Preliminary analysis of cellular sociology of co-cultured glioma initiating cells and macrophages in vitro
2016-11-16MingxiaZhangJunjieChenLinWangXiaoyanJiLinYangYujingShengHairuiLiuHaiyangWangAidongWangXingliangDaiXiaonanLiQiangHuangJunDong
Mingxia Zhang, Junjie Chen, Lin Wang, Xiaoyan Ji, Lin Yang, Yujing Sheng, Hairui Liu, Haiyang Wang, Aidong Wang, Xingliang Dai, Xiaonan Li, Qiang Huang, Jun Dong(✉)
1Department of Neurology, the Second Affiliated Hospital of Soochow University, Suzhou 215004, China
2Department of Neurology, Xishan People's Hospital, Wuxi 214000, China
3Laboratory of Molecular Neuron-oncology, Texas Children's Cancer Center, Baylor College of Medicine, Houston, TX, USA
§These authors contributed equally to this work.
Preliminary analysis of cellular sociology of co-cultured glioma initiating cells and macrophages in vitro
Mingxia Zhang1,§, Junjie Chen2,§, Lin Wang1, Xiaoyan Ji1, Lin Yang1, Yujing Sheng1, Hairui Liu1, Haiyang Wang1, Aidong Wang1, Xingliang Dai1, Xiaonan Li3, Qiang Huang1, Jun Dong1(✉)
1Department of Neurology, the Second Affiliated Hospital of Soochow University, Suzhou 215004, China
2Department of Neurology, Xishan People's Hospital, Wuxi 214000, China
3Laboratory of Molecular Neuron-oncology, Texas Children's Cancer Center, Baylor College of Medicine, Houston, TX, USA
§These authors contributed equally to this work.
ARTICLE INFO
Received: 20 May 2016
Revised: 30 May 2016
Accepted: 31 May 2016
© The authors 2016. This article
is published with open access
at www.TNCjournal.com
glioma initiating cells;
macrophages;
cellular interactions;
live cell time-lapse shoot;
dual fluorescence tracing
technique
Objective: Real-time monitoring of cytokine secretion at the single immunocyte level,based on the concept of immune cells, sociology has been recently reported. However,the relationships between glioma-initiating cells (GICs) and host immune cells and their mutual interactions in the tumor microenvironment have not been directly observed and remain unclear.
Methods: The dual fluorescence tracing technique was applied to label the co-cultured GICs and host macrophages (Mø), and the interactions between the two types of cells were observed using a live cell imaging system. Fusion cells in the co-culture system were monocloned and proliferated in vitroand their social interactions were observed and recorded.
Results: Using real-time dynamic observation of target cells, 6 types of intercellular conjunction microtubes were found to function in the transfer of intercellular information between GICs and Mø; GICs and host Mø can fuse into hybrid cells after several rounds of mutual interactions, and then these fusion cells fused with each other; Fusion cells generated offspring cells through symmetrical and asymmetrical division or underwent apoptosis. A “cell in cell”phenomenon was observed in the fusion cells, which was often followed by cell release, namely entosis.
Conclusions: Preliminary studies revealed the patterns of cell conjunction via microtubes between GICs and host Mø and the processes of cell fusion, division, and entosis. The results revealed malignant transformation of host Mø, induced by GICs, suggesting complex social relationships among tumor-immune cells in gliomas.
1 Introduction
Cellular sociology is a new field that addresses total behavior including recognition, communication assembly and mutual interactions among whole cells and cell groups. Several studies have examined the biological changes among body cells from the perspective of cellular sociology, including some studiesof tumors; however, there have been no studies involving the dynamic and continuous observations of in vitro mutual interactions between glioma- initiating cells (GICs) and macrophages (Mø).
In this study, after co-culturing GICs with host Mø in vitro, social behaviors such as cell conjunction, cell fusion, and entosis among these two cells types were evaluated.
It is well known that in addition to GICs and their progeny cells, gliomas also contain microglia (tumor- associated macrophages). GICs have the ability to remodel the surrounding stromal environment by recruiting nonmalignant host cells including Mø that provide physiological resources to facilitate tumor progression[1]. Tumor-associated macrophages, and mutual inter-reaction with glioma cells, as well as the effects on promoting tumor cell proliferation, invasion, and metastasis have been reviewed previously[2]. However, no continuous and dynamic observations of the processes of direct mutual interactions between GICs and Mø have been reported. Our goal was to describe dynamically social activities of co-cultured GICs and Mø using a dual-color tracing technique and to evaluate the interactions between the two types of cells from a new perspective to understand GICs-Mø relationships during GIC initiation of tumor remodeling processes.
2 Methods
2.1 Preparation of athymic nude mice with whole body expression of green fluorescence protein (GFP)
Transgenic C57BL/6J GFP-mice were crossed with athymic NC nude mice, and then the male progenies expressing GFP were selected and crossed with the female nude mice again. After the 8thgeneration of propagation, the organs and cells clearly expressed GFP in the GFP nude mice, which were named NCC57BL/ 6JGFP athymic nude mice. These mice were bred in an IVC isolation device (Fengshi Laboratory Animal Equipment Co., Ltd., Suzhou, China) according to specific pathogen free level management requirements.
2.2 GIC cell line
Lentiviral vectors (Genechem Chemical Technology Co., Ltd., Shanghai, China) were used to transfect the red fluorescence protein (RFP) gene into human SU3 GICs as previously reported[3]. A total of 1 × 106SU3 RFP cells were injected directly into the abdominal cavity of 6-week-old NC C57BL/6J GFP athymic nude mice, tumors formed over approxiamtely 4 weeks.
2.3 Preparation of GFP + Mø
Preheated DMEM culture medium was used for washing and injected into the abdominal cavity of 6-week-old NC C57BL/6J GFP nude mice after general anesthesia. This fluid was collected to harvest Mø. Immunocytochemistry was performed to detect the expression of the Mø specific marker protein CD68 using rat anti-mouse monoclonal antibody (1:200; Abcam, Cambridge, UK).
2.4 Cell co-culture in vitro
In vitro, SU3-RFP cells and normal Mø were co-cultured in DMEM in a 5% CO2incubator. For co-cultured cells, the optimal ratio of SU3/Mø was 1:10, and the culture medium was changed every 3-4 days. Active mutual interactions between the two cell types were observed and recorded using a live-cell imaging system(Olympus, Tokyo, Japan)by live cell time-lapse shoot. Yellow fusion cells were observed under a fluorescence microscope (Carl Zeiss, Jena, Germany) 3 weeks later, and the number of yellow cells gradually increased over time. Yellow cells were monocloned by micro-pipetting techniques and further cultured in vitro.
2.5 Live cell time-lapse shoot
Cell nuclei were dyed with Hoechst 33342 (live-cell fluorescence dye) (Sigma, St. Louis, MO, USA), and then the cells were digested by trypsin to prepare single-cell suspensions (1 × 105cells /mL) in DMEM medium. Next, 1 mL of cell suspension was transferred to a culture dish prior to video microscopy. The microscope was surrounded by an enclosure to maintain a constant temperature (37°C) and atmosphere (5% CO2and 95% air). Cells were observed under an inverted microscope with a 20× objective lens. The filter sets used were as follows: Hoechst 33342, 405 nm (excitation), GFP, 488 nm (excitation), and RFP 561 nm (excitation). The time-lapse images were recorded every 10 min for 48-60 h, and then a video was made at a speed of 6/12 frames per second.
2.6 Flowchart of experimental methods
A flowchart of the experiment methods is shown in Figure 1.

Figure 1 Flowchart of the study.
3 Results
Four types of cellular interactions between GICs and Mø were observed as follows.
3.1 Cell conjunction
Some of the observed cells lived alone and were nearly stationary, while others showed various patterns of cell contacts. Some cells directly contacted each other via their cell bodies; other types of cell communication were achieved via direct anatomical microtubes among cells. The intercellular microtubes varied in length and diameter; there were both short-distance conjunctions and long-distance conjunctions, single conjunctions between two cells, and multiple conjunctions among a few cells. Further, some multi-cellular conjunctions formed network-like structures to communicate with a cluster of cells. No conjunctions were permanent, but were highly dynamic, and moved and changed through the observation period. The conjunctions were formed by extending filamentous pseudopodia between cells, starting at one cell and ending at another, resulting in the development of microtubes between cells. Some microtubes were formed through the separation of two cells that were linked together tightly (Figure 2).
3.2 Cell fusion
We observed three types of intercellular fusion. (1) GICs fused actively with Mø, which accounted for approximately 11% of the fusion events. (2) Mø actively fused with GICs, which was accounting for 17% of fusion events. (3) Fusion cell fused with other cells (GIC, Mø, or fusion cell), which was the most common fusion form, accounting for 72%.
Pattern 1: A very large red GIC with 4 nuclei extended its pseudopodia to attack several Mø. 970 min later, at least 3 Mø were captured. Next the cell became a yellow (RFP/GFP double positive) and its body was elongated with tapering of the middle portion. The cell gradually entered into prophase of cell division, and 1,020 min were required to produce two offspring cells after cell division (Figures 3a-3d).
Pattern 2: A GIC was enclosed by several Mø. The Mø (including No. ① Mø) first approached the GIC, and then hit the cell continuously. It initially appeared that breakthrough point would not occur, but the No. ① Mø used several points of attack. After 1,870 min, the shape of the Mø cells changed. Suddenly the No. ② Mø quickly approached and directly inserted into the GIC. Next, the color of the GIC became light yellow, and then several Mø around the GIC hit and entered easily into the GIC invidually. Soon afterwards, the GIC became bright yellow (Figures 3e-3l).
Pattern 3: A second fusion could occur between two fusion cells. At first, one fusion cell rapidly migrated close to another cell, untill the cells contracted each other. Nearly simultaneously, rupture of the cell membrane initiated the fusion process. This procedure was relatively short, occuring over only 60 min (Figures 3m-3o).
3.3 Outcomes of fusion cell
The fate of the fused cells showed two opposite outcomes: reproduction and apoptosis. The former was dominant, occuring in 91.4% of the cases, and apoptosis occured in 8.6% of cases.
Cell reproduction and division was observed in a very large RFP/GFP double positive yellow fusion cell. In approximately 440 min the body and pseudopodia were reduced to a round cell. Next, the cell divided into two independent green cells after an additional 890 min, both cells gradually became yellow by 760 min later (Figure 4).
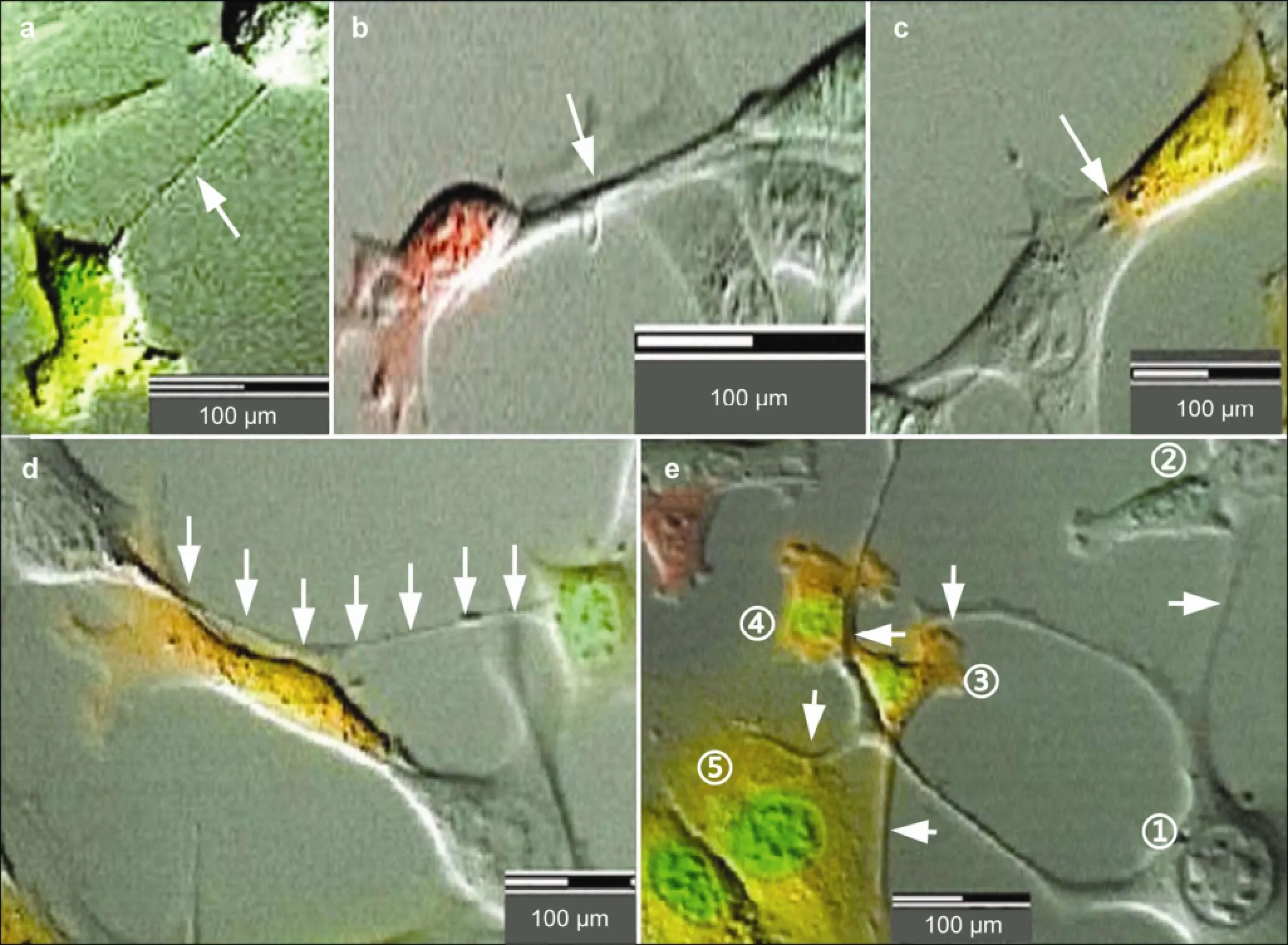
Figure 2 Various types of cell conjunctions. Diameters and lengths of conjunction microtubes varied, with slim (a arrow) and thick tubes (b, c arrow) observed conjunctions connected nearby cells (a-c) and distant cells (d arrow) according to the positions of relevant cells. These conjunctions can be also divided into single cell conjunctions (a-d) and multiple cell conjunctions (e, cell ① connected with ②, ③, ④, ⑤cells, respectively, via conjunction tubes). None of the conjunctions was permanent.
Apoptosis of fusion cells were also observed. First, the fusion cells retraced and migrated irregularly, after which, the cell body instantly collapsed into many pieces, and then the debris was engulfed by nearby Mø. Another fusion cell underwent a different apoptosis process, in which the cell slowly became static garbage, and no obvious changes were observed over long-term observation (Figure 5).
3.4 Phenomenon and outcomes of entosis
There were two types of entosis, a GIC in a Mø; and a Mø in a GIC (Figure 6).
The Outcomes of entosis were as follows.
Release: A deep yellow round cell released a light yellow short spindle cell. The newly released cell rapidly exited the parent cell. During the release process, the shape of parent cell changed from round to spindle, when the release process was complete, the shape of the parent cell was restored to its original state. A second release process was observed. The parent cell migrated irregularly with multiple small prominences stretching in different directions. Next, the cell contracted rapidly, quickly releasing a long, spindle, pale yellow offspring cell which moved actively around the parent cell. After 370 min, its appearance changed from spindle-like to spherical, which was similar to its parent cell and was yellow in color (Figure 7).
Digestion: The phenomenon of “being digested” by the intracellular enzymes after entosis can occur, and was the second common phenomenon.

Figure 3 Cell fusion between GICs and Mø. (a-d) GIC actively fused with Mø. A GIC cell extended its pseudopodia to attack several Mø (a). Next, some Mø were fused successfully by the GICs (b), after which another GIC completely fused with Mø and became an RFP/GFP double positive fusion cell. The cell body of the fusion cell gradually became prolonged with a tapered middle section (c). Next, the cell entered prophase of mitosis. Approximately 17 h were required for the cell to divide into two offspring cells (d). (e-l) Mø actively fused GIC. Initially a GIC was enclosed by several Mø (e), and then the Mø (including No. ① cell) further approached and attacked the GIC continuously. It appeared that the cells could not find a breakthrough point, so the No. ① cell changed its attack point (f). After 1,870 min of “fighting”, the shape of Mø (No. ①) changed and had more filamentous pseudopodium and did not maintain close to inserted into the GIC directly (j). Next, the GIC changed from red to light yellow, and several Mø entered the GIC individually, causing the GIC to become dark yellow. Both the RFP and GFP markers were positive (l). (m-o) Re-fusion occurred between the GIC-Mø fusion cells. There were 4 yellow cells in the vision field. The two round cells fused together. Initially, one cell rapidly approached another cell (m). The cells came into contact and began to combine (n). This fusion process took approximately 60 min and the fusion cells were 2× larger than the size of the parent cell (o).

Figure 4 Reproduction of fusion cells. A very large multi-nuclei re-fusion cell was observed (a). After 440 min its body turned into a sphaerocyst with retracted pseudopodia (b). Another 890 min later, this cell divided into two cells (c); however, after cell division, the fluorescence of these two cells was initially green and then became yellow after 760 min (d).
4 Conclusions
In recent years, the new concept of tumor microecology and tumor cellular sociology was introduced to explore the mechanisms of tumor occurrence and development. This new research field has attracted great attentions in the oncology field[2, 4]. Tumor stem cells have the ability to remodel the surrounding stromal environment by recruiting nonmalignant host cells including Mø that provide physiological resources to facilitate tumor progression. On the other hand, immune cells might modulate the biology of tumor cells[1]. In order to comprehensively image the interactions between tumor stem cells and cells in their microenvironment, cells are labeled, with different fluorescence marker for accurate tracing. Using this technique, our research team has performed a series studies on glioma tissue remodeling initiated by GICs[5], including trans- differentiation of GICs into tumor vasculature[6], and malignant transformation of host immune cells and glial cells induced by GICs[1, 7]. Few studies have examined the mutual interactions between GICs and macrophages in dynamic enviroments. In our study, by observing co-cultured GICs and macrophages using a live cell imaging system, the main cellular events were evaluated from the perspective of cellular sociology.
Cell-cell communication is critical for the development, maintenance, and function of multi-cellular organisms. Six different types of cell conjunctions between GICs and macrophages were observed, all of which were highly dynamic, unstable, and changed continuously. Similar cell-cell connections via membrane tubes have been reported previously, including intercellular nanotubes, membrane nanotube, tunneling nanotubes, or cytonemes[8]. These interactions play important roles in the transport of organelles, cell membrane components, cytoplasmic molecules, and spread of infectious particles. Additionally, microvesicle and stem signals may also be transmitted through intercellular microtubes[8-10]. Many membrane tubesformed among tumor cells in astrocytomas, creating functional multi-cellular network structures, that can be applied as routes for brain invasion, proliferation, inter-connection, and radioresistance[9]. However, the exact functions of microtube connections between GICs and macrophages require further analysis.
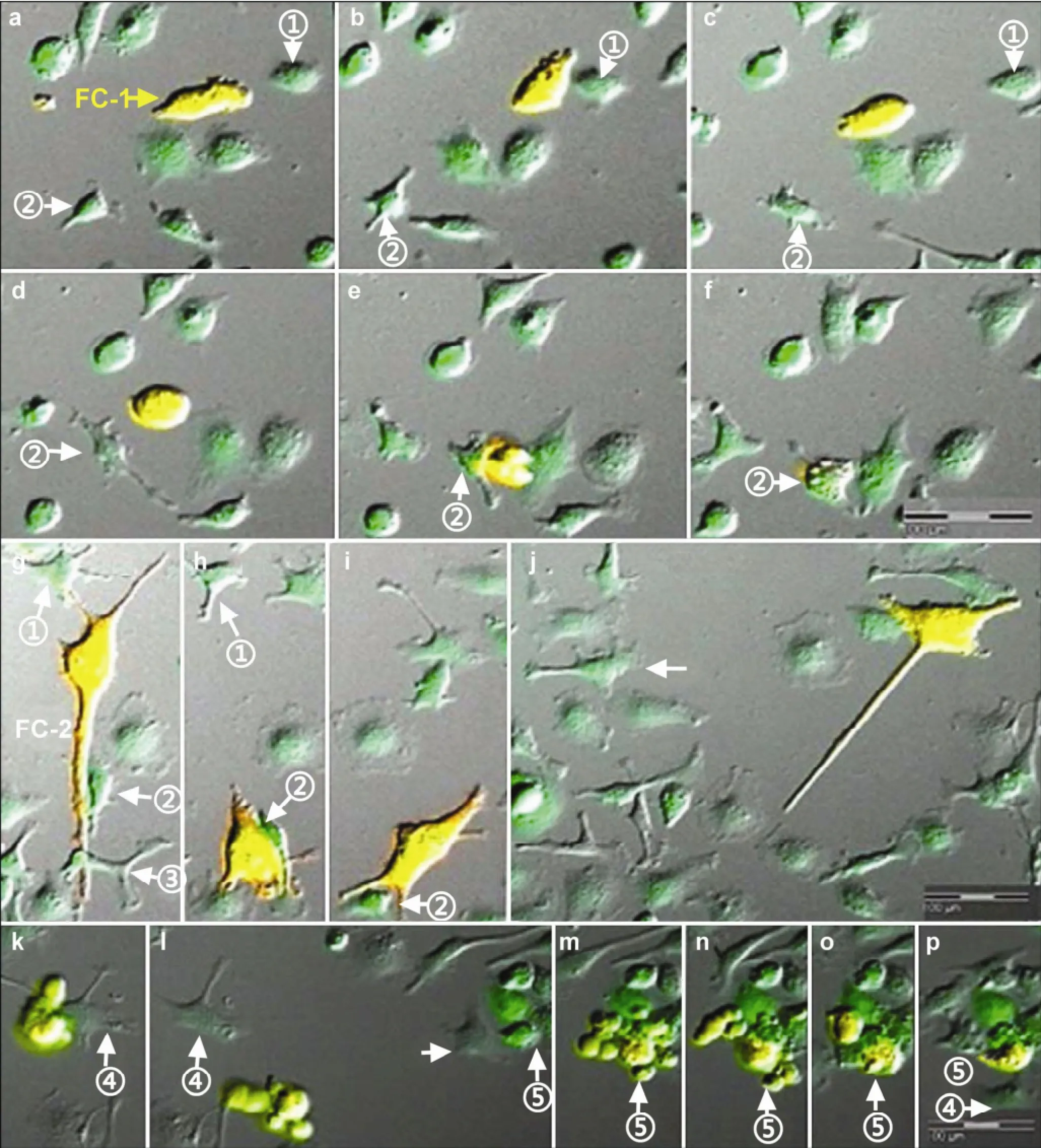
Figure 5 Instant apoptosis of fusion cells. One of the fusion cells, the FC-1 cell migrated irregularly before apoptosis. Initially, it moved close to the No. ① cell (a), but the cells were not in close contact. The migrating cell continued towards the left front and turned back (b). Next, the FC-1 cell approached to the No. ② cell (c), which extended its pseudopodia, stopped during migration (d) and moved close to the FC-1 cell (e). Nearly simultaneously, the FC-1 cell collapsed suddenly and its debris was engulfed by the No. ② cell (f). Another fusion cell, the FC-2 cell, migrated while showing continuous morphologic changes of its cell body. Initially, the cell contacted the surrounding No. ①-③ Mø cells (g). Only the No. ② cell followed the FC-2 cell closely (h), but they eventually separated (i). The No. ④ cell approached the FC-2 cell and extended its pseudopodia (j), after which the FC-2 broke into pieces (k), but phagocytosis did not occur. The FC-2 cell debris approached the No. ⑤ cell (l) gradually, and phagocytosis occurred in No. ⑤ (m-o), while No. ④ had no further changes (p).
Cell fusion is a major and important cellular event in cellular sociology. Previous studies reported that the incidence of cell fusion in a tumor model was 1%[11]. Our data showed that following in vitro co-culture of GICs and macrophages, the spontaneous fusion incidence between the two types of cell was 2.7%. Different fusion rates may be observed in different co-culture systems.
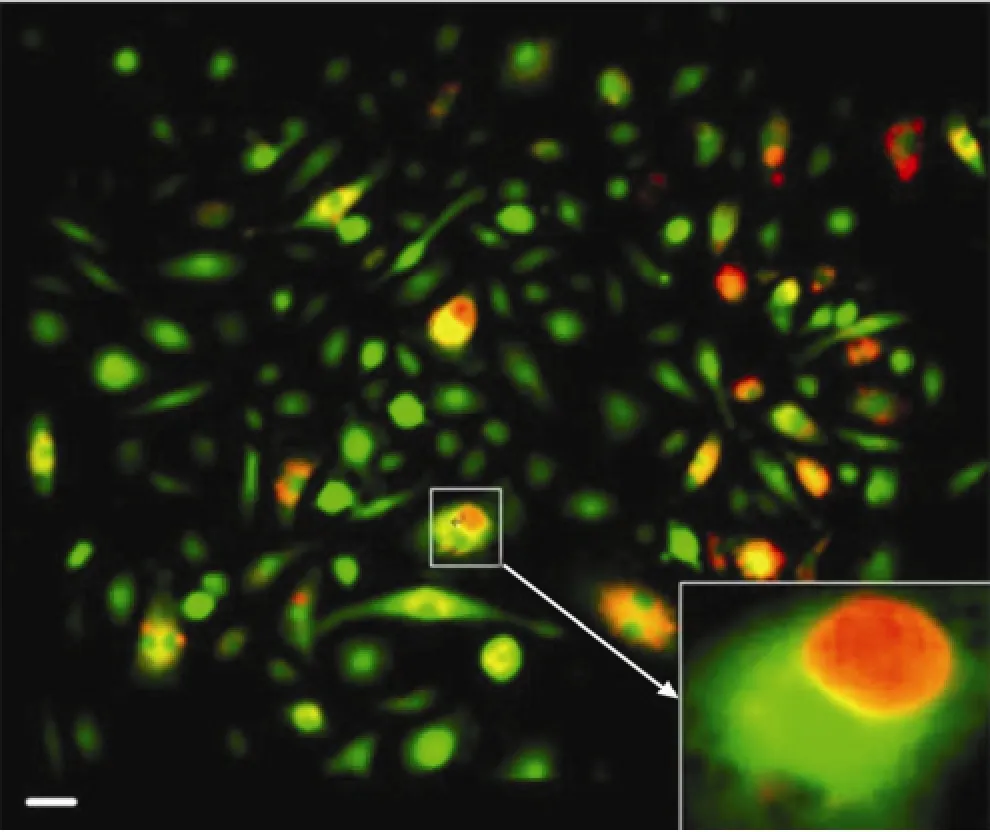
Figure 6 Phenomenon of entosis (scale bar 20 μm). The transient state in which a live GIC was contained within a Mø (Arrows marked engulfed cells).
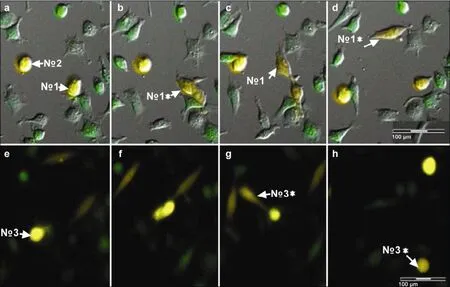
Figure 7 Fate of entotic cells. Entosis occurred in two yellow spherocytes, No. 1 (a) and No. 3 (e). The deep yellow round cell No.1 released a slight yellow short spindle cell (b, c), after which the offspring cells rapidly exited. During release, the shape of the parent cell changed from round to spindle. After release,the shape of the parent cell was restored to its original state (b: No. 1*; d: No. 1*). The No.3 (e) parent cell migrated and stretched its small prominences in different directions (f), and then it retracted and released a long spindle pale yellow offspring (g: No. 3*). The offspring cell moved actively around its parent cell. After 370 min the cell became a spherocyte, similar to its parent cell (h: No. 3*).
The pathways of cell fusion vary between different cell types, suggesting that different systems have evolved separately in various cell type[12-16]. Recent studies have revealed that fusion can occur followingphagocytosis of cancer cells or apoptotic bodies by tumor-associated macrophages or other phagocytes[17]. In fact, macrophages may fuse with cancer cells through their inherent fusion capability; similarly, cancer cells may be prone to fusion because of aberrant expression of fusion-associated receptors or ligands[18]. Our data suggested that GICs can actively fuse to host macrophages and that macrophage cell occasionally actively fuse to GICs. Regardless of the interaction type, cell fusions occured at the level of whole cells.
Tumor fusion plays an important role in the progression and metastasis of cancer[17-22], which represents a non-genetic mutational mechanism that may explain the aberrant gene expression patterns associated with malignant cells. Hybrids produced by fusion in vitro or in vivo fusion were aneuploid and showed higher invasiveness potential. Additionally, GICs fusion may be an important cause of glioma remodeling and progression, particulaly in the vicious transformation of tumor mesenchaymal cells and heterogenicity of tumor cells.
Fusion cells with the biological effects should have potential to divide, differentiate, and trans-differentiate, although their number in the cell sociology was very low; however, similarly to tumor stem cells, cell fusion was very effective in achieving these goals and may play very important roles in tumor remodeling driven by GICs[23]. Our results showed that fusion cells could produce equal or unequal offspring cells in symmetrical and asymmertrical forms, confirming that the differentiation ability described above occurred in gliomagenesis.
Programmed cell death, particularly apoptosis, is important in tissue shaping, eliminating unnecessary cells, and tumor suppression[24]. Our data revealved two types of apoptosis processes of fusion cells. The rate of apoptosis was very rapid, suggesting that rapid apoptosis of fusion cells is relevant to the regulation and suppression of tumors.
Another mode of non-apoptotic cell elimination known as entosis resembles cell cannibalism and the cell-in-cell phenotype was observed in some tumors[25]. During entosis, a live cell accommodates a host cell that comes from the extra-cellular matrix; the viability of the internalized cell is sustained over the short term, with two results: digestion by the intracellular enzymes or extrication out of the host cell[24]. Our results showed the latter destination, in which the cell was released. It was previously unclear whether entosis was a means for cell elimination or cannibalism of the invading cell, which can provide certain advantages to the host cell through nutrient recycling during metabolic stress. In tumor occurrence and evolution, entosis inhibited tumor growth[25].
In summary, valuable information was obtained related to the direct and dynamic processes of interactions between GICs and macrophages in vitro. these data may help to reveal the phenomenon of malignant transformation of host macrophages, which we reported previously[1], and was an important part of glioma cellular sociology.
Conflict of interests
All contributing authors have no conflict of interests.
[1] Wang AD, Dai XL, Cui BQ, Fei XF, Chen YM, Zhang JS, Zhang QB, Zhao YD, Wang ZM, Chen H, et al. Experimental research of host macrophage canceration induced by glioma stem progenitor cells. Mol Med Rep 2015, 11(4): 2435-2442.
[2] Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia 2012, 60(3): 502-514.
[3] Wan Y, Fei XF, Wang ZM, Jiang DY, Chen HC, Yang J, Shi L, Huang Q. Expression of miR-125b in the new highly invasive glioma stem cell and progenitor cell line SU3. Chin J Cancer 2012, 31(4): 207-214.
[4] Shirasaki Y, Yamagishi M, Shimura N, Hijikata A, Ohara O. Toward an understanding of immune cell sociology: Real- time monitoring of cytokine secretion at the single-cell level. IUBMB Life 2013, 65(1): 28-34.
[5] Dong J, Zhang QB, Huang Q, Chen H, Shen YT, Fei XF, Zhang TY, Diao Y, Wu ZC, Qin ZH, et al. Glioma stem cells involved in tumor tissue remodeling in a xenograft model. J Neurosurg 2010, 113(2): 249-260.
[6] Dong J, Zhao YD, Huang Q, Fei XF, Diao Y, Shen YT, Xiao H, Zhang TY, Lan Q, Gu XS. Glioma stem/progenitor cells contribute to neovascularization via transdifferentiation. Stem Cell Rev 2011, 7(1): 141-152.
[7] Chen YM, Fei XF, Wang AD, Dai XL, Zhang JS, Cui BQ, Zhang QB, Zhao YD, Chen H, Wang ZM, et al. Host glialcell canceration induced by glioma stem cells in GFP/RFP dual fluorescence orthotopic glioma models in nude mice. Chin J Oncol 2013, 35(1): 5-10.
[8] Hurtig J, Chiu DT, Önfelt B. Intercellular nanotubes: Insights from imaging studies and beyond. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2010, 2(3): 260-276.
[9] Osswald M, Jung E, Sahm F, Solecki G, Venkataramani V, Blaes J, Weil S, Horstmann H, Wiestler B, Syed M, et al. Brain tumour cells interconnect to a functional and resistant network. Nature 2015, 528(7580): 93-98.
[10] Antonyak MA, Li B, Boroughs LK, Johnson JL, Druso JE, Bryant KL, Holowka DA, Cerione RA. Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc Natl Acad Sci USA 2011, 108(12): 4852-4857.
[11] Duelli D, Lazebnik Y. Cell fusion: A hidden enemy? Cancer Cell 2003, 3(5): 445-448.
[12] Goldenberg DM, Zagzag D, Heselmeyer-Haddad KM, Berroa Garcia LY, Ried T, Loo M, Chang CH, Gold DV. Horizontal transmission and retention of malignancy, as well as functional human genes, after spontaneous fusion of human glioblastoma and hamster host cells in vivo. Int J Cancer 2012, 131(1): 49-58.
[13] Schichor C, Albrecht V, Korte B, Buchner A, Riesenberg R, Mysliwietz J, Paron I, Motaln H, Turnšek TL, Jürchott K, et al. Mesenchymal stem cells and glioma cells form a structural as well as a functional syncytium in vitro. Exp Neurol 2012, 234(1): 208-219.
[14] Chen EH, Olson EN. Unveiling the mechanisms of cell-cell fusion. Science 2005, 308(5720): 369-373.
[15] Vignery A. Macrophage fusion: The making of osteoclasts and giant cells. J Exp Med 2005, 202(3): 337-340.
[16] Vignery A, Gilgenkrantz S. Macrophage fusion: Are somatic and cancer cells possible partners? Med Sci (Paris) 2005, 21(12): 1070-1075.
[17] Pawelek JM. Tumour cell hybridization and metastasis revisited. Melanoma Res 2000, 10(6): 507-514.
[18] Pawelek JM, Chakraborty AK. Fusion of tumour cells with bone marrow-derived cells: A unifying explanation for metastasis. Nat Rev Cancer 2008, 8(5): 377-386.
[19] Pawelek JM. Tumour-cell fusion as a source of myeloid traits in cancer. Lancet Oncol 2005, 6(12): 988-993.
[20] Pawelek J, Chakraborty A, Lazova R, Yilmaz Y, Cooper D, Brash D, Handerson T. Co-opting macrophage traits in cancer progression: A consequence of tumor cell fusion? Contrib Microbiol 2006, 13: 138-155.
[21] Duelli D, Lazebnik Y. Cell-to-cell fusion as a link between viruses and cancer. Nat Rev Cancer 2007, 7(12): 968-976.
[22] Holmgren L, Bergsmedh A, Spetz AL. Horizontal transfer of DNA by the uptake of apoptotic bodies. Vox Sang 2002, 83(Suppl. 1): 305-306.
[23] Dittmar T, Nagler C, Schwitalla S, Reith G, Niggemann B, Zänker KS. Recurrence cancer stem cells-made by cell fusion? Med Hypotheses 2009, 73(4): 542-547.
[24] White E. Entosis: It's a cell-eat-cell world. Cell 2007, 131(5): 840-842.
[25] Overholtzer M, Mailleux AA, Mouneimne G, Normand G, Schnitt SJ, King RW, Cibas ES, Brugge JS. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell 2007, 131(5): 966-979.
Zhang MX, Chen JJ, Wang L, Ji XY, Yang L, Sheng YJ, Liu HR, Wang HY, Wang AD, Dai XL, Li XN, Huang Q, Dong J. Preliminary analysis of cellular sociology of co-cultured glioma initiating cells and macrophages in vitro. Transl. Neurosci. Clin. 2016, 2(2): 77-86.
✉ Corresponding author: Jun Dong, E-mail: djdongjun@163.com
Supported by the National Natural Science Foundation of China (Grant No. 81472739) and the Natural Science Foundation of Jiangsu Province (Grant No. BK20151214).
杂志排行
Translational Neuroscience and Clinics的其它文章
- Behavioral features of mice fed with a cholesterol-enriched diet: Deficient novelty exploration and unaltered aggressive behavior
- A study of the effects of 3,5-diiodo-L-thyronine in the tail suspension and forced swim models of depression
- Transplantation of neural progenitor cells differentiated from adipose tissue-derived stem cells for treatment of sciatic nerve injury
- New frontiers in biomaterials research for tissue repair and regeneration
- A newly developed open-end intracranial hematoma drainage tube
- Are life sciences all about life?
