Mineralocorticoid receptor gene-2G/C polymorphism in central serous chorioretinopathy and relation of polymorphism with plasma cortisol levels
2016-11-04AlperYaziciEsinSogutluSariBetulEserGozdeSahinMedineAlpdemirAdilKilicMuhammetKazimErolSitkiSametErmis
Alper Yazici, Esin Sogutlu Sari, Betul Eser, Gozde Sahin, Medine Alpdemir, Adil Kilic, Muhammet Kazim Erol , Sitki Samet Ermis
·Original article·
Mineralocorticoid receptor gene-2G/C polymorphism in central serous chorioretinopathy and relation of polymorphism with plasma cortisol levels
Alper Yazici1, Esin Sogutlu Sari1, Betul Eser2, Gozde Sahin1, Medine Alpdemir3, Adil Kilic1, Muhammet Kazim Erol4, Sitki Samet Ermis1
1Department of Ophthalmology, School of Medicine, Balikesir University, Balikesir 10150, Turkey2Department of Genetics, School of Medicine, Balikesir University, Balikesir 10150, Turkey3Department of Biochemistry, Bigadic State Hospital, Balikesir 10010, Turkey4Department of Ophthalmology, Antalya Training and Research Hospital, Antalya, Turkey
方法:选取60例中心性浆液性脉络膜视网膜病变患者和50例正常人作为研究对象。患者皆患有急性中心性浆液性脉络膜视网膜病变,即浆液性视网膜脱离和视网膜色素上皮脱离或功能障碍(排除其它可能导致渗出的疾病,比如脉络膜新生血管、炎症或浸润病变)。为避免皮质醇水平的昼夜变化,上午8时到10时之间采集外周血样,检测盐皮质激素受体基因多态性(rs2070951)和血浆皮质醇水平。
结果:CSCR组的基因型频率分布为G/C (46.6% ), G/G (26.7%) 和 C/C (26.7%)。两组间基因型分布无统计学差异 (P=0.96)。研究结果显示,CSCR组的血浆皮质醇水平为401.2±162.1 nmol/L ,对照组为296.8±130.1 nmol/L,两组间差异有统计学意义(P<0.01)。血浆皮质醇水平在G/C (345.0±137.0 nmol/L), G/G (369.2±165.3 nmol/L) 和 C/C (395.3±188.8 nmol/L) 基因型之间不存在差异(P=0.50)。
结论:盐皮质激素受体基因多态性与中心性浆液性脉络膜视网膜病变和血浆皮质醇水平无关。
•AIM:To evaluate the mineralocorticoid receptor (MR) gene - 2G/C single nucleotide polymorphism in central serous chorioretinopathy (CSCR), polymorphism and plasma cortisol level relationship.
•METHODS:Sixty CSCR patients and 50 controls were included in the study. Inclusion criteria for patients were acute manifestation of CSCR characterized by serous retinal detachment, RPE detachment or dysfunction without evidence of any other possible cause of fluid exudation, such as choroidal neovascularization, inflammation or infiltration. Peripheric blood sample was collected from the participants between 8 and 10 a.m. to avoid the diurnal changes of cortisol levels.MR(NR3C2) gene polymorphism (rs2070951) and plasma cortisol levels sere studied.
•RESULTS: The genotype frequencies in CSCR group were G/C (46.6% ), G/G (26.7%) and C/C (26.7%).There was no statistically significant difference in terms of genotype distribution among groups (P=0.96). The plasma cortisol levels were also studied and the results were 401.2±162.1 nmol/L in the CSCR group and 296.8±130.1 nmol/L in the control group and the difference was statistically significant (P<0.01). The plasma cortisol levels also did not differ between G/C (345.0±137.0 nmol/L), G/G (369.2±165.3 nmol/L) and C/C (395.3±188.8 nmol/L) genotypes (P=0.50).
•CONCLUSION: TheMR(NR3C2) gene polymorphism is not associated with CSCR and the plasma cortisol levels.
mineralocorticoid; macula; central serous chorioretinopathy; polymorphism; genetics
INTRODUCTION
Central serous chorioretinopathy (CSCR) is a disorder characterized by the detachment of the neurosensory retina due to the accumulation of the fluid in the subretinal space[1]. Generally, it is believed to be due to a focal leak from retina pigment epithelium (RPE)[2]. Although the etiology is unclear glucocorticoids, pregnancy, hypertension, type A personality are some of the mostly recognized risk factors. Recent findings suggest that the pathology is the dysregulation or focal thrombosis of the choroidal blood flow, which results in ischemia, vasodilation, and hyperpermeability of the choroidal vessels. As a result, choroid gets thicker and the increased hydrostatic pressure overwhelms the barrier function of the RPE and this result with the leak of fluid from the choroid to the subretinal space[3].
Exogenous or endogenous steroids are believed to have a critical role in the development of CSCR. They show their affect thru glucocorticoid receptor (GR) and mineralocorticoid receptor (MR).MRhas similar high affinities to both mineralocorticoid and glucocorticoid and is typically expressed in kidney cells, hypothalamus-pituitary-adrenal axis and vascular system[4-5]. Zhaoetal[4]have found thatMRwas also present in neuroretina, muller glial cells and choroid. They showed thatMRactivation in rats resulted in choriocapillary vasodilation and focal leakage which in turn resulted in choroidal thickening. In clinical practice, the studies performed withMRantagonists revealed promising results in CSCR patients that further increased the interest in these receptors[4,6]. Studies performed withMRalso revealed that they are involved in stress coping, behavioral adaptation and modulating brain plasticity[7]. From this point on numerous studies were performed to evaluate the polymorphism of theMRgene in neuro-psychiatric disorders involving depression, attention deficit hyperactivity disorder, bipolar disease and schizophrenia[7-9]. Type A personality which can be accepted as stressful condition is also frequently seen in CSCR patients[10]and associated with higher levels of plasma cortisol and catecholamine levels[11]probably due to the difference in the perception of stress. Therefore, due to both possible involvement ofMRin type A personality and established role ofMRantagonists in the treatment of CSCR, we evaluated single nucleotide polymorphisms (SNPs) that could be associated with CSCR. In this particular study, we investigated theMR(NR3C2) gene polymorphism (rs2070951) in CSCR patients.
SUBJECTS AND METHODS
Patients who were diagnosed as CSCR in a tertiary referral hospital between Jan. 2013 and Aug. 2013 were enrolled. The samples were previously studied for 4G/5G polymorphism of plasminogen activator inhibitor (PAI-1) gene[12]. Institutional Review Board and ethics committee approval was obtained and informed consent were gathered (Ethics committee of Balikesir University School of Medicine, 2013/56). Sixty CSCR patients and 50 healthy individuals were included in the study. Body mass index(BMI) was calculated according to the World Health Organisation guidelines[13]. Inclusion criteria for patients were acute manifestation of CSCR (first attack or acute attack of recurrent disease) characterized by serous retinal detachment, RPE detachment or dysfunction without evidence of any other possible cause of fluid exudation, such as choroidal neovascularization, inflammation or infiltration. Detailed ophthalmic examination including best-corrected visual acuity, slit-lamp biomicroscopy, and dilated fundus examination was performed to all patients. CSCR diagnosis was confirmed by fluorescein angiography and neurosensory or RPE detachment was also documented with optical coherence tomography imaging (Cirrus HD-OCT Model 4000, Carl Zeiss Meditec Inc., Dublin, CA, USA).
Subjects with concurrent ocular or retinal disease, history of coagulation abnormalities such as thromboembolism, pregnancy, congestive heart failure, diabetes mellitus, coronary artery disease, uncontrolled arterial hypertension, smoking, hyperlipidemia, cancer, autoimmune inflammatory diseases, renal and hepatic abnormalities, endocrine pathology, and concomitant treatment affecting fibrinolysis metabolism (such as glucocorticoids and oral contraceptives), drug and/or alcohol intake were excluded from the study. Peripheric blood sample were collected from the patients and controls between 8 and 10 a.m. to avoid the diurnal changes of cortisol levels.
2G/C Polymorphism GenotypingGenomic DNA was extracted from a 200 μL peripheral venous blood sample according to the standard protocol by using GeneJet DNA Purification Kit (Thermo Fisher Scientific Inc., USA). DNA samples were stored at -20℃ until use. Light Cycler Nano®(Roche Diagnostics GmbH, Mannheim Germany) were used for analysing the - 2G/C polymorphism in theMR(NR3C2) gene. This polymorphism is amplified with specific primers and detected with hydrolysis probes (TIB MOLBIOL GmbH, Berlin, Germany). Single-nucleotide polymorphism amplification assays were performed according to the manufacturer’s instructions. Cycling conditions for amplification of theMR(NR3C2) gene were initial denaturation at 95℃ for 10 min, followed by 45 cycles with at 95℃ for 10 s, at 40 ℃ for 10 s and at 72℃ for 15 s.
Biochemical AnalysisSerum cortisol level was measured with the electrochemiluminescence immunoassay method by cobas e 411 immunoassay analysers. (Roche Diagnostics GmbH, Mannheim Germany, Kit Measuring Range 1-1750 nmol/L) The assays were performed according to the manufacturer’s recommendations. Serum samples were obtained from the patients within one week after the diagnosis was confirmed with fluorescein angiography and optical coherence tomography. Peripheral venous blood was collected in the morning and was centrifuged at 1500g for 15min at 4℃. After centrifugation, serum was separated from the sediment and stored frozen (-80℃) until use.
Data AnalysesQuantitative data was described as mean±standard deviation (SD) and qualitative data was described as percentage. SPSS for Windows software (version 11.0, SPSS, Inc., USA) was used for statistical analysis. Statistical analyses of the differences in gender and genotype frequencies between groups were analysed with Chi-square test. Normality of the quantitative data sets was assessed by the Shapiro-Wilk test. Student’st-test for parametric analysis and Mann - Whitney U test for nonparametric analysis was used for searching intergroup difference. One-way ANOVA test was used for comparison of plasma cortisol levels among 3 different polymorphism types.
RESULTS
There were not statistically significant between the groups on age, gender and the BMI . The demographic characteristics of the groups were presented in Table 1. The genotype frequencies in CSCR group were G/C (46.6%), G/G (26.7%) and C/C (26.7%). The frequencies in the control group were G/C (44.0%), G/G (28.0%) and C/C (28.0%). There was no statistically significant difference in terms of genotype distribution among groups (P=0.96). The allele frequencies were 52% for G and 48% for C in the CSCR group and 49% for G and 51% for C allele in the control group (P>0.05).
The plasma cortisol levels were also studied and the results were 401.2±162.1 nmol/L in the CSCR group and 296.8±130.1 nmol/L in the control group and the difference was statistically significant (P<0.01). The plasma cortisol levels also did not differ between G/C (345.0±137.0 nmol/L), G/G (369.2±165.3 nmol/L) and C/C (395.3±188.8nmol/L) genotypes (P=0.50).
DISCUSSION
CSCR is the 4thmost common retinopathy after age related macular degeneration, diabetic retinopathy, and branch retinal vein occlusion[2]. To better understand the disease, many studies were conducted for epidemiology, pathophysiology, systemic associations, risk factors and treatment options[2,4, 13-16]. The possible genetic contribution in the disease development was evaluated with some familial CSCR reports in the literature[17-20]. Additionally the racial predilection to white, Hispanic and Asian populations also supports a genetic tendency. However, the genetic background of the disease was not exclusively studied and the literature search reveals limited number of studies mostly of familial involvement basis[12,21-22]. In a recent review, this issue is also addressed and the need for further studies investigating SNPs was expressed[3]. Studies of SNPs might help identifying the individuals at risk and be useful to predict the ones to progress to chronic form of the disease.
Table 1Characteristics of groups
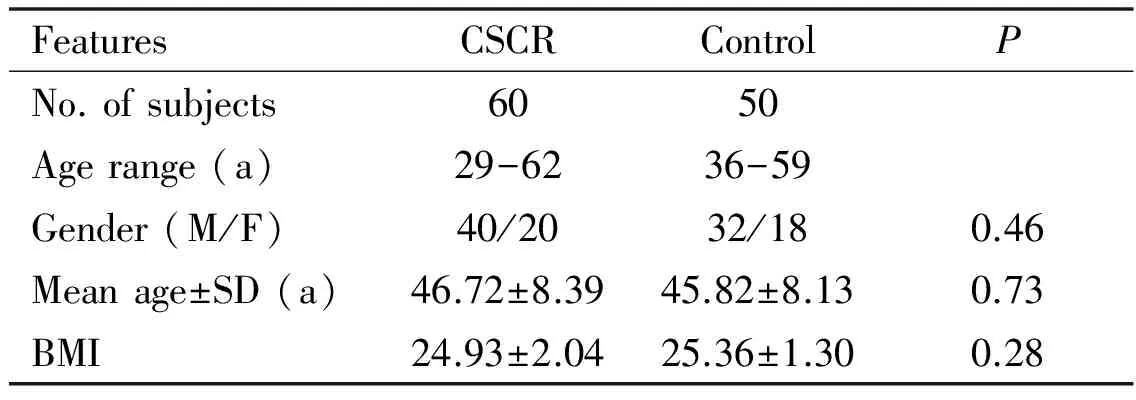
FeaturesCSCRControlPNo.ofsubjects6050Agerange(a)29-6236-59Gender(M/F)40/2032/180.46Meanage±SD(a)46.72±8.3945.82±8.130.73BMI24.93±2.0425.36±1.300.28
The results of the current study revealed that there is no statistically significant difference between CSCR patients and healthy controls in terms of theMR- 2G/C polymorphism. The rates were very similar and nearly every 1 of 2 cases had-2G/C polymorphism in both CSCR and controls. The allelic frequency also did not differ between the groups.MRis critical in hypothalamic-pituitary-adrenal(HPA) axis and response to clinical stress which we believe might cause increased cortisol levels in type A personality CSCR patients. As expected, we found that plasma cortisol levels were significantly higher in the CSCR patients. However, the cortisol levels were not significantly different among G/G, G/C and C/C polymorphisms. Contrary to our findings, previous studies have found a relationship between polymorphisms and plasma cortisol levels. Muhtzetal[23]investigated howMRgene variants affected basal cortisol secretion in 133 healthy adults and have found thatMR- 2G/G gene polymorphism was associated with higher plasma cortisol levels in healthy adults. They emphasized that this polymorphism might have a role in interindividual variability in stress responsiveness and be involved in stress related disorder. Another study conducted on 166 school teacher, a stressful occupation, has found that subjects having C/C polymorphism has highest plasma cortisol levels[5]. Apart from chronic perceived stress, this study also performed acute experimental psychosocial stress test and found that individuals with C/C polymorphism showed higher salivary cortisol, plasma cortisol, adrenocorticotropic hormone(ACTH) levels and heart rate responses. Two different studies conducted on the effect of theMRgene polymorphism on cortisol levels found higher cortisol levels with homozygote allele carriers but one found C/C and the other found G/G to be related. In our study we failed to find any association of theMR-2G/C polymorphisms with plasma cortisol levels. Therefore, the hypothesis that type A personality, one of the earliest risk factor for developing CSCR with higher levels of cortisol ( x 40) compared to type B personality, andMRgene might be related andMRgene polymorphism might have a role in the development of CSCR has failed.
The literature search has resulted with only 3 studies for SNPs in CSCR which were published recently. One of these studies belonged to us in which we investigated plasminogen activator inhibitor (PAI-1) gene 4G/5G polymorphism in the same patient group. The study has failed to find any difference between CSCR and controls in terms ofPAI-1 gene 4G/5G polymorphism. The second study was related with cadherin 5 (CDH5) protein which stands as the major cell to cell adhesion molecules in the vascular endothelium[21]. The study confirmed that CDH5 protein was down-regulated with steroids and also found significant association of four common CDH5 SNPs with CSCR in male patients. They proposed that genetic variation in CDH5 protein might create a tendency for CSCR with triggering events such as hypercortisolism. The third study investigated the complement H protein that binds adrenomedullin, a strong vasodilator in the choroidal vasculature[22]. They found an association between CSCR and common complement H SNPs and imply that some variants in this protein may act as susceptibility elements for CSCR development. Both CDH5 and complement H studies were performed with higher number of patients and controls (400 and 140 CSCR patients and 1400 and 934 controls respectively). The small sample size in our study is the major limitation and might have an impact on our results.
As a conclusion, we did not find any difference in genotypes ofMRgene (rs2070951) polymorphism between CSCR patients and healthy controls. Our current study is first to examine the association between theMRgene polymorphisms and CSCR in Turkish population. Similar studies with larger sample size are needed to reveal the genetic predispositions in CSCR.
1 Kitaya N, Nagaoka T, Hikichi T, Sugawara R, Fukui K, Ishiko S, Yoshida A. Features of abnormal choroidal circulation in central serous chorioretinopathy.BrJOphthalmol2003;87(6):709-712
2 Wang M, Munch IC, Hasler PW, Prünte C, Larsen M. Central serous chorioretinopathy.ActaOphthalmol2008;86(2):126-145
3 Nicholson B, Noble J, Forooghian F, Meyerle C. Central serous chorioretinopathy: update on pathophysiology and treatment.SurvOphthalmol2013;58(2):103-126
4 Zhao M, Célérier I, Bousquet E, Jeanny JC, Jonet L, Savoldelli M, Offret O, Curan A, Farman N, Jaisser F, Behar-Cohen F. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy.JClinInvest2012;122(7):2672-2679
5 van Leeuwen N, Bellingrath S, de Kloet ER, Zitman FG, DeRijk RH, Kudielka BM, Wüst S. Human mineralocorticoid receptor (MR) gene haplotypes modulateMRexpression and transactivation: implication for the stress response.Psychoneuroendocrinology2011;36(5):699-709
6 Bousquet E, Beydoun T, Zhao M, Hassan L, Offret O, Behar-Cohen F. Mineralocorticoid receptor antagonism in the treatment of chronic central serous chorioretinopathy: a pilot study.Retina(Philadelphia,Pa) 2013;33(10):2096-2102
8 Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments.TrendsNeurosci2008;31(9):464-468
9 Spijker AT, Giltay EJ, van Rossum EF, Manenschijn L, DeRijk RH, Haffmans J, Zitman FG, Hoencamp E. Glucocorticoid and mineralocorticoid receptor polymorphisms and clinical characteristics in bipolar disorder patients.Psychoneuroendocrinology2011;36(10):1460-1469
10 Yannuzzi LA. Type A behavior and central serous chorioretinopathy.TransAmOphthalmolSoc1986;84:799-845
11 Ross A, Ross AH, Mohamed Q. Review and update of central serous chorioretinopathy.CurrOpinOphthalmol2011;22(3):166-173
12 Sogutlu Sari E, Yazici A, Eser B, Erol MK, Kilic A, Ermis SS, Koytak A, Akit H, Yakut T. The prevalence of 4G/5G polymorphism of plasminogen activator inhibitor-1 (PAI-1) gene in central serous chorioretinopathy and its association with plasmaPAI-1 levels.CutanOculToxicol2014;33(4):270-274
13 WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies.Lancet2004;363(9403):157-163
14 Caccavale A, Romanazzi F, Imparato M, Negri A, Morano A, Ferentini F. Central serous chorioretinopathy: a pathogenetic model.ClinOphthalmol2011;5:239-243
15 Alkin Z, Ozkaya A, Agca A, Yazici AT, Demirok A. Early visual and morphologic changes after half-fluence photodynamic therapy in chronic central serous chorioretinopathy.JOculPharmacolTher2014;30(4):359-365
16 Erol MK, Özdemir Ö, Çoban DT, Karacor A, Bulut M, Sari ES. Akut ve Kronik Santral Seröz Koryoretinopatide Fundus Otofloresansi.TurkJOphthalmol2013;43:94-98
17 Haik GM, Perez LF, Murtagh JJ. Central serous retinopathy. Consecutive development in daughter and mother.AmJOphthalmol1968;65(4):612-615
18 Lin E, Arrigg PG, Kim RY. Familial central serous choroidopathy.GraefesArchClinExpOphthalmol2000;238(11):930-931
19 Park DW, Schatz H, Gaffney MM, McDonald HR, Johnson RN, Schaeffer D. Central serous chorioretinopathy in two families.EurJOphthalmol1998;8(1):42-47
20 Weenink AC, Borsje RA, Oosterhuis JA. Familial chronic central serous chorioretinopathy.Ophthalmologica2001;215(3):183-187
21 Schubert C, Pryds A, Zeng S, Xie Y, Freund KB, Spaide RF, Merriam JC, Barbazetto I, Slakter JS, Chang S, Munch IC, Drack AV, Hernandez J, Yzer S, Merriam JE, Linneberg A, Larsen M, Yannuzzi LA, Mullins RF, Allikmets R. Cadherin 5 is regulated by corticosteroids and associated with central serous chorioretinopathy.HumMutat2014;35(7):859-867
22 Miki A, Kondo N, Yanagisawa S, Bessho H, Honda S, Negi A. Common variants in the complement factor H gene confer genetic susceptibility to central serous chorioretinopathy.Ophthalmology2014;121(5):1067-1072
23 Muhtz C, Zyriax BC, Bondy B, Windler E, Otte C. Association of a common mineralocorticoid receptor gene polymorphism with salivary cortisol in healthy adults.Psychoneuroendocrinology2011;36(2):298-301
CSCR中盐皮质激素受体基因-2G/C的多态性及其与血浆皮质醇水平的关系
Alper Yazici1, Esin Sogutlu Sari1, Betul Eser2, Gozde Sahin1, Medine Alpdemir3, Adil Kilic1, Muhammet Kazim Erol4, Sitki Samet Ermis1
(作者单位:110150土耳其巴勒凯西尔巴勒凯西尔大学医学院眼科;210150土耳其巴勒凯西尔巴勒凯西尔大学医学院遗传学系;310010土耳其巴勒凯西尔比格达奇州医院生物化学系;4土耳其安塔利亚安塔利亚培训与研究医院眼科)
Alper Yazici. lpryzc@yahoo.com
目的:评估中心性浆液性脉络膜视网膜(CSCR)病变中盐皮质激素受体基因-2G/C单核苷酸多态性,以及基因多态性和血浆皮质醇水平的关系。
盐皮质激素;黄斑;中心性浆液性脉络膜视网膜病变;多态性;遗传学
Alper Yazici. School of Medicine, Cagis Campus, Balikesir University, Balikesir 10150, Turkey. lpryzc@yahoo.com
2015-04-09Accepted: 2016-04-19
10.3980/j.issn.1672-5123.2016.7.01
Yazici A, Sari ES, Eser B, Sahin G, Alpdemir M, Kilic A, Erol MK, Ermis SS. Mineralocorticoid receptor gene-2G/C polymorphism in central serous chorioretinopathy and relation of polymorphism with plasma cortisol levels.GuojiYankeZazhi(IntEyeSci) 2016;16(7):1203-1206
引用:Yazici A, Sari ES, Eser B, Sahin G, Alpdemir M, Kilic A, Erol MK, Ermis SS. CSCR中盐皮质激素受体基因-2G/C的多态性及其与血浆皮质醇水平的关系.国际眼科杂志2016;16(7):1203-1206
