolds but may be infected with virus carried by the donor and may cause host rejection. To avoid these problems, significant efforts have been made to develop decellularized tissues. Nerve allografts prepared by the “cold preserved”[55]and “freezing and freeze- thaw”[56]techniques have important limitations such as residual cells. Therefore, a technique that makes use of chemical detergents was pioneered by Johnson et al.[57]. Recently, Sun et al. investigated the effects of decellularized tissue on an 8 mm facial nerve branch lesion by transplanting a decellularized allogeneic artery conduit containing autologous transdifferentiated adipose-derived stem cells (dADSCs) into a rat model[58]. After 8 weeks, nerve regeneration was assessed by performing functional evaluation of vibrissae movements and electrophysiological assessment, retrograde labeling of facial motoneurons, and morphological analysis. The results showed beneficial effects, especially in promoting nerve regeneration and functional restoration, which provide strong evidence in favor of dADSCs as an alternative and promising approach for the reconstruction of peripheral facial nerve defects. In another study, Wang et al. evaluated the effects of transplantation of a nerve derived from an acellular allogenic nerve graft, combined with autologous bone marrow stromal cells (MSCs), on peripheral nerve defects[59]. Experimental results indicated that the group treated with cultured MSCs showed a statistically higher number of nerve fibers, with well-shaped remyelinated axons. The acellular allogenic nerve used as a support for MSC growth showed no graft rejection but rather provided a favorable environment for the growth and myelination of regenerating axons.
4.2 Bone and cartilage
Decellularized bone tissues were introduced to treat orthopedic diseases[60]because of the deterioration thatoccurs during natural wound healing in full-thickness cartilage defects[61]. Demineralized bone matrices can be prepared by removing soft tissue, blood, and lipids, followed by antibiotic soaks, acid demineralization, and several rounds of freeze-drying[62]. A number of studies have demonstrated that decellularized bone matrices can support and guide osteogenic differentiation of mesenchymal stem cells, human embryonic stem cells[63], and induced pluripotent stem cells[64]. Kheir et al. developed a technique to decellularize porcine cartilage bone structure with the aim of using it as a scaffold for cartilage substitution[65]. They produced a biocompatible acellular cartilage bone matrix scaffold for osteochondral defect repair. The decellularized porcine cartilage tissue thus produced was found to exhibit favorable compatibility in both in vitro and in vivo tests. Additionally, this was the first study to demonstrate that whole cells and α-Gal can be successfully removed from xenogeneic cartilage attached to bone tissue.
4.3 Skin
Allogeneic skin transplantation offers a new option for the treatment of skin wounds. The constructs[66], which have moved into clinical use as treatments for large losses and nonhealing wounds, can be generated by cellular reseeding of decellularized skin[67]. Tang et al. evaluated the biocompatibility of an acellular porcine dermis using in vitro methods[68]. Results obtained using morphological assessment and methyl thiazolyl tetrazolium (MTT) assay indicated good biocompatibility of acellular porcine dermis, which allowed adhesion and proliferation of examined cell types. Gupta et al. fabricated a scaffold from cadaver goat lung tissue and evaluated it for skin tissue engineering applications[69]. The prepared goat lung scaffolds, which were successfully decellularized, possessed high porosity, good pore-to-pore interconnectivity, and adequate pore size for the attachment and proliferation of skin-derived mesenchymal stem cells, strongly suggesting their applicability as skin regenerating templates.
4.4 Cornea
Although scaffolds made of collagen or other synthetic polymeric materials are being prepared to mimic the composition of native corneal stroma, the biomechanical properties of these biomaterials are inferior to those of native cornea. Decellularization treatment has been employed to remove cells from a tissue or an organ without interfering with the complex mixture of structural and functional proteins that constitute the extracellular matrix, while simultaneously eliminating tissue immunogenicity[70]. Wu et al. developed a method using phospholipase A2 (PLA2) to prepare acellular porcine corneal stroma (APCS) for tissue engineering[71]. The decellularization method using PLA2 and Sodium deoxycholate (SD) could remove almost all xenogeneic cells. The prepared APCS, which shows favorable biocompatibility, undetectable immunogenicity, adequate biomechanical intension, high transparency, and long-term stability, is a highly suitable scaffold for corneal tissue engineering and other applications, such as treatment of corneal ulcers.
5 Metamaterials
Metamaterials are artificial materials designed to have properties that are not found in nature. These materials are generally arranged in repeating patterns with multiple elements fashioned from composite materials such as metals or plastics. Their unique properties are often determined by their organized structure and not by the base materials used.
Typical metamaterials include aerogels, perovskite, and stanene. Graphene-based materials and other types of metamaterials used in neuroscience and other biomedical applications are introduced in further detail below.
5.1 Nerve
Because of their unique electroconductive properties, graphene-based materials are designed as scaffolds to provide cell guidance cues within a 3D microenvironment. Recently, a graphene foam with a 3D porous structure was employed as a novel scaffold for neural stem cells (NSC) in vitro[72]. It was found that the graphene foam not only supported NSC growth, but also maintained cells in an active proliferation state. There is also evidence to show that graphene foam can enhance NSCs' differentiation towards astrocytes and neurons (Figure 3).
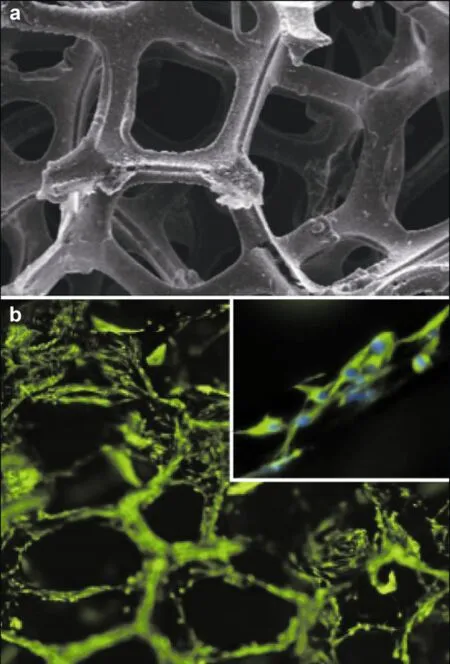
Figure 3 (a) SEM image of 3D graphene foams, reproduced with permission from Ref. [72]. (b) Fluorescence images of NSCs proliferated on 3D graphene foams for 5 days. Immunostaining markers are nestin (green) for neural stem cells and DAPI (blue) for nuclei, reproduced with permission from Ref. [72].
For improved detection and diagnosis of neurological diseases, genetic and biochemical analyses are increasingly being used in neurology and neurosurgery. Due to their high electron mobility, graphene- based electrodes can detect DNA strands with high accuracy. Nelson et al. inserted nucleobases into a pore in a graphene nanoribbon and the electrical current and conductance spectra were calculated as functions of voltage applied across the nanoribbon[73]. The conductance spectra and charge densities were analyzed, and the results indicated that the device had adequate sensitivity to discriminate between different nucleotides. These results demonstrate that graphene nanopores are a novel biomaterial that can be used in genetic and biochemical analyses.
A recent therapeutic improvement is the continuous monitoring of acute brain injuries. Few biosensors are capable of providing accurate, reliable information about the actual state of the microenvironment in the brain[74]. To improve neurointensive care for brain injuries, graphene with high electrocatalytic activity was developed for integration in devices for continuous monitoring. A novel amperometric biosensor for hydrogen peroxide was fabricated using layer-by- layer (LBL) self-assembly of functionalized graphene by covalent bonding[75]. Positive and negative charges were introduced onto the surface of graphene separately, and the resultant material displayed an excellent response to glucose. By detecting glucose in the brain, variations in glucose concentration were successfully observed.
5.2 Other applications
Metamaterials have been successfully employed as biomaterials due to their unmatched flexibility in building supramolecular architectures[76]. A microstructured asymmetric array that formed the core hydrodynamic element was used as a building block to construct microfluidic metamaterials[77]. Particle size replaced the concept of polarization, thereby allowing particles to choose separate paths through the asymmetric array based on their size. Particles larger than critical size moved along the asymmetric array at an angle to the flow while smaller particles moved along streamline paths. Depending on the design criteria, streams of beads, cells, and DNA could move to targets deterministically based on their size. Another research team found that a multicomponent nanocomposite material, formed by graphene layers and Au nanoparticles supported on the surface of hydroxyapatite nanoparticles, exhibited good biocompatibility and an ability to induce excellent cell proliferation in bone cells[78]. The Au/HA@graphene system can be the foundation of highly active scaffolds for tissue and bone regeneration because of their excellent biocompatibility with cellular proliferation and growth.
6 Synthetic biology and genomics: New approaches for designing and fabricating biomaterials
6.1 Synthetic biology
The concept of “synthetic biology” is extremely broadand encompasses all aspects of designing biological systems, from modifying natural materials to creating complex synthetic materials or processes that are based on naturally occurring counterparts. It is considered to be an emerging research field that will provide new opportunities for tissue repair and regeneration. Protein- or peptide-based biomaterials are capable of transmitting rich biochemical information to direct cell fate, in addition to providing structural support. For these reasons, the development of such materials has seen tremendous growth[79]. Viruses and virus- like particles represent another overarching trend for synthetic biology. Figure 4 has shown the current and anticipated development of evolutionary biomaterials in 3 steps that's based on viruses and virus-like particles[80]. First, genetically programmable rod or shaped and icosahedral particles self-assemble into multiple 3D morphologies in various ways. Second, the materials obtained in last step interact with cells to tunable and controllable cell behaviors, including adhesion, growth and proliferation. Third, organs or tissues with higher-order structures and functions were obtained by programmed cell differentiation. At each stage, it's possible to create feedback mechanisms to allow selection of desired construction or properties from the directed evolution of building blocks.
Martino et al.[81]fused the growth factor domains of bone morphogenetic protein-2 (BMP-2) and platelet-derived growth factor-BB (PDGF-BB) with the fibronectin cell binding domains. Interestingly, the authors found potent synergistic signaling and morphogenesis between α5β1 integrin and the growth factor receptors. The multifunctional polypeptide chain FN III9-10/12-14 greatly enhanced the regenerative effects of the growth factors in a rat model of critical- size bone defects. This approach proved to be a significant enhancement of the regenerative effects of the growth factors for bone repair.
Clinically sized, anatomically shaped, viable human bone grafts[82]have been engineered using human mesenchymal stem cells (hMSCs) and a biomimetic scaffold-bioreactor system. Because of its clinical importance and complex shape, the temporomandibular joint (TMJ) condylar bone was selected as a model and cultured in a bioreactor. After 5 weeks of cultivation, tissue growth was evidenced by the formation of confluent layers of lamellar bone and osteoids. This biological approach has the potential to overcome a key hurdle of in vitro cultivation of viable bone grafts with complex geometries, providing patient-specific bone grafts for craniofacial and orthopedic reconstructions.

Figure 4 Schematic diagram of the development of evolutionary biomaterials based on viruses and virus-like particles. (a) Ingredients with different shape can be self-assembled into different surface and three-dimensional morphologies. (b) The materials obtained in last step interact with cells leading to controlled cell behaviors. (c) Organs or tissues with higher-order structures and functions were obtained by programmed cell differentiation.
In addition to orthopedic applications, Martino et al.[81]also presented an approach for skin regenerationby engineering the cellular microenvironment to greatly accentuate the effects of vascular endothelial growth factor-A (VEGF-A) and PDGF-BB (primarily through an angiogenic mechanism). The in vivo experiment was performed in a diabetic mouse model with chronic wounds where the growth factors that were delivered within fibrin only had no significant effects.
In addition to tissue repair and regeneration using synthetic biological materials, biological surface engineering also has great potential for advancing our understanding of complex biological phenomena. A simple system to engineer biologically relevant surfaces was developed by Zhang et al.[83]using a combination of self-assembling oligopeptide monolayers and microcontact printing (μ-CP). Well-defined pattern formation in a variety of cell types can be obtained by this biological surface engineering technique. The authors also noted that this simple and versatile protocol has the potential to open new research opportunities to further the study of cell- material interaction, cell migration, cell mechanical compliance, cell-cell communication, and cell behavior.
6.2 Synthetic genomics
A minimal genome consists of the minimal number of genes that are essential for survival of the respective organism under defined conditions. Non-essential genes and non-encoding regions are usually removed; for example, genetic elements encoding alternative metabolic pathways or those encoding responses to stress situations are usually deleted. Minimal genomes may serve as efficient platforms to endow organisms with new functions.
With the rapid development of the technology for synthesizing oligonucleotides, in conjunction with the steady reduction of synthesis cost and constant improvement of synthesis length and precision, large- scale DNA synthesis from an oligonucleotide chain may be possible[84]. DNA synthesis not only provides researchers with the ability to create artificial life systems[85], but also provides opportunities for advancements in the life sciences (e.g., genetics, molecular biology, and synthetic biology)[86, 87]in terms of codon optimization for heterologous gene expression, construction of heterologous metabolic pathways, synthesis of artificial genomes, and synthesis of attenuated artificial viruses for vaccine research (Figure 5).
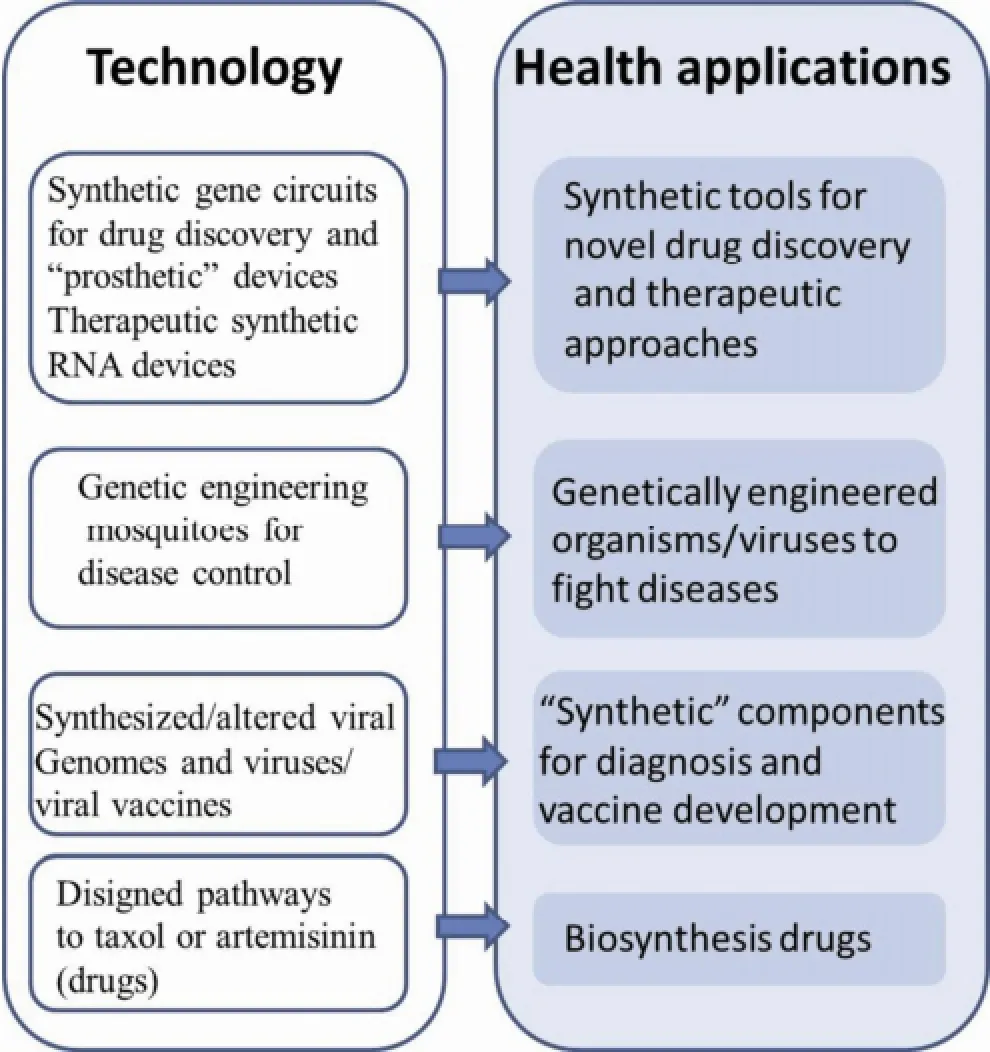
Figure 5 Synthetic genomics and synthetic biology technologies and the applications in health.
Compared to recombinant DNA technology, chemical synthesis of DNA is used to construct synthetic genomes and rationally designed biosynthetic pathways, leading to creation of new DNA sequences to optimize gene expression in related systems. For example, using chemical synthesis, the 1918 Spanish pandemic influenza virus, bearing all eight gene segments of the pandemic virus, was reconstructed and used for studying the properties associated with its unusual virulence[88]. Gibson et al. combined all their previously established procedures, yielding a breakthrough innovation. They reported the synthesis, assembly, cloning, and successful transplantation of the 1.08-Mbp Mycoplasma mycoides JCVI-syn1.0 genome, creating a new cell controlled by the synthetic genome[85].
A synthetic yeast genome project, Sc2.0[89], reported the first partially synthetic eukaryotic chromosomes: Saccharomyces cerevisiae chromosome synIXR, and semi-synVIL. The authors defined three design principles for a synthetic genome, to which their constructed chromosomes adhered. These included an inducible evolution system, synthetic chromosome rearrangement, and modification by loxP-mediated evolution, which is a novel method of combinatorial mutagenesis to generate complex genotypes and variousphenotypes. The authors suggested that this completely synthetic method will allow massive restructuring of the yeast genome and may open the door to a new type of combinatorial genetics based entirely on variations in gene content and copy number.
Significant progress has been made in DNA synthesis technologies. Synthetic oligonucleotide chains are widely used in the optimization of heterologous gene expression, metabolic engineering, genome rearrangement, and genome construction. Future research will focus on cooperation between different scientific disciplines, new synthesis strategies, and development and application of new technologies.
Figure 5 summarizes various synthetic genomics/ biology-related approaches to address specific health applications and challenges[90-92]. First, complex or multi-input sensing, gene expression regulatory circuits, and synthetic RNA molecules have been designed to detect diseases associated with molecular changes in cells (e.g., cancer cells) and activate cell-death pathways to eliminate diseased cells. For example, Culler et al.[91]engineered RNA devices that detected signaling through the nuclear factor κB and Wnt signaling pathways in human cells and rewired these pathways to produce new behaviors, thereby linking disease markers to noninvasive sensing and reprogrammed cellular fates. Their work provided a genetic platform that could build programmable sensing-actuation devices enabling autonomous control over cellular behavior.
Second, a combination of classical genetic engineering (through the modification or transfer of one or two natural genes) and the synthetic biology concept of rationally designing functions not present in nature have both been employed to reengineer viruses to fight disease. For example, in a clinical trial, JX-594[92], a dose-specific oncolytic poxvirus, could selectively infect, replicate, and express transgene products in cancer tissue after intravenous infusion. However, normal tissues were not clinically affected. This approach opens up the possibility of multifunctional products that selectively express high concentrations of several complementary therapeutic and imaging molecules in metastatic solid tumors in humans.
In order to diagnose diseases and develop vaccines, complex DNA synthesis and genome assembly techniques have been used to generate entire viral genomes and to address the etiology and pathogenicity mechanisms of corresponding viruses, such as the SARS viruses[93]. A model was articulated to predict and directly test tenable emergence pathways. Paired with a greater availability of reagents and therapeutics, these studies represent an approach for rapid recovery and testing of newly identified pathogens, which may improve public health preparedness and intervention strategies against natural or artificial zoonotic-human epidemics.
7 Summary and outlook
Over the past several decades, there has been tremendous progress in the development of engineering and biomimetic materials that can functionally substitute or help regenerate organs and tissues of the human body. Major efforts directed toward biomedical and biotechnological applications have been reviewed in this article, including self-assembly and macromolecular biomaterials, biomaterials constructed via synthetic biology, hydrogels, metamaterials, and decellularized tissues, used either for tissue repair and regeneration or for therapeutic use by exploiting other mechanisms of healing. All these efforts aim to create functional materials, devices, systems, and/or organisms with novel and useful functions on the basis of catalogued and standardized biological building blocks[94].
The field of biomaterials is becoming increasingly complex from both a materials science perspective as well as a medical perspective. Future advances in this field require more thorough understanding of natural tissue and development of better engineering materials for tissue substitution and regeneration, as well as the application of new engineering principles to strengthen or advance current biomimetic strategies.
Moreover, three-dimensional printing (3D printing) is an emerging strategy for creating engineered tissue constructs[95]. This technique is a versatile tool for building 3D structures layer by layer with specific conformation and shape using the materials mentioned above seeded with cells, drugs or other factors in the fabricating procedure. All the biomaterials (i.e., collagen, hydrogel, and metamaterials) can be introduced into the 3D printing system to fabricate tissues or organs ofcustomized shape with controlled and interconnected porous structures. For example, collagen can be incorporated in low-temperature 3D printing to form scaffolds for bone regeneration in a critically sized femoral defect, with different porosities for new bone and vascular growth[96]. More importantly, the scaffolds are degradable and osteoconductive, which are useful for new bone formation and incorporation with the scaffold. The need for custom medical devices that can be tailored to specific clinical needs provides motivation for future research to combine biomaterials and 3D printing to form completely functional tissue.
The biomaterials that are currently available and being used in clinical treatments are few, and are limited by various complications such as biological compatibility, biological function, and service life. The key to solving this problem is composite materials. Therefore, future biomaterials will most likely develop in the following direction: stronger biological compatibility and more functional capacity leading to more comprehensive and cohesive versions of current technologies. Due to the trend of interdisciplinary research and development involving biological materials, improvements in biomaterials will promote rapid development of new materials and their applications. Furthermore, rules for commercialization and application and government regulations should also be simultaneously introduced. Biomaterials should meet clinical requirements with more performance and multi-functional diversity, leading to comprehensive and spontaneous tissue repair and regeneration.
Conflict of interests
All contributing authors have no conflict of interests.
[1] Hinderer S, Layland SL, Schenke-Layland K. ECM and ECM- like materials-Biomaterials for applications in regenerative medicine and cancer therapy. Adv Drug Deliv Rev 2016, 97: 260-269.
[2] Chen QZ, Thouas GA. Metallic implant biomaterials. Mater Sci Eng R-Rep 2015, 87: 1-57.
[3] Sionkowska A. Current research on the blends of natural and synthetic polymers as new biomaterials: Review. Prog Polym Sci 2011, 36(9): 1254-1276.
[4] Kinnari TJ, Esteban J, Gomez-Barrena E, Zamora N, Fernandez-Roblas R, Nieto A, Doadrio JC, López-Noriega A, Ruiz-Hernández E, Arcos D, et al. Bacterial adherence to SiO2-based multifunctional bioceramics. J Biomed Mater Res A 2009, 89A(1): 215-223.
[5] Kopeček J. Hydrogel biomaterials: A smart future? Biomaterials 2007, 28(34): 5185-5192.
[6] Branco MC, Schneider JP. Self-assembling materials for therapeutic delivery. Acta Biomater 2009, 5(3): 817-831.
[7] Sridharan R, Reilly RB, Buckley CT. Decellularized grafts with axially aligned channels for peripheral nerve regeneration. J Mech Behav Biomed Mater 2015, 41: 124-135.
[8] Fairman R, Åkerfeldt K. Peptides as novel smart materials. Curr Opin Struct Biol 2005, 15(4): 453-463.
[9] Zhang SG, Holmes TC, DiPersio C M, Hynes RO, Su X, Rich A. Self-complementary oligopeptide matrices support mammalian cell attachment. Biomaterials 1995, 16(18): 1385-1393.
[10] Aggarwal S, Yurlova L, Simons M. Central nervous system myelin: Structure, synthesis and assembly. Trends Cell Biol 2011, 21: 585-593.
[11] Taraballi F, Natalello A, Campione M, Villa O, Doglia SM, Paleari A, Gelain F. Glycine-spacers influence functional motifs exposure and self-assembling propensity of functionalized substrates tailored for neural stem cell cultures. Front Neuroengineering 2010, 3: 1.
[12] Cunha C, Panseri S, Antonini S. Emerging nanotechnology approaches in tissue engineering for peripheral nerve regeneration. Nanomedicine 2011, 7(1): 50-59.
[13] Palmer LC, Newcomb CJ, Kaltz SR, Spoerke ED, Stupp SI. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem Rev 2008, 108(11): 4754-4783.
[14] Spoerke ED, Anthony SG, Stupp SI. Enzyme directed templating of artificial bone mineral. Adv Mater 2009, 21(4): 425-430.
[15] Salem AK, Rose FRAJ, Oreffo ROC, Yang X, Davies MC, Mitchell JR, Roberts CJ, Stolnik-Trenkic S, Tendler SJB, Williams PM, et al. Porous polymer and cell composites that self-assemble in situ. Adv Mater 2003, 15(3): 210-213.
[16] Dubey P, Murab S, Karmakar S, Chowdhury PK, Ghosh S. Modulation of self-assembly process of Fibroin: An insight for regulating the conformation of silk biomaterials. Biomacromolecules 2015, 16(12): 3936-3944.
[17] Forstater JH, Kleinhammes A, Wu Y. Self-assembly of protein-based biomaterials initiated by titania nanotubes. Langmuir 2013, 29(48): 15013-15021.
[18] Mishra A, Loo Y, Deng RS, Chuah YJ, Hee HT, Ying JY, Hauser CAE. Ultrasmall natural peptides self-assemble to strong temperature-resistant helical fibers in scaffolds suitable for tissue engineering. Nano Today 2011, 6(3): 232-239.
[19] Sargeant TD, Guler MO, Oppenheimer SM, Mata A, Satcher RL, Dunand DC, Stupp SI. Hybrid bone implants: Self- assembly of peptide amphiphile nanofibers within porous titanium. Biomaterials 2008, 29(2): 161-171.
[20] Priya SG, Jungvid H, Kumar A. Skin tissue engineering for tissue repair and regeneration. Tissue Eng Part B Rev 2008, 14(1): 105-118.
[21] Jean J, Bernard G, Duque-Fernandez A, Auger FA, Pouliot R. Effects of serum-free culture at the air-liquid interface in a human tissue-engineered skin substitute. Tissue Eng Part A 2011, 17(7-8): 877-888.
[22] Black AF, Bouez C, Perrier E, Schlotmann K, Chapuis F, Damour O. Optimization and characterization of an engineered human skin equivalent. Tissue Eng 2005, 11(5-6): 723-733.
[23] Jean J, Garcia-Pérez ME, Pouliot R. Bioengineered skin: The self-assembly approach. J Tissue Sci Eng 2011, DOI 10.4172/2157-7552.S5-001.
[24] Zhang Y, Jin T, Zhuo R-X. Methotrexate-loaded biodegradable polymeric micelles: Preparation, physicochemical properties and in vitro drug release. Colloids Surf B: Biointerfaces 2005, 44(2-3): 104-109.
[25] Jeong WJ, Lim YB. Macrocyclic peptides self-assemble into robust vesicles with molecular recognition capabilities. Bioconjugate Chem 2014, 25(11): 1996-2003.
[26] Majithia R, Patterson J, Bondos SE, Meissner KE. On the design of composite protein-quantum dot biomaterials via self-assembly. Biomacromolecules 2011, 12(10): 3629-3637.
[27] Li W, Wang JS, Ren JS, Qu XG. 3D graphene oxide- polymer hydrogel: Near-infrared light-triggered active scaffold for reversible cell capture and on-demand release. Adv Mater 2013, 25(46): 6737-6743.
[28] Wichterle O, Lím D. Hydrophilic gels for biological use. Nature 1960, 185(4706): 117-118.
[29] Vermonden T, Klumperman B. The past, present and future of hydrogels. Eur Polym J 2015, 72: 341-343.
[30] Amini AA, Nair LS. Injectable hydrogels for bone and cartilage repair. Biomed Mater 2012, 7(2): 024105.
[31] Belkas JS, Munro CA, Shoichet MS, Midha R. Peripheral nerve regeneration through a synthetic hydrogel nerve tube. Restor Neurol Neurosci 2005, 23(1): 19-29.
[32] Dalton PD, Shoichet MS. Creating porous tubes by centrifugal forces for soft tissue application. Biomaterials 2001, 22(19): 2661-2669.
[33] Midha R, Munro CA, Dalton PD, Tator CH, Shoichet MS. Growth factor enhancement of peripheral nerve regeneration through a novel synthetic hydrogel tube. J Neurosurg 2003, 99(3): 555-565.
[34] Wei GJ, Yao M, Wang YS, Zhou CW, Wan DY, Lei PZ, Wen J, Lei HW, Dong DM. Promotion of peripheral nerve regeneration of a peptide compound hydrogel scaffold. Int J Nanomedicine 2013, 8: 3217-3225.
[35] Magnaghi V, Conte V, Procacci P, Pivato G, Cortese P, Cavalli E, Pajardi G, Ranucci E, Fenili F, Manfredi A, et al. Biological performance of a novel biodegradable polyamidoamine hydrogel as guide for peripheral nerve regeneration. J Biomed Mater Res A 2011, 98(1): 19-30.
[36] Bai SM, Zhang WM, Lu Q, Ma QH, Kaplan DL, Zhu HS. Silk nanofiber hydrogels with tunable modulus to regulate nerve stem cell fate. J Mater Chem B 2014, 2: 6590-6600.
[37] Martínez-Sanz E, Ossipov DA, Hilborn J, Larsson S, Jonsson KB, Varghese OP. Bone reservoir: Injectable hyaluronic acid hydrogel for minimal invasive bone augmentation. J Control Release 2011, 152(2): 232-240.
[38] Chung YI, Ahn KM, Jeon SH, Lee SY, Lee JH, Tae G. Enhanced bone regeneration with BMP-2 loaded functional nanoparticle-hydrogel complex. J Control Release 2007, 121(1-2): 91-99.
[39] Betz MW, Yeatts AB, Richbourg WJ, Caccamese JF, Coletti DP, Falco EE, Fisher JP. Macroporous hydrogels upregulate osteogenic signal expression and promote bone regeneration. Biomacromolecules 2010, 11(5): 1160-1168.
[40] Takahashi Y, Yamamoto M, Yamada K, Kawakami O, Tabata Y. Skull bone regeneration in nonhuman primates by controlled release of bone morphogenetic protein-2 from a biodegradable hydrogel. Tissue Eng 2007, 13(2): 293-300.
[41] Sasaki JI, Matsumoto T, Imazato S. Oriented bone formation using biomimetic fibrin hydrogels with three-dimensional patterned bone matrices. J Biomed Mater Res A 2015, 103(2): 622-627.
[42] Patterson J, Siew R, Herring SW, Lin ASP, Guldberg R, Stayton PS. Hyaluronic acid hydrogels with controlled degradation properties for oriented bone regeneration. Biomaterials 2010, 31(26): 6772-6781.
[43] Wegman F, Bijenhof A, Schuijff L, Oner FC, Dhert WJ, Alblas J. Osteogenic differentiation as a result of BMP-2 plasmid DNA based gene therapy in vitro and in vivo. Eur Cell Mater 2011, 21: 230-242.
[44] Yeom J, Hwang BW, Dong JY, Shin HI, Hahn SK. Effect of osteoconductive hyaluronate hydrogels on calvarial bone regeneration. Biomater Res 2014, 18: 8.
[45] Bai X, Lü SY, Cao Z, Gao CM, Duan HG, Xu XB, Sun L, Gao NN, Feng C, Liu MZ. Self-reinforcing injectable hydrogel with both high water content and mechanical strength for bone repair. Chem Eng J 2016, 288: 546-556.
[46] Sadat-Shojai M, Khorasani M-T, Jamshidi A. A new strategy for fabrication of bone scaffolds using electrospun nano-HAp/ PHB fibers and protein hydrogels. Chem Eng J 2016, 289: 38-47.
[47] Sánchez-Ferrero A, Mata A, Mateos-Timoneda MA, Rodríguez-Cabello JC, Alonso M, Planell J, Engel E.Development of tailored and self-mineralizing citric acid-crosslinked hydrogels for in situ bone regeneration. Biomaterials 2015, 68: 42-53.
[48] Kim IL, Mauck RL, Burdick JA. Hydrogel design for cartilage tissue engineering: A case study with hyaluronic acid. Biomaterials 2011, 32(34): 8771-8782.
[49] Bryant SJ, Durand KL, Anseth KS. Manipulations in hydrogel chemistry control photo encapsulated chondrocyte behavior and their extracellular matrix production. J Biomed Mater Res Part A 2003, 67A(4): 1430-1436.
[50] Salinas CN, Anseth KS. The enhancement of chondrogenic differentiation of human mesenchymal stem cells by enzymatically regulated RGD functionalities. Biomaterials 2008, 29(15): 2370-2377.
[51] Perez RA, Kim JH, Buitrago JO, Wall IB, Kim HW. Novel therapeutic core-shell hydrogel scaffolds with sequential delivery of cobalt and bone morphogenetic protein-2 for synergistic bone regeneration. Acta Biomater 2015, 23: 295-308.
[52] Park KM, Lee SY, Joung YK, Na JS, Lee MC, Park KD. Thermosensitive chitosan-Pluronic hydrogel as an injectable cell delivery carrier for cartilage regeneration. Acta Biomater 2009, 5(6): 1956-1965.
[53] Boucard N, Viton C, Agay D, Mari E, Roger T, Chancerelle Y, Domard A. The use of physical hydrogels of chitosan for skin regeneration following third-degree burns. Biomaterials 2007, 28(24): 3478-3488.
[54] Miguel SP, Ribeiro MP, Brancal H, Coutinho P, Correia IJ. Thermoresponsive chitosan-agarose hydrogel for skin regeneration. Carbohyd Polym 2014, 111: 366-373.
[55] Sanders FK, Young JZ. The degeneration and re-innervation of grafted nerves. J Anat 1942, 76(Pt 2): 143-166.7.
[56] Singh R. Experience with allografts in the surgery of peripheral nerves (experimental study). Acta Neurochir 1976, 34(1-4): 195-201.
[57] Johnson PC, Duhamel RC, Meezan E, Brendel K. Preparation of cell-free extracellular matrix from human peripheral nerve. Muscle Nerve 1982, 5(4): 335-344.
[58] Sun F, Zhou K, Mi WJ, Qiu JH. Combined use of decellularized allogeneic artery conduits with autologous transdifferentiated adipose-derived stem cells for facial nerve regeneration in rats. Biomaterials 2011, 32(32): 8118-8128.
[59] Wang D, Liu XL, Zhu JK, Jiang L, Hu J, Zhang Y, Yang LM, Wang HG, Yi JH. Bridging small-gap peripheral nerve defects using acellular nerve allograft implanted with autologous bone marrow stromal cells in primates. Brain Res 2008, 1188: 44-53.
[60] Cheng CW, Solorio LD, Alsberg E. Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnol Adv 2014, 32(2): 462-484.
[61] Benders KEM, van Weeren PR, Badylak SF, Saris DBF, Dhert WJA, Malda J. Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol 2013, 31(3): 169-176.
[62] Elliott G, Doll BA, Futrell FW, Schmitz JP, Hollinger JO. Demineralized bone matrix in bone repair: History and use. Adv Drug Deliv Rev 2012, 64(12): 1063-1077.
[63] Marcos-Campos I, Marolt D, Petridis P, Bhumiratana S, Schmidt D, Vunjak-Novakovic G. Bone scaffold architecture modulates the development of mineralized bone matrix by human embryonic stem cells. Biomaterials 2012, 33(33): 8329-8342.
[64] de Peppo GM, Sladkova M, Sjövall P, Palmquist A, Oudina K, Hyllner J, Thomsen P, Petite H, Karlsson C. Human embryonic stem cell-derived mesodermal progenitors display substantially increased tissue formation compared to human mesenchymal stem cells under dynamic culture conditions in a packed bed/column bioreactor. Tissue Eng Part A 2013, 19(1-2): 175-187.
[65] Kheir E, Stapleton T, Shaw D, Jin Z, Fisher J, Ingham E. Development and characterization of an acellular porcine cartilage bone matrix for use in tissue engineering. J Biomed Mater Res Part A 2011, 99(2): 283-294.
[66] Nyame TT, Chiang H A, Orgill DP. Clinical applications of skin substitutes. Surg Clin N Am 2014, 94(4): 839-850.
[67] Wells A, Nuschke A, Yates CC. Skin tissue repair: Matrix microenvironmental influences. Matrix Biol 2016, 49: 25-36.
[68] Tang LL, Liu H, Wang YL, Xian CY, Su AH. Evaluation of the biocompatibility of acellular porcine dermis. Colloids Surf B: Biointerfaces 2007, 57(2): 215-218.
[69] Gupta SK, Dinda AK, Potdar PD, Mishra NC. Fabrication and characterization of scaffold from cadaver goat-lung tissue for skin tissue engineering applications. Mater Sci Eng C Mater Biol Appl 2013, 33(7): 4032-4038.
[70] Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials 2006, 27(19): 3675-3683.
[71] Wu Z, Zhou Y, Li NY, Huang MH, Duan HY, Ge J, Xiang P, Wang ZC. The use of phospholipase A2 to prepare acellular porcine corneal stroma as a tissue engineering scaffold. Biomaterials 2009, 30(21): 3513-3522.
[72] Li N, Zhang Q, Gao S, Song Q, Huang R, Wang L, Liu LW, Dai JW, Tang ML, Cheng GS. Three-dimensional graphene foam as a biocompatible and conductive scaffold for neural stem cells. Sci Rep 2013, 3: 1604.
[73] Nelson T, Zhang B, Prezhdo OV. Detection of nucleic acids with graphene nanopores: Ab initio characterization of a novel sequencing device. Nano Lett 2010, 10(9): 3237-3242.
[74] Mattei TA, Rehman AA. Technological developments and future perspectives on graphene-based metamaterials: A primer for neurosurgeons. Neurosurgery 2014, 74(5): 499-516.
[75] Gu H, Yu YY, Liu XQ, Ni B, Zhou TS, Shi GY. Layer-by- layer self-assembly of functionalized graphene nanoplates for glucose sensing in vivo integrated with on-line microdialysis system. Biosens Bioelectron 2012, 32(1): 118-126.
[76] DuFort CC, Dragnea B. Bio-enabled synthesis of metamaterials. Ann Rev Phys Chem 2010, 61: 323-344.
[77] Morton KJ, Loutherback K, Inglis DW, Tsui OK, Sturm JC, Chou SY, Austin RH. Hydrodynamic metamaterials: Microfabricated arrays to steer, refract, and focus streams of biomaterials. Proc Natl Acad Sci USA 2008, 105(21): 7434-7438.
[78] Biris AR, Mahmood M, Lazar MD, Dervishi E, Watanabe F, Mustafa T, Baciut G, Baciut M, Bran S, Ali S, et al. Novel multicomponent and biocompatible nanocomposite materials based on few-layer graphenes synthesized on a gold/hydroxyapatite catalytic system with applications in bone regeneration. J Phys Chem C 2011, 115(39): 18967-18976.
[79] DiMarco RL, Heilshorn SC. Multifunctional materials through modular protein engineering. Adv Mater 2012 24(29): 3923-3940.
[80] Bryksin AV, Brown AC, Baksh MM, Finn MG, Barker TH. Learning from nature—Novel synthetic biology approaches for biomaterial design. Acta Biomater 2014, 10(4): 1761-1769.
[81] Martino MM, Tortelli F, Mochizuki M, Traub S, Ben-David D, Kuhn GA, Müller R, Livne E, Eming SA, Hubbell JA. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci Transl Med 2011, 3(100): 100ra89.
[82] Grayson WL, Fröhlich M, Yeager K, Bhumiratana S, Chan ME, Cannizzaro C, Wan LQ, Liu XS, Guo XE, Vunjak- Novakovic G. Engineering anatomically shaped human bone grafts. Proc Natl Acad Sci USA 2010, 107(8): 3299-3304.
[83] Zhang SG, Yan L, Altman M, Lassle M, Nugent H, Frankel F, Lauffenburger DA, Whitesides GM, Rich A. Biological surface engineering: A simple system for cell pattern formation. Biomaterials 1999, 20(13): 1213-1220.
[84] Reese CB. Oligo- and poly-nucleotides: 50 years of chemical synthesis. Org Biomol Chem 2005, 3: 3851-3868.
[85] Gibson DG, Glass JI, Lartigue C, Noskov VN, Chuang R-Y Algire MA, Benders GA, Montague MG, Ma L, Moodie MM, et al. Creation of a bacterial cell controlled by a chemically synthesized genome. Science 2010, 329(5987): 52-56.
[86] Merryman C, Gibson DG. Methods and applications for assembling large DNA constructs. Metab Eng 2012, 14(3): 196-204.
[87] Ma SY, Tang N, Tian JD. DNA synthesis, assembly and applications in synthetic biology. Curr Opin Chem Biol 2012, 16(3-4): 260-267.
[88] Tumpey TM, Basler CF, Aguilar PV, Zeng H, Solórzano A, Swayne DE, Cox NJ, Katz JM, Taubenberger JK, Palese P, et al. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science 2005, 310(5745): 77-80.
[89] Dymond JS, Richardson SM, Coombes CE, Babatz T, Muller H, Annaluru N, Blake WJ, Schwerzmann JW, Dai JB, Lindstrom DL, et al. Synthetic chromosome arms function in yeast and generate phenotypic diversity by design. Nature 2011, 477(7365): 471-476.
[90] König H, Frank D, Heil R, Coenen C. Synthetic genomics and synthetic biology applications between hopes and concerns. Current Genomics 2013, 14: 11-24.
[91] Culler SJ, Hoff KG, Smolke CD. Reprogramming cellular behavior with RNA controllers responsive to endogenous proteins. Science 2010, 330(6008): 1251-1255.
[92] Breitbach CJ, Burke J, Jonker D, Stephenson J, Haas AR, Chow LQM, Nieva J, Hwang TH, Moon A, Patt R, et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature 2011, 477(7362): 99-102.
[93] Becker MM, Graham RL, Donaldson EF, Rockx B, Sims AC, Sheahan T, Pickles RJ, Corti D, Johnston RE, Baric RS, et al. Synthetic recombinant bat SARS-like coronavirus is infectious in cultured cells and in mice. Proc Natl Acad Sci USA 2008, 105(50): 19944-19949.
[94] Weber W, Fussenegger M. Emerging biomedical applications of synthetic biology. Nat Rev Genet 2012, 13: 21-35.
[95] Derby B. Printing and prototyping of tissues and scaffolds. Science 2012, 338(6109): 921-926.
[96] Inzana JA, Olvera D, Fuller SM, Kelly JP, Graeve OA, Schwarz EM, Kates SL, Awad HA. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014, 35(13): 4026-4034.
Liu HL, Liu HR, Clasky A, Yang HL, Yang L. New frontiers in biomaterials research for tissue repair and regeneration. Transl. Neurosci. Clin. 2016, 2(2): 120-137.
✉ Corresponding author: Lei Yang, E-mail: leiy@suda.edu.cn
Supported by the Jiangsu Provincial Special Program of Medical Science (BL2012004), Jiangsu Innovation and Entrepreneurship Program 2015, the National Basic Research (973) Program of China (2014CB748600), the Priority Academic Program Development of Jiangsu High Education Institutions (PAPD), Jiangsu Provincial R&D Innovation Program (BY2014059-07), the National Natural Science Foundation of China (51472279), and Jiangsu Six Peak of Talents Program (2013-WSW-056).
杂志排行
Translational Neuroscience and Clinics的其它文章
- Preliminary analysis of cellular sociology of co-cultured glioma initiating cells and macrophages in vitro
- Behavioral features of mice fed with a cholesterol-enriched diet: Deficient novelty exploration and unaltered aggressive behavior
- A study of the effects of 3,5-diiodo-L-thyronine in the tail suspension and forced swim models of depression
- Transplantation of neural progenitor cells differentiated from adipose tissue-derived stem cells for treatment of sciatic nerve injury
- A newly developed open-end intracranial hematoma drainage tube
- Are life sciences all about life?
