Towards Personalized Intervention for Alzheimer’s Disease
2016-11-09XingPengPeiqiXingXiuhuiLiYingQianFuhaiSongZhouxianBaiGuangchunHanHongxingLei
Xing PengPeiqi XingXiuhui LiYing QianFuhai SongZhouxian BaiGuangchun HanHongxing Lei*h
1CAS Key Laboratory of Genome Sciences and Information,Beijing Institute of Genomics,Chinese Academy of Sciences,Beijing 100101,China
2Cunji Medical School,University of Chinese Academy of Sciences,Beijing 100049,China
3Center of Alzheimer's Disease,Beijing Institute for Brain Disorders,Beijing 100053,China
REVIEW
Towards Personalized Intervention for Alzheimer’s Disease
Xing Peng1,2,a,Peiqi Xing1,2,b,Xiuhui Li1,2,c,Ying Qian1,2,d,Fuhai Song1,2,e,Zhouxian Bai1,2,f,Guangchun Han1,g,Hongxing Lei1,2,3,*,h
1CAS Key Laboratory of Genome Sciences and Information,Beijing Institute of Genomics,Chinese Academy of Sciences,Beijing 100101,China
2Cunji Medical School,University of Chinese Academy of Sciences,Beijing 100049,China
3Center of Alzheimer's Disease,Beijing Institute for Brain Disorders,Beijing 100053,China
Alzheimer's disease;
Demographic information;
Genome;
Peripheral biomarkers;
iPSC technology
Alzheimer’s disease(AD)remains to be a grand challenge for the international community despite over a century of exploration.A key factor likely accounting for such a situation is the vast heterogeneity in the disease etiology,which involves very complex and divergent pathways. Therefore,intervention strategies shall be tailored for subgroups of AD patients.Both demographic and in-depth information is needed for patient stratification.The demographic information includes primarily APOE genotype,age,gender,education,environmental exposure,life style,and medical history,whereas in-depth information stems from genome sequencing,brain imaging,peripheral biomarkers,and even functional assays on neurons derived from patient-specific induced pluripotent cells(iPSCs).Comprehensive information collection,better understanding of the disease mechanisms,and diversified strategies of drug development would help with more effective intervention in the foreseeable future.
aORCID:0000-0002-3645-8115.
bORCID:0000-0002-5675-1036.
cORCID:0000-0002-9808-1173.
dORCID:0000-0002-4622-1904.
eORCID:0000-0003-0848-8349.
fORCID:0000-0001-7071-666X.
gORCID:0000-0001-9277-2507.
hORCID:0000-0003-0496-0386.
Peer review under responsibility of Beijing Institute of Genomics,Chinese Academy of Sciences and Genetics Society of China.
http://dx.doi.org/10.1016/j.gpb.2016.01.006
1672-0229Ⓒ2016 The Authors.Production and hosting by Elsevier B.V.on behalf of Beijing Institute of Genomics,Chinese Academy of Sciences and Genetics Society of China.
This is an open access article under the CC BY license(http://creativecommons.org/licenses/by/4.0/).
Introduction
Alzheimer's disease(AD)is one of the leading causes of death in senior people.Caring for AD patients with deteriorating cognitive and daily functions poses a great economic and psychological burden for the families as well as society.Initially discovered in 1906,the pathological hallmarks of AD,namely amyloid plaques and neurofibrillary tangles,have been well documented over a century.However,little had been known about the disease mechanisms at molecular level until the identification of the gene encoding amyloid precursor protein(APP)and the genetic mutations causing familial AD[1,2]. Nonetheless,familial AD only constitutes~2%of AD patients[3],while the vast majority of AD cases are not caused by the genetic mutations affecting the coding or processing of APP.For sporadic AD,the molecular pathogenesis seems to be far from understood as yet.
Up till now,only four drugs have been approved for AD treatment by the Food and Drug Administration(FDA)in USA and its counterparts in Europe[4].These drugs target neural transmission and are all used for symptom relief,sometimes even with unbearable side effects.They are unable to modify the disease trajectory,not even slowing down the disease progression.Therefore,the‘neural transmission”hypothesis for AD has not been well supported by the human trials,and deficiency in neural transmission may merely be a downstream and symptomatic problem in AD.In the past couple of decades,most of the efforts on drug development have been devoted to the clearance of amyloid or the aggregating oligomers,which is believed to be a major causal factor according to the‘amyloid cascade hypothesis”[5].Although recent studies targeting amyloid showed marginal progress in the early stage AD[6,7],most of the clinical trials along this line have been very disappointing.Beyond amyloid clearance,other prevention or treatment strategies have also been initiated with no conclusive evidence of success so far[8].
To achieve more positive outcome,better patient stratification shall be adopted in the future based on comprehensive collection of patient information.The details will be discussed in the following sections(Figure 1).
Demographic information for AD
Currently,the well-recognized demographic information about AD includes APOE genotype,age,gender,education,environmental exposure,life style,and medical history.
APOE genotype
APOE ε4 allele is the major genetic risk of sporadic AD,which confers risks 3-4 folds higher for people carrying one ε4 allele and~10 folds higher for peoples carrying two ε4 alleles compared to non-carriers[2].Although mainly viewed as a gene involved in the lipid metabolism pathway,APOE seems to be associated with many AD-related processes.Most notably,APOE is involved in amyloid β(Aβ)metabolism and amyloid clearance[9].APOE may also be involved in brain development.For example,infants carrying APOE ε4 allele have different brain structure compared to non-carriers[10].The altered brain structures at early developmental stage may confer susceptibility for AD at the old age.APOE ε4 genotype is also associated with reduced glucose metabolism in the brain independent of amyloid aggregation[11].Since deficiency in energy metabolism is considered as one of the major upstream factors in AD pathogenesis,this study suggests that APOE genotype itself can be a causal factor for AD.Thus,AD patients with 0,1,or 2 ε4 alleles may follow different paths to the disease stage and therefore shall be treated differently. Due to the critical roles of APOE in the development ofsporadic AD,APOE-centered drug development has also been attempted,and notably drugs targeting APOE have demonstrated efficiency in mouse model of AD[12].
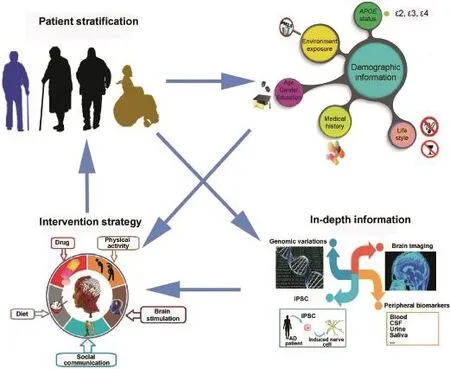
Figure 1 Path from patient stratification to personalized intervention for ADCollection of demographic information is the basis for patient stratification.Incorporation of in-depth information will greatly facilitate the design of personalized intervention.AD,Alzheimer's disease;iPSC,induced pluripotent stem cell;CSF,cerebrospinal fluid;PM 2.5,particulate matter(≤2.5 μm).
Age
Apart from APOE,age is the most recognized risk factor for sporadic AD.Sporadic AD mostly starts after the age of 65,while the chance of developing AD climbs to~40%for people aged over 85[13].Aging is accompanied by the systematic deterioration of the condition over the whole body.Most notably,brain perfusion(blood supply to the whole brain)continues to decline starting at the age of 22,which is likely the root cause of many problems due to the critical role of energy supply in brain functions.Studies have shown that brain atrophy and amyloid deposition are part of the normal aging process.Even in normal elderly people without dementia,over 1/3 has amyloid plaque in the brain[14].In addition,brain transcriptome studies showed that the aging brain is characterized by up-regulation of immune response and down-regulation of synaptic transmission,which is similar to the gene dysregulation pattern in AD[15].To some extent,AD can be viewed as accelerated aging.Interestingly,aging is lately considered by some people as a treatable disease and anti-aging clinical trials have been proposed[16].
Gender
Epidemiological studies suggest that females have a higher risk of developing AD than males do[17],although the exact reason remains unclear at this moment.It has been postulated that decreased estrogen levels at old age may be partially responsible for higher incidence of AD in females[18].Estrogen is neuroprotective and estrogen receptor β plays a critical role in brain function[19].Studies on the human brain transcriptome have also shown that immune response is more widely spread in aged female brains than aged male brains,consistent with the accelerated aging scenario in female brains[20].There is interaction between gender and APOE in both brain connection and cerebrospinal fluid(CSF)markers[21],which may be partially attributed to the involvement of both estrogen receptor β and APOE in certain brain functions.Further elucidation of the gender risk of AD may require better understanding of the brain network through ongoing big brain projects worldwide.
Education
Lower education level is associated with higher risk of developing AD[22].Education process is accompanied by sophisticated brain wiring.Lower education likely leads to less complex brain network which is more susceptible for breakdown,which is the so-called‘brain reserve”hypothesis[23]. However,it has also been reported that the rate of cognitive decline is higher for people with higher education once AD is diagnosed[24].This is likely due to the depletion of‘brain reserve”after the onset of AD symptoms even for people with higher education.
Environmental exposure
Exposure to non-healthy environment is also associated with higher risk of developing AD.For example,long-term exposure to high concentration of fine particulate matter(≤2.5 μm)environment can lead to peripheral biomarker change linked to AD[25].Mechanistic studies have shown that similar environment can lead to elevated inflammation and AD pathologies in mice[26].Additionally,high concentration of pesticide,such as dichlorodiphenyldichloroethylene(DDE),in the blood is also associated with higher AD risk[27].Overall,environmental exposure may affect the brain either directly through the inhalation process or indirectly through the blood-brain barrier[25].
Life style
Life style can have major effects on the risk of developing AD. Cigarette smoking and alcohol drinking are associated with higher risk,both of which may disrupt the global metabolism and in the meantime exert epigenetic modification in the brain[28].On the other hand,more active lifestyle,such as physical exercise[29],social communication,and brain stimulating activities like music and arts[30],has a protective effect.The beneficial effect may arise from better energy supply and more synaptic activities.
Medical history
Certain medical histories are associated with elevated AD risk. For instance,hypertension is associated with both vascular dementia and AD[31],which is consistent with the critical role of the cardiovascular system and energy supply in AD development.In a population-based study over 25 years,late-life atrial fibrillation was found to be the highest risk of AD among several heart diseases examined[32].In another longitudinal study,the duration of type 2 diabetes was found to be a risk factor for AD[33].In addition,periodontal disease is also associated with higher risk of AD through increased brain amyloid load[34].In a meta-analysis over studies on the relationship between bacterial infection and AD,certain bacterial infection such as by Chlamydophila pneumoniae was found to increase the risk of 4-10 fold,likely through sustained high level of immune response[35].Notably sleep has been recently linked to AD because of its role in amyloid clearance[36,37],and sleep postures may affect efficiency in amyloid removal[38].
In-depth information for AD
Demographic information alone is insufficient to achieve precise assessment of AD patients,which would be greatly facilitated by collecting in-depth information including genome,brain imaging,and peripheral biomarkers.In addition,recent development in induced pluripotent stem cell(iPSC)technology makes it possible to conduct functional assays on patient-derived neurons.
Genomic variations
Although APOE ε4 allele confers high risk of developing AD,it is neither necessary nor sufficient for AD development[39]. For instance,some people without APOE ε4 alleles can develop AD,while others carrying one or two APOE ε4 alleles may never develop AD throughout their lives.Genome-wide association studies(GWAS)have revealed 20 additional risk loci such as those encoding clusterin(CLU)and bridging integrator 1(BIN1),all with moderate risk[40].A recent work showed that marginally-significant SNPs(P<10-3)from GWAS studies may contribute significantly to the prediction of genetic risk of AD[41].Other than the genetic risk from common variations examined in GWAS studies,rare variations detected by sequencing technologies may also confer risk of AD.For instance,rare variations in genes encoding triggering receptor expressed on myeloid cells 2(TREM2)[42]and phospholipase D family,member 3(PLD3)[43]from exome sequencing studies could significantly increase the risk of developing AD,albeit limited to a small population of the rare variant carriers.The relative contribution from common and rare variations is still under debate,which could be tackled using whole-genome sequencing(WGS)since compared to GWAS,WGS can detect both common and rare variations once sufficient sequencing depth is reached.
Brain imaging
Brain imaging offers the direct measurement of the patients' brain structure or function.The degree of brain atrophy can be evaluated by magnetic resonance imaging(MRI).Glucose uptake as an indicator of energy metabolism level can be measured by 18F-fluorodeoxiglucouse positron emission tomography(PET).For more specific diagnosis of AD,various PET technologies such as Pittsburgh compound B(PIB)have been developed for the measurement of Aβ amyloid[44]and tangle[45,46].PET and MRI have also been combined to elucidate the causal relationship between amyloid deposition and regional cerebral blood flow[47].
Peripheral biomarkers
Brain imaging is currently not suitable for early diagnosis due to its poor accessibility especially in China.Therefore,peripheral biomarkers from CSF,blood,urine[48],and saliva[49]are highly desirable.Most notably,low level of Aβ42 and high level of p-tau in CSF have been demonstrated to be sensitive biomarkers for AD diagnosis[50].Aside from these wellrecognized biomarkers,other biomarkers,mostly in the peripheral blood,have also been investigated.For example,the expression levels of several microRNAs including miR-15a in the blood are associated with AD[51].Lower expression level of ribosome genes has also been reported in the blood of AD patients[52].Apart from gene expression,the level of immune-related proteins or analytes in the plasma or serum has also been associated with AD.For example,complement C3 has been replicated in independent studies[53].A panel of 21 serum proteins has also been replicated in independent sample sets[54].A panel of blood analytes including pancreatic polypeptide(PPY)shows high correlation with brain amyloid load[55].In addition,a protein microarray study identified a panel of 20 autoantibodies with high accuracy in discriminating AD from control[56].A multi-tissue study showed that calpain activity is increased in CSF and decreased in blood of AD patients[57].Interestingly,a rare study of telomereinbuccalcellsshowedshortertelomeresand increased number of telomeres in AD patients compared to controls[58].
Functional assays on patient-derived neurons
The most relevant cell type for AD studies is neuron,which is not accessible in patients.Notably,the development of iPSC technology opens the door to examine patient-specific neurons derived from the skin fibroblast.Various assays can be conducted on these neurons.For instance,Aβ42/Aβ40 ratio and gene expression profiles were compared on iPSC-derived neural progenitor cells from PSEN1 mutation carriers and controls[59].A PSEN1 ΔE9 mutation was shown to specifically affect the γ-secretase activity of PSEN1[60].Neurotoxicity caused by various forms of Aβ can be examined too[61]. Moreover,these neurons can also be used to test whether drugs like γ-secretase modulators are effective in reversing certain cellular phenotypes[62].In the past few years,3D culture models have been under development,which may better model the 3D neuronal network in vitro[63].
Intervention strategies for AD
Although there are limited options available for treating AD,proper intervention as discussed in this section is highly recommended.Prevention is the best medicine[64].Therefore,it is a common view to treat the disease at its early stage,with or without medication.
Physical activity
The effect of physical exercises on AD has been extensively studied in both human and mouse models,with the beneficial effect reported at different stages of AD.For example,regular physical exercises can lower the rate of conversion from mild cognitive impairment(MCI)to dementia[65].The exact mechanism underlying such beneficial effect is likely very complex. Obviously,exercise can improve the cardiovascular functions and provide better blood supply to the brain,whereas at the molecular level,it may involve elevated level of BDNF in the serum[66].Interestingly,conventional exercises such as Tai-Chi have also been demonstrated to confer a wide spectrum of beneficial effects[67].
Brain stimulation
Proper brain stimulation such as music is protective for AD.It may help maintain existing neural networks or develop new ones[68].Music and arts are the common approaches for brain stimulation.Studies showed that certain sound waves may help clear amyloid from the blood stream[69].In addition,non-invasive stimulations such as transcranial magnetic stimulation can improve cognition[70].To restore certain neuronal circuits in patients with confirmed neurodegeneration,invasive deep brain stimulation has been tested in AD andParkinson's disease[71].To reduce the side effects from invasive operation,some minimally invasive brain stimulation approaches are also currently under development[72].
Social communication
Social communication can improve cognitive function for AD patients.Active cognitive life style such as social engagement has been shown to be associated with reduced risk of developing AD[73],whereas elderly people living a solitary life tend to have higher risk of developing depression[74],which is associated with higher risk of AD[75].Thus,engaging elderly people in community activities is strongly recommended.
Diet

Table 1 Stage-specific intervention strategies for AD
Western diet is associated with higher risk of developing AD,while Mediterranean diet is protective[76,77].Moreover,Mediterranean diet and physical activity are independently protective for AD[29].The Mediterranean diet likely favors a stronger cardiovascular system which can provide better energy and nutrient supply to the brain.In addition,dietary supplements have also been widely studied.For example,omega3 has been shown by many studies to slow down cognitive decline[78]and consumption of cocoa flavanol can improve cognitive function[79].Other than these supplements,Selenium as an anti-aging nutrient is also widely studied in the field of aging and AD[80].
Drugs
The four FDA-approved drugs for AD mainly target synaptic stimulation.In the past few years,the most actively pursued drug development for AD is Aβ immunotherapy that targets the clearance of amyloid plaques or Aβ oligomers[6].Along the same line,targeting the pathological processing of Aβ has also been pursued,mainly focusing on γ-secretase[81]. Tau,another major hallmark of AD,is also an activelypursued drug target[82].Clinical trials for drugs targeting γsecretase or tau have not been successful so far[83,84].While these drugs target specific molecules,Chinese traditional medicine likely has broader impact on the brain and the body as a whole.For example,some of the widely-used Chinese traditional medicine for AD such as ginseng can stimulate blood flow in the brain[85],while some traditional Chinese medicine may also enhance amyloid clearance[86].
Stage-specific intervention strategy for AD
Based on current findings,we here summarize a stage-specific intervention strategy for AD[87,88].Please note that some of the intervention approaches may be applicable to other stages as well(Table 1).
Preimplantation stage
Intervention at the preimplantation stage is highly controversial due to the ethical issues.Currently,preimplantation diagnosis is only available for families with inheritable diseases[89],including familiar AD.It is foreseeable that genome editing may be used to correct the causal mutations in familiar AD in the future.However,genome editing is not yet a mature technology for clinical practice at this moment due to the concerns including off-targeting.In addition,the ethical issue in human germline genome editing is extremely sensitive due to the risk of‘playing God”by some people.
Childhood,youth,and adulthood
Proactive prevention could be performed at stages of childhood,youth,and adulthood.This is particularly important for people who carry APOE ε4 allele or other high risk genetic variations.A good lifelong education can also help build a resilient brain network[24].Furthermore,it is necessary to avoid non-healthy living and working environment.It is equally important to stick to a healthy diet and stay away from bad habits such as cigarette smoking and alcohol drinking.
MCI and preclinical AD
With confirmed diagnosis from cognitive tests,peripheral biomarkers,or brain imaging,proper intervention shall be applied for MCI and preclinical AD.A good starting point is to have regular physical exercise.This can be supplemented with brain stimulating activities such as music and arts.Early stage patients should be actively involved in social communication with family,friends,or local community.Certain Chinese traditional medicine may also be helpful[86].In addition,iPSC technology may be needed to properly design personalized treatment approaches.
Mild AD
Once the patients progress to the clinical AD stage,medical intervention shall be added on top of the non-medical treatments described above.Several factors shall be taken into account for such intervention,including neural transmission,energy supply,and amyloid clearance.While prescribed drugs can stimulate neural transmission,nutritional supplementssuch as coenzyme Q10 may provide more energy supply to the brain[90].Furthermore,the most heavily investedAβ immunotherapy can remove the pathological amyloid buildup[6].
Moderate AD
At the moderate AD stage,neural inflammation caused by prolonged stimulation by amyloid deposit may be a more pressing issue to deal with[91].AD brain and blood transcriptome studies suggest that elevated level of the innate immune system may be a suitable target for further drug development[92].For example,glucogon-like peptide 1 analogs,the widely-studiedneuro-protectiveagent,areinvolvedin reducing neural inflammation among many other protective functions[93].More studies directly targeting neural inflammation including with non-steroidal anti-inflammatory drugs are underway[94].
Severe AD
At the late stage of the disease,neuronal cell death becomes a major issue[95].Neuronal cell death results in the permanent breakdown of the brain network,which is responsible for cognitive and daily functions.Thus,neural regeneration therapy could be more effective for patients at this stage.Interestingly,a recent study demonstrated enhanced learning and memory in AD mice after transplantation of inhibitory interneuron progenitors in the brain[96].Stem cell technology will likely play an increasingly important role in neural regeneration.In addition to stem cell transplantation,activation of endogenous neural stem cells or converting the inflammatory astrocytes to neurons are other viable options[97].However,these studies are still at the preclinical stages and the efficacy on human AD is thus unknown as yet.
Concluding remarks
Although convergent AD symptoms have been observed,the underlying heterogeneous pathways for AD development require personalized intervention strategy.Adjustment of life styles may be a lifelong battle especially for those who carry causal mutations for familial AD or high risk genetic variations for sporadic AD.The combination of proper education,diet,life style,and environment seems to be the best prescription to prevent or delay the onset of AD.For patients at the early stage of the disease,physical and mental exercises,social communication,and traditional Chinese medicine could be effective to slow down the disease progression.For patients at the mild or moderate clinical AD stage,medical interventions,including those targeting neural transmission,energy supply,Aβ aggregation,and neural inflammation,should come into play.For patients at the severe stage with considerable loss of neurons,neural regeneration therapy could be critical to restore the normal function of the brain,although clinical trials have not been reported.
At each of the disease stages,intervention can be further tailored toward the patients according to the demographic and in-depth information described in this review.For example,drugs especially APOE-targeting drugs may act differently in patients with different APOE genotypes.As more drugs become available,it may be necessary to test the drug efficiency on patients with different genotypes.For such kind of test,patient-derived neurons can be used for preliminary tests. Other than the demographic and genetic factors,brain imaging and peripheral biomarkers may also be employed for patient stratification.In addition,age factor shall be considered when designing programs of physical activities because some activities may not be suitable for the highly-aged patients.For elderly females,hormone replacement therapy may be beneficial as well.Furthermore,for people with specific medical conditions such as hypertension or type 2 diabetes,management of these specific medical conditions is good for both AD prevention and treatment.
Competing interest
The authors declare that they have no competing interests.
Acknowledgments
This work was supported by the National High-tech R&D Program of China(863 Program;Grant No.2015AA020108)and the National Basic Research Program of China(973 Program;Grant No.2014CB964901)funded by the Ministry of Science and Technology of China.
References
[1]Patterson D,Gardiner K,Kao FT,Tanzi R,Watkins P,Gusella JF.Mapping of the gene encoding the beta-amyloid precursor protein and its relationship to the Down syndrome region of chromosome 21.Proc Natl Acad Sci U S A 1988;85:8266-70.
[2]Guerreiro R,Bras J,Hardy J.SnapShot:genetics of Alzheimer's disease.Cell 2013;155:968.e1.
[3]Bird TD.Genetic factors in Alzheimer's disease.N Engl J Med 2005;352:862-4.
[4]Townsend M.When will Alzheimer's disease be cured?A pharmaceutical perspective.J Alzheimers Dis 2011;24:43-52.
[5]Musiek ES,Holtzman DM.Three dimensions of the amyloid hypothesis: time, spaceand‘wingmen'.NatNeurosci 2015;18:800-6.
[6]Reardon S.Antibody drugs for Alzheimer's show glimmers of promise.Nature 2015;523:509-10.
[7]Sevigny J,Chiao P,Bussiere T,Weinreb PH,Williams L,Maier M,et al.The antibody aducanumab reduces Abeta plaques in Alzheimer's disease.Nature 2016;537:50-6.
[8]Herrup K,Carrillo MC,Schenk D,Cacace A,Desanti S,Fremeau R,et al.Beyond amyloid:getting real about nonamyloid targets in Alzheimer's disease.Alzheimers Dement 2013;9:452-8.e1.
[9]Kanekiyo T,Xu H,Bu G.ApoE and Abeta in Alzheimer's disease:accidental encounters or partners?Neuron 2014;81:740-54.
[10]Dean DC,Jerskey BA,Chen K,Protas H,Thiyyagura P,Roontiva A,et al.Brain differences in infants at differential genetic risk for late-onset Alzheimer disease:a cross-sectional imaging study.JAMA Neurol 2014;71:11-22.
[11]Jagust WJ,Landau SM.Alzheimer's disease neuroimaging I. Apolipoprotein E,not fibrillar beta-amyloid,reduces cerebral glucosemetabolisminnormalaging.JNeurosci 2012;32:18227-33.
[12]Cramer PE,Cirrito JR,Wesson DW,Lee CY,Karlo JC,Zinn AE,etal.ApoE-directedtherapeuticsrapidlyclearbetaamyloid and reverse deficits in AD mouse models.Science 2012;335:1503-6.
[13]Herrup K.The case for rejecting the amyloid cascade hypothesis. Nat Neurosci 2015;18:794-9.
[14]Burnham SC,Bourgeat P,Dore V,Savage G,Brown B,Laws S,et al.Clinical and cognitive trajectories in cognitively healthy elderly individuals with suspected non-Alzheimer's disease pathophysiology(SNAP)or Alzheimer's disease pathology:a longitudinal study.Lancet Neurol 2016;15:1044-53.
[15]Sun J,Feng X,Liang D,Duan Y,Lei H.Down-regulation of energy metabolism in Alzheimer's disease is a protective response ofneuronstothemicroenvironment.JAlzheimersDis 2012;28:389-402.
[16]Hall SS.A trial for the ages.Science 2015;349:1274-8.
[17]Finch CE,Shams S.Apolipoprotein E and sex bias in cerebrovascularagingofmenandmice.TrendsNeurosci 2016;39:625-37.
[18]Lan YL,Zhao J,Li S.Update on the neuroprotective effect of estrogen receptor alpha against Alzheimer's disease.J Alzheimers Dis 2015;43:1137-48.
[19]Zhao L,Woody SK,Chhibber A.Estrogen receptor beta in Alzheimer's disease:from mechanisms to therapeutics.Ageing Res Rev 2015;24:178-90.
[20]Berchtold NC,Cribbs DH,Coleman PD,Rogers J,Head E,Kim R,et al.Gene expression changes in the course of normal brain aging are sexually dimorphic.Proc Natl Acad Sci U S A 2008;105:15605-10.
[21]Damoiseaux JS,Seeley WW,Zhou J,Shirer WR,Coppola G,Karydas A,et al.Gender modulates the APOE epsilon4 effect in healthy older adults:convergent evidence from functional brain connectivityandspinalfluidtaulevels.JNeurosci 2012;32:8254-62.
[22]Xu W,Yu JT,Tan MS,Tan L.Cognitive reserve and Alzheimer's disease.Mol Neurobiol 2015;51:187-208.
[23]Foubert-Samier A,Catheline G,Amieva H,Dilharreguy B,Helmer C,Allard M,et al.Education,occupation,leisure activities,and brain reserve:a population-based study.Neurobiol Aging 2012;33:423.e15-25.
[24]Guo LH,Alexopoulos P,Wagenpfeil S,Kurz A,Perneczky R. Alzheimer's disease neuroimaging I.Brain size and the compensation of Alzheimer's disease symptoms:a longitudinal cohort study.Alzheimers Dement 2013;9:580-6.
[25]Calderon-Garciduenas L,Franco-Lira M,D'Angiulli A,Rodriguez-Diaz J,Blaurock-Busch E,Busch Y,et al.Mexico City normal weight children exposed to high concentrations of ambient PM2.5 show high blood leptin and endothelin-1,vitamin D deficiency,and food reward hormone dysregulation versus low pollution controls.Relevance for obesity and Alzheimer disease. Environ Res 2015;140:579-92.
[26]Bhatt DP,Puig KL,Gorr MW,Wold LE,Combs CK.A pilot study to assess effects of long-term inhalation of airborne particulate matter on early Alzheimer-like changes in the mouse brain.PLoS One 2015;10:e0127102.
[27]Richardson JR,Roy A,Shalat SL,von Stein RT,Hossain MM,Buckley B,et al.Elevated serum pesticide levels and risk for Alzheimer disease.JAMA Neurol 2014;71:284-90.
[28]Zhou S,Zhou R,Zhong T,Li R,Tan J,Zhou H.Association of smoking and alcohol drinking with dementia risk among elderly men in China.Curr Alzheimer Res 2014;11:899-907.
[29]Scarmeas N,Luchsinger JA,Schupf N,Brickman AM,Cosentino S,Tang MX,et al.Physical activity,diet,and risk of Alzheimer disease.JAMA 2009;302:627-37.
[30]Ray KD,Mittelman MS.Music therapy:a nonpharmacological approach to the care of agitation and depressive symptoms for nursing home residents with dementia.Dementia(London)2015;9:155-73.
[31]Nelson L,Gard P,Tabet N.Hypertension and inflammation in Alzheimer's disease:close partners in disease development and progression!J Alzheimers Dis 2014;41:331-43.
[32]Rusanen M,Kivipelto M,Levalahti E,Laatikainen T,Tuomilehto J,Soininen H,et al.Heart diseases and long-term risk of dementia and Alzheimer's disease:a population-based CAIDE study.J Alzheimers Dis 2014;42:183-91.
[33]Bruce DG,Davis WA,Starkstein SE,Davis TM.Mid-life predictors of cognitive impairment and dementia in type 2 diabetes mellitus:the Fremantle Diabetes Study.J Alzheimers Dis 2014;42:S63-70.
[34]Kamer AR,Pirraglia E,Tsui W,Rusinek H,Vallabhajosula S,Mosconi L,et al.Periodontal disease associates with higher brainamyloidloadinnormalelderly.NeurobiolAging 2015;36:627-33.
[35]Maheshwari P,Eslick GD.Bacterial infection and Alzheimer's disease:a meta-analysis.J Alzheimers Dis 2015;43:957-66.
[36]Ju YE,Lucey BP,Holtzman DM.Sleep and Alzheimer disease pathology-abidirectionalrelationship.NatRevNeurol 2014;10:115-9.
[37]Xie L,Kang H,Xu Q,Chen MJ,Liao Y,Thiyagarajan M,et al. Sleep drives metabolite clearance from the adult brain.Science 2013;342:373-7.
[38]Lee H,Xie L,Yu M,Kang H,Feng T,Deane R,et al.The effect of body posture on brain glymphatic transport.J Neurosci 2015;35:11034-44.
[39]Genin E,Hannequin D,Wallon D,Sleegers K,Hiltunen M,Combarros O,et al.APOE and Alzheimer disease:a major gene with semi-dominant inheritance.Mol Psychiatry 2011;16:903-7.
[40]Lambert JC,Ibrahim-Verbaas CA,Harold D,Naj AC,Sims R,Bellenguez C,et al.Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease.Nat Genet 2013;45:1452-8.
[41]Escott-Price V,Sims R,Bannister C,Harold D,Vronskaya M,Majounie E,et al.Common polygenic variation enhances risk prediction for Alzheimer's disease.Brain 2015;138:3673-84.
[42]Guerreiro R,Wojtas A,Bras J,Carrasquillo M,Rogaeva E,Majounie E,et al.TREM2 variants in Alzheimer's disease.N Engl J Med 2012;368:117-27.
[43]Cruchaga C,Karch CM,Jin SC,Benitez BA,Cai Y,Guerreiro R,et al.Rare coding variants in the phospholipase D3 gene confer risk for Alzheimer's disease.Nature 2014;505:550-4.
[44]Liu E,Schmidt ME,Margolin R,Sperling R,Koeppe R,Mason NS,et al.Amyloid-beta 11C-PiB-PET imaging results from 2 randomizedbapineuzumabphase3ADtrials.Neurology 2015;85:692-700.
[45]Cook CN,Murray ME,Petrucelli L.Understanding biomarkers of neurodegeneration:novel approaches to detecting tau pathology.Nat Med 2015;21:219-20.
[46]Okamura N,Furumoto S,Fodero-Tavoletti MT,Mulligan RS,Harada R,Yates P,et al.Non-invasive assessment of Alzheimer's disease neurofibrillary pathology using 18F-THK5105 PET.Brain 2014;137:1762-71.
[47]Maier FC,Wehrl HF,Schmid AM,Mannheim JG,Wiehr S,Lerdkrai C,et al.Longitudinal PET-MRI reveals beta-amyloid deposition and rCBF dynamics and connects vascular amyloidosis to quantitative loss of perfusion.Nat Med 2014;20:1485-92.
[48]Ma L,Chen J,Wang R,Han Y,Zhang J,Dong W,et al.The level of Alzheimer-associated neuronal thread protein in urine may be an important biomarker of mild cognitive impairment.J Clin Neurosci 2015;22:649-52.
[49]Tsuruoka M,Hara J,Hirayama A,Sugimoto M,Soga T,Shankle WR,et al.Capillary electrophoresis-mass spectrometry-based metabolome analysis of serum and saliva from neurodegenerative dementia patients.Electrophoresis 2013;34:2865-72.
[50]Steenland K,Zhao L,Goldstein F,Cellar J,Lah J.Biomarkers for predicting cognitive decline in those with normal cognition.J Alzheimers Dis 2014;40:587-94.
[51]Bekris LM,Lutz F,Montine TJ,Yu CE,Tsuang D,Peskind ER,etal.MicroRNAinAlzheimer'sdisease:anexploratory study in brain,cerebrospinal fluid and plasma.Biomarkers 2013;18:455-66.
[52]Luo H,Han G,Wang J,Zeng F,Li Y,Shao S,et al.Common aging signature in the peripheral blood of vascular dementia and Alzheimer's disease.Mol Neurobiol 2016;53:3596-605.
[53]Kiddle SJ,Sattlecker M,Proitsi P,Simmons A,Westman E,BazenetC,etal.Candidatebloodproteomemarkersof Alzheimer's disease onset and progression:a systematic review and replication study.J Alzheimers Dis 2014;38:515-31.
[54]O'Bryant SE,Xiao G,Zhang F,Edwards M,German DC,Yin X,et al.Validation of a serum screen for Alzheimer's disease across assayplatforms,species,andtissues.JAlzheimersDis 2014;42:1325-35.
[55]Burnham SC,Faux NG,Wilson W,Laws SM,Ames D,Bedo J,et al.A blood-based predictor for neocortical Abeta burden in Alzheimer's disease:results from the AIBL study.Mol Psychiatry 2014;19:519-26.
[56]Nagele E,Han M,Demarshall C,Belinka B,Nagele R.Diagnosis of Alzheimer's disease based on disease-specific autoantibody profiles in human sera.PLoS One 2011;6:e23112.
[57]Laske C,Stellos K,Kempter I,Stransky E,Maetzler W,Fleming I,et al.Increased cerebrospinal fluid calpain activity and microparticle levels in Alzheimer's disease.Alzheimers Dement 2015;11:465-74.
[58]Mathur S,Glogowska A,McAvoy E,Righolt C,Rutherford J,Willing C,et al.Three-dimensional quantitative imaging of telomeres in buccal cells identifies mild,moderate,and severe Alzheimer's disease patients.J Alzheimers Dis 2014;39:35-48.
[59]Sproul AA,Jacob S,Pre D,Kim SH,Nestor MW,Navarro-Sobrino M,et al.Characterization and molecular profiling of PSEN1 familial Alzheimer's disease iPSC-derived neural progenitors.PLoS One 2014;9:e84547.
[60]Woodruff G,Young JE,Martinez FJ,Buen F,Gore A,Kinaga J,et al.The presenilin-1 DeltaE9 mutation results in reduced gamma-secretase activity,but not total loss of PS1 function,in isogenic human stem cells.Cell Rep 2013;5:974-85.
[61]Vazin T,Ball KA,Lu H,Park H,Ataeijannati Y,Head-Gordon T,et al.Efficient derivation of cortical glutamatergic neurons from human pluripotent stem cells:a model system to study neurotoxicityinAlzheimer'sdisease.NeurobiolDis 2014;62:62-72.
[62]Liu Q,Waltz S,Woodruff G,Ouyang J,Israel MA,Herrera C,et al.Effect of potent gamma-secretase modulator in human neurons derived from multiple presenilin 1-induced pluripotent stem cell mutant carriers.JAMA Neurol 2014;71:1481-9.
[63]Zhang D,Pekkanen-Mattila M,Shahsavani M,Falk A,Teixeira AI,Herland A.A 3D Alzheimer's disease culture model and the induction of P21-activated kinase mediated sensing in iPSC derived neurons.Biomaterials 2014;35:1420-8.
[64]SelkoeDJ.PreventingAlzheimer'sdisease.Science 2012;337:1488-92.
[65]Grande G,Vanacore N,Maggiore L,Cucumo V,Ghiretti R,Galimberti D,et al.Physical activity reduces the risk of dementia inmildcognitiveimpairmentsubjects:acohortstudy.J Alzheimers Dis 2014;39:833-9.
[66]Coelho FG,Vital TM,Stein AM,Arantes FJ,Rueda AV,Camarini R,et al.Acute aerobic exercise increases brain-derived neurotrophic factor levels in elderly with Alzheimer's disease.J Alzheimers Dis 2014;39:401-8.
[67]Wu E,Barnes DE,Ackerman SL,Lee J,Chesney M,Mehling WE.Preventing Loss of Independence through Exercise(PLIE):qualitative analysis of a clinical trial in older adults with dementia. Aging Ment Health 2015;19:353-62.
[68]Rowland NC,Sammartino F,Tomaszczyk JC,Lozano AM.Deep brain stimulation of the fornix:engaging therapeutic circuits and networks in Alzheimer disease.Neurosurgery 2016;63:1-5.
[69]Underwood E.Neuroscience.Can sound open the brain for therapies?Science 2015;347:1186-7.
[70]Parkin BL,Ekhtiari H,Walsh VF.Non-invasive Human brain stimulationincognitiveneuroscience:aprimer.Neuron 2015;87:932-45.
[71]Kuhn J,Hardenacke K,Lenartz D,Gruendler T,Ullsperger M,Bartsch C,et al.Deep brain stimulation of the nucleus basalis of MeynertinAlzheimer'sdementia.MolPsychiatry 2015;20:353-60.
[72]Chen R,Romero G,Christiansen MG,Mohr A,Anikeeva P. Wirelessmagnetothermaldeepbrainstimulation.Science 2015;347:1477-80.
[73]Marioni RE,van den Hout A,Valenzuela MJ,Brayne C,Matthews FE,Function MRCC,et al.Active cognitive lifestyle associates with cognitive recovery and a reduced risk of cognitive decline.J Alzheimers Dis 2012;28:223-30.
[74]Gan P,Xie Y,Duan W,Deng Q,Yu X.Rumination and loneliness independently predict six-month later depression symptoms among Chinese elderly in nursing homes.PLoS One 2015;10:e0137176.
[75]Cherbuin N,Kim S,Anstey KJ.Dementia risk estimates associated with measures of depression:a systematic review and meta-analysis.BMJ Open 2015;5:e008853.
[76]Gardener SL,Rainey-Smith SR,Barnes MB,Sohrabi HR,Weinborn M,Lim YY,et al.Dietary patterns and cognitive decline in an Australian study of ageing.Mol Psychiatry 2015;20:860-6.
[77]Singh B,Parsaik AK,Mielke MM,Erwin PJ,Knopman DS,Petersen RC,et al.Association of Mediterranean diet with mild cognitive impairment and Alzheimer's disease:a systematic review and meta-analysis.J Alzheimers Dis 2014;39:271-82.
[78]Shinto L,Quinn J,Montine T,Dodge HH,Woodward W,Baldauf-Wagner S,et al.A randomized placebo-controlled pilot trial of omega-3 fatty acids and alpha lipoic acid in Alzheimer's disease.J Alzheimers Dis 2014;38:111-20.
[79]Brickman AM,Khan UA,Provenzano FA,Yeung LK,Suzuki W,Schroeter H,et al.Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults.Nat Neurosci 2014;17:1798-803.
[80]Song G,Zhang Z,Wen L,Chen C,Shi Q,Zhang Y,et al. Selenomethionine ameliorates cognitive decline,reduces tau hyperphosphorylation,and reverses synaptic deficit in the triple transgenic mouse model of Alzheimer's disease.J Alzheimers Dis 2014;41:85-99.
[81]Brendel M,Jaworska A,Herms J,Trambauer J,Rotzer C,Gildehaus FJ,et al.Amyloid-PET predicts inhibition of de novo plaque formation upon chronic gamma-secretase modulator treatment.Mol Psychiatry 2015;20:1179-87.
[82]Kondo A,Shahpasand K,Mannix R,Qiu J,Moncaster J,Chen CH,et al.Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy.Nature 2015;523:431-6.
[83]Coric V,Salloway S,van Dyck CH,Dubois B,Andreasen N,Brody M,et al.Targeting prodromal Alzheimer disease with Avagacestat:arandomizedclinicaltrial.JAMANeurol 2015;72:1324-33.
[84]Iqbal K,Liu F,Gong CX.Tau and neurodegenerative disease:the story so far.Nat Rev Neurol 2016;12:15-27.
[85]Wang Y,Yang G,Gong J,Lu F,Diao Q,Sun J,et al.Ginseng for Alzheimer's disease:a systematic review and meta-analysis of randomizedcontrolledtrials.CurrTopMedChem 2016;16:529-36.
[86]Hugel HM.Brain food for Alzheimer-free ageing:focus on herbal medicines.Adv Exp Med Biol 2015;863:95-116.
[87]Bu XL,Jiao SS,Lian Y,Wang YJ.Perspectives on the tertiary prevention strategy for Alzheimer's disease.Curr Alzheimer Res 2016;13:307-16.
[88]Wang YJ.Alzheimer disease:lessons from immunotherapy for Alzheimer disease.Nat Rev Neurol 2014;10:188-9.
[89]Tur-Kaspa I,Jeelani R,Doraiswamy PM.Preimplantation genetic diagnosis for inherited neurological disorders.Nat Rev Neurol 2014;10:417-24.
[90]Garcia-Corzo L,Luna-Sanchez M,Doerrier C,Ortiz F,Escames G,Acuna-Castroviejo D,et al.Ubiquinol-10 ameliorates mitochondrial encephalopathy associated with CoQ deficiency.Biochim Biophys Acta 2014;1842:893-901.
[91]Wisniewski T,Goni F.Immunotherapeutic approaches for Alzheimer's disease.Neuron 2015;85:1162-76.
[92]Song F,Qian Y,Peng X,Han G,Wang J,Bai Z,et al. Perturbation of the transcriptome:implications of the innate immune system in Alzheimer's disease.Curr Opin Pharmacol 2015;26:47-53.
[93]Femminella GD,Edison P.Evaluation of neuroprotective effect of glucagon-like peptide 1 analogs using neuroimaging.Alzheimers Dement 2014;10:S55-61.
[94]Deardorff WJ,Grossberg GT.Targeting neuroinflammation in Alzheimer's disease:evidence for NSAIDs and novel therapeutics. Expert Rev Neurother 2016:1-16.
[95]Krstic D,Knuesel I.Deciphering the mechanism underlying lateonset Alzheimer disease.Nat Rev Neurol 2013;9:25-34.
[96]Tong LM,Djukic B,Arnold C,Gillespie AK,Yoon SY,Wang MM,et al.Inhibitory interneuron progenitor transplantation restores normal learning and memory in ApoE4 knock-in mice withoutorwithAbetaaccumulation.JNeurosci 2014;34:9506-15.
[97]Bali P,Lahiri DK,Banik A,Nehru B,Anand A.Potential for stem cells therapy in Alzheimer's disease:do neurotrophic factors play critical role?Curr Alzheimer Res 2016;13:1-13.
18 December 2015;revised 14 January 2016;accepted 31 January 2016
Available online 28 September 2016
Handled by Ge Gao
.
E-mail:leihx@big.ac.cn(Lei H).
杂志排行
Genomics,Proteomics & Bioinformatics的其它文章
- Construction and Analysis of Functional Networks in the Gut Microbiome of Type 2 Diabetes Patients
- Pharmacogenomics of Drug Metabolizing Enzymes and Transporters:Relevance to Precision Medicine
- Long Non-coding RNAs and Their Roles in Non-small-cell Lung Cancer
- Oxford Nanopore MinION Sequencing and Genome Assembly
- Precision Medicine:What Challenges Are We Facing?
- Cancer Precision Medicine in China
