Yb(OTf)3促进的螺环吲哚类衍生物合成研究
2016-10-24王梦佳徐慧婷谢佳慧吴小雅曲喜胜吴春雷
王梦佳 徐慧婷 陈 飞 谢佳慧 吴小雅 曲喜胜 吴春雷
(绍兴文理学院 化学化工学院,浙江 绍兴312000)
Yb(OTf)3促进的螺环吲哚类衍生物合成研究
王梦佳徐慧婷陈飞谢佳慧吴小雅曲喜胜吴春雷
(绍兴文理学院化学化工学院,浙江绍兴312000)
以三氟甲磺酸镱(Yb(OTf)3)为催化剂,PEG-400为溶剂,靛红、β-环己二酮与丙二腈(或氰基乙酸乙酯)三组分于60℃反应30 min合成一系列螺环吲哚类衍生物,收率为87%~95%,其结构经1H NMR和13C NMR确证.Yb(OTf)3/ PEG-400可回收套用五次.
靛红;螺环吲哚;PEG-400;三氟甲磺酸镱
吲哚是一类很重要的杂环化合物,也是组成很多药物和天然化合物的核心结构,而在吲哚的三号位形成螺环后能增强整个结构的生物活性,如spirotryprostatins A、恩卡林碱和异恩卡林碱都表现出很好的调节毒蕈碱样5-羟色胺受体的功能.[1]由于此类物质极高的生物活性,因此有关它的合成也是研究的一大热点,尤其是吲哚的三号位连接苯并吡喃所构成的螺环吲哚类衍生物,其中NaCl(超声),[2]HAuCl4·3H2O,[3]硬脂酸钠,[4]InCl3,[5]NH4Cl,[6]三乙基苄铵,[7]L-脯氨酸[8]等物质都已被用作催化剂来促进此类物质的合成,但绿色、高效的合成方法仍有待进一步开发.
三氟甲磺酸金属盐是一类高效的Lewis酸催化剂,已被广泛应用于各类有机反应中.[9-11]近年来,聚乙二醇(PEG)由于具有无毒、溶解能力好、低蒸气压、易回收、易处理、环境友好和原料易得等优点而成为很多有机反应的绿色替代溶剂.[12-14]本文以三氟甲磺酸镱为催化剂,PEG-400为溶剂,靛红、环己二酮和丙二腈(或氰基乙酸乙酯)三组份一锅法合成螺环吲哚类衍生物(4a~4n,Scheme 1),其结构经1H NMR和13C NMR确证.
该方法具有反应速度快、收率高和环境友好的特点,符合“绿色化学”的要求.

Scheme 1
1 实验部分
1.1主要仪器与试剂
瑞士Buchi M-560型熔点仪(温度计未经校正);Avance DMX Ⅲ 400 M 核磁共振仪(CDCl3为溶剂,TMS为内标,瑞士Bruker公司);反应进程用TLC跟踪检测.
所用试剂均为化学纯或分析纯.
1.2化合物4的合成通法
在圆底烧瓶中,加入5 mL PEG-400,再将靛红(10 mmol)、β-环己二酮(10 mmol)和丙二腈(10 mmol)加入,开启搅拌,加入催化剂Yb(OTf)3,60℃下反应(TLC跟踪).反应结束后冷却至室温,过滤,滤饼用50%乙醇水溶液洗涤,滤液于80℃烘干至恒重回收PEG-400/Yb(OTf)3(套用).
4a.1H NMR δ: 1.00 (s, 3H, CH3), 1.03 (s, 3H, CH3), 2.09 (d,J= 16.0 Hz, 1H, CHAHB), 2.13 (d,J= 16.0 Hz, 1H, CHAHB), 2.56 (s, 2H, CH2), 6.78 (d,J= 7.6 Hz, 1H, ArH), 6.88 (t,J= 7.4 Hz, 1H, ArH), 6.98 (d,J= 6.8 Hz, 1H, ArH), 7.14 (t,J= 8.2 Hz, 1H, ArH), 7.12 (s, 2H, NH2), 10.39 (s, 1H, NH);13C NMR δ: 27.5, 28.1, 32.4, 47.3, 50.4, 57.9, 109.7, 111.2, 117.8, 122.1, 123.5, 128.6, 134.9, 142.5, 159.2, 164.6, 178.5, 195.3.
4a~4n的实验结果见表1,4b~4n的表征数据与Scheme l预期吻合.
表14a~4n的实验结果

Product外 观m.p.(lit)/℃收率a(%)4a白色固体290-292(290-292)5914b白色固体280-281(278-280)5924c白色固体279-280(278-280)5904d白色固体284-286(282-284)5894e白色固体293-295(294-296)5954f白色固体292-293(292-294)5914g白色固体290-291(291-293)7924h白色固体278-280934i黄色固体279-281874j白色固体296-297934k白色固体289-290914l白色固体292-293(291-294)20954m白色固体>300914n白色固体250-253(251-254)988
反应条件同1.2
2 结果与讨论
2.1反应条件筛选
首先,以靛红、丙二腈、1,3-环己二酮三组份合成4a为例,考察了不同催化剂、催化剂用量和不同的反应温度对收率的影响(见表2).
表2反应条件的优化

EntryCatalystMol%TempTime(min)Yielda%1--60180Trace2FeCl3.6H2O1060120473ZnCl21060120534MgBr21060120515Zn(OTf)2106030786Cu(OTf)2106030857Yb(OTf)356030838Yb(OTf)3106030919Yb(OTf)31560309010Yb(OTf)310r.t1208511Yb(OTf)310803089
由表2可知,不加催化剂时,只能检测到微量的产物生成,而反应在FeCl3.6H2O、ZnCl2和MgBr2的作用下能进行,但即使延长反应时间至120 min,收率仍然偏低,Zn(OTf)2、Cu(OTf)2和Yb(OTf)3等三氟甲磺酸盐作催化剂时,反应基本在30 min内就能快速完成,并且收率良好,尤其是Yb(OTf)3较另外两种盐催化效果更好,因此选用Yb(OTf)3作为本反应的催化剂.其次,催化剂用量也对反应有着很大影响,由表2的Entry 7-9可以看出,Yb(OTf)3的用量为10 mol%较为合适.最后,实验过程中还显示温度对该反应的影响也较大,反应在60℃下进行较合适,反应温度过低需要延长反应时间(Entry 10),过高则反应液颜色过深(Entry 11),副产物较多.
在上述优化条件下,以不同的取代靛红、环己二酮和丙二腈(氰基乙酸乙酯)为底物合成了一系列螺环吲哚类衍生物,反应在30min内完成,且收率良好.靛红上的不同取代基对反应基本没有影响,1,3-环己二酮上五位的二甲基取代以及丙二腈和氰基乙酸乙酯的变换也都能使反应顺利进行.
2.2催化剂/溶剂体系回收套用
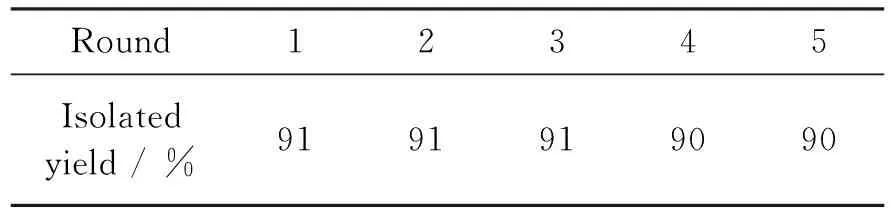
以合成4a为例,考察Yb(OTf)3/PEG-400的回收套用情况,结果见表3.从表3可见,Yb(OTf)3/PEG-400体系回收套用四次,其催化活性未见明显降低.
表3Yb(OTf)3/PEG-400体系的回收使用结果
Round12345Isolatedyield/%9191919090
*Yb(OTf)310 mol%(以1计算),于60℃反应30 min,其余反应条件同1.2.
3 结论
以三氟甲磺酸镱为催化剂,PEG-400为溶剂,靛红,β-环己二酮与丙二腈(或氰基乙酸乙酯)三组分于60℃反应30 min合成了一系列螺环吲哚类衍生物,收率为87%~95%,其结构经1H NMR和13C NMR确证.Yb(OTf)3/ PEG-400可回收重复使用,收率几乎不变.
[1]KANG T H, MATSUMOTO K, TOHDA M, et al. Pteropodine and isopteropodine positively modulate the function of rat muscarinic M1and 5-HT2receptors expressed in Xenopus oocyte.[J]. European Journal of Pharmacology, 2002, 444(1-2):39-45.
[2] ANSHU, ANUJ K J, DHARMENDRA. NaCl as a Novel and Green Catalyst for the Synthesis of Biodynamic Spiro Heterocycles in Water Under Sonication[J]. Synthetic Communications, 2011, 41(19):2905-2919.
[3] KIDWAI M, JAHAN A, MISHRA N K. Gold(III) chloride (HAuCl4.3H2O) in PEG: A new and efficient catalytic system for the synthesis of functionalized spirochromenes[J]. Applied Catalysis A General, 2012, s 425-426:35-43.
[4] WANG L M, JIAO N, QIU J, et al. Sodium stearate-catalyzed multicomponent reactions for efficient synthesis of spirooxindoles in aqueous micellar media [J]. Tetrahedron, 2010, 66: 339-343.
[5] SHANTHI G, SUBBULAKSHMI G, PERUMAL P T. A New InCl 3 -Catalyzed, Facile and Efficient Method for the Synthesis of Spirooxindoles under Conventional and Solvent-Free Microwave Conditions.[J]. Cheminform, 2007, 63(9):2057-2063.
[6] DABIRI M, BAHRAMNEJAD M, BAGHBANZADEH M. Ammonium salt catalyzed multicomponent transformation: simple route to functionalized spirochromenes and spiroacridines[J]. Cheminform, 2009, 65(45):9443-9447.
[7] ZHU S L, JI S J, ZHANG Y. A Simple and Clean Procedure for Three-Component Synthesis of Spirooxindoles in Aqueous Medium.[J]. Cheminform, 2007, 38(51):9365-9372.
[8] LI Y, HUI C, SHI C, et al. Efficient One-Pot Synthesis of Spirooxindole Derivatives Catalyzed by l-Proline in Aqueous Medium[J]. Journal of Combinatorial Chemistry, 2010, 12(2):231-237.
[9] ARASAMPATTU S N, BOREDDY S R R Efficient One-Pot Synthesis of Substituted Lactams via Three-Component Solvent-Free Reaction Catalyzed by Ytterbium Triflate[J]. Synthetic Communications, 2013, 43(9):1229-1236.
[10]HEO Y, CHO D H, MISHRA M K, et al. Erratum: Efficient one-pot synthesis of α-aminophosphonates from aldehydes and ketones catalyzed by ytterbium(III)triflate [J]. Tetrahedron Lett, 2013, 54(14):1899-1899.
[11] ADRIANO M, SALVATORE G, FRANCESCO P,et al. Ytterbium triflate catalysed Meerwein-Ponndorf-Verley (MPV) reduction[J]. Tetrahedron Letters, 2012, 53(7):890-892.
[12]曹瑞伟,陈朝辉,吴春雷,等.CAN/PEG-400体系催化合成氧杂蒽二酮类衍生物[J].合成化学,2012,20(4):511-514.
[13]RAGHU M, RAJASEKHAR M, REDDY B C O, et al. ChemInform Abstract: Polyethylene Glycol (PEG-400): A Mild and Efficient Reaction Medium for One-Pot Synthesis of 3-Hydroxy-3-(pyridin-2-ylmethyl)indolin-2-ones.[J]. Tetrahedron Letters, 2013, 54(27):3503-3506.
[14] FIROUZABADI H, IRANPOOR N, KAZEMI F, et al. Palladium nano-particles supported on agarose as efficient catalyst and bioorganic ligand for C C bond formation via solventless Mizoroki-Heck reaction and Sonogashira-Hagihara reaction in polyethylene glycol (PEG 400)[J]. Journal of Molecular Catalysis A Chemical, 2012, 357:154-161.
附录:氢谱 4b:1H NMR δ: 0.79 (t,J= 6.8 Hz, 3H, CH3), 0.94 (s, 3H, CH3), 1.01 (s, 3H, CH3), 2.00 (d,J= 16.0 Hz, 1H, CHAHB), 2.14 (d,J= 16.0 Hz, 1H, CHAHB), 2.52 (m, 2H, CH2), 3.69 (q,J= 6.8 Hz, 2H, CH2), 6.66 (d,J= 7.6 Hz, 1H, ArH), 6.75 (t,J= 7.2 Hz, 1H, ArH), 6.82 (d,J= 7.2 Hz, 1H, ArH), 7.03 (t,J= 7.2 Hz, 1H, ArH), 7.84 (s, 2H, NH2), 10.12 (s, 1H, NH);13C NMR δ: 13.6, 27.1, 28.2, 32.0, 47.1, 51.1, 59.3, 76.8, 108.6, 113.6, 121.0, 122.7, 127.6, 136.4, 144.5, 159.6, 162.8, 168.1, 180.2, 195.1. 4c:1H NMR δ: 1.00 (s, 3H, CH3), 1.02 (s, 3H, CH3), 2.12 (m, 2H, CH2), 2.19 (s, 3H, Ar-CH3), 2.55 (m, 2H, CH2), 6.67 (d,J= 7.2 Hz, 1H, ArH), 6.78 (s, 1H, ArH), 6.93 (d,J= 8.4 Hz, 1H, ArH), 7.19 (s, 2H, NH2), 10.27 (s, 1H, NH).13C NMR δ: 21.1, 27.7, 27.9, 32.4, 47.3, 50.5, 58.2, 109.4, 111.3, 117.8, 124.1, 128.9, 130.9, 135.0, 140.1, 159.2, 164.5, 178.4, 195.3. 4d.1H NMR δ: 0.82 (t,J= 7.2 Hz, 3H, CH3), 0.96 (s, 3H, CH3), 1.01 (s, 3H, CH3), 2.03 (d,J= 15.6 Hz, 1H, CHAHB), 2.13 (d,J= 15.6 Hz, 1H, CHAHB), 2.15 (s, 3H, CH3), 2.48-2.54 (m, 2H, CH2), 3.70 (q,J= 7.2 Hz, 2H, CH2), 6.55 (d,J= 8.0 Hz, 1H, ArH), 6.64 (s, 1H, ArH), 6.84 (d,J= 7.6 Hz, 1H, ArH), 7.84 (s, 2H, NH2), 10.02 (s, 1H, NH).13C NMR δ: 13.5, 21.1, 27.3, 28.1, 32.0, 47.1, 51.1, 59.3, 76.9, 108.3, 113.6, 123.4, 127.9, 129.5, 136.5, 142.1, 159.5, 162.7, 168.2, 180.2, 195.1. 4e.1H NMR δ: 1.00 (s, 3H, CH3), 1.01 (s, 3H, CH3), 2.14 (s, 2H, CH2), 2.49-2.56 (m, 2H, CH2), 6.78 (d,J= 8.0 Hz, 1H, ArH), 7.08 (s, 1H, ArH), 7.17 (d,J= 5.6 Hz,1H, ArH), 7.29 (s, 2H, NH2), 10.51 (s, 1H, NH).13C NMR δ: 27.7, 27.9, 32.4, 47.5, 50.4, 57.1, 110.6, 111.1, 117.7, 123.7, 126.1, 128.5, 136.9, 141.5, 159.3, 165.1, 178.3, 195.6. 4f.1H NMR δ: 0.82 (t,J= 6.2 Hz, 3H, CH3), 0.95 (s, 3H, CH3), 0.98 (s, 3H, CH3), 2.07-2.13 (m, 2H, CH2), 2.48-2.55 (m, 2H, CH2), 3.70 (q,J= 6.0 Hz, 2H, CH2), 6.66 (d,J= 8.8 Hz, 1H, ArH), 6.88 (s, 1H, ArH), 7.10 (d,J= 8.0 Hz, 1H, ArH), 7.91 (s, 2H, NH2), 10.29 (s, 1H, NH).13C NMR δ: 13.6, 27.5, 27.9, 32.0, 47.4, 51.0, 59.4, 76.1, 109.8, 112.9, 122.9, 124.8, 127.5, 138.6, 143.6, 159.7, 163.4, 167.9, 180.0, 195.3. 4g.1H NMR δ: 0.99 (s, 3H, CH3), 1.03 (s, 3H, CH3), 2.10 (d,J=16.0 Hz, 1H, CHAHB), 2.18 (d,J=16.0 Hz, 1H, CHAHB), 2.49-2.62 (m, 2H, CH2), 6.92 (t,J= 8.0 Hz, 1H, ArH), 6.98 (d,J= 6.8 Hz, 1H, ArH), 7.21 (m, 1H, ArH), 7.32 (s, 2H, NH2), 10.84 (s, 1H, NH).13C NMR δ: 27.5, 28.0, 32.4, 48.1, 50.3, 57.3, 110.9, 114.0, 117.6, 122.2, 123.5, 128.7, 136.6, 140.3, 159.3, 164.9, 178.4, 195.5. 4h.1H NMR δ: 0.83 (t,J= 7.2 Hz, 3H, CH3), 0.95 (s, 3H, CH3), 1.02 (s, 3H, CH3), 2.03 (d,J=15.6 Hz, 1H, CHAHB), 2.18 (d,J=15.0 Hz, 1H, CHAHB), 2.47-2.62 (m, 2H, CH2), 3.64-3.78 (m, 2H, CH2), 6.79 (t,J= 7.6 Hz, 1H, ArH), 6.83 (d,J= 6.4 Hz, 1H, ArH), 7.10 (m, 1H, ArH), 7.92 (s, 2H, NH2), 10.55 (s, 1H, NH).13C NMR δ: 13.4, 27.2, 28.2, 32.0, 47.9, 51.0, 59.4, 76.3, 113.1, 121.3, 122.3, 127.6, 138.3, 142.2, 159.6, 163.2, 167.9, 180.2, 195.3. 4i.1H NMR δ: 1.01 (s, 3H, CH3), 1.04 (s, 3H, CH3), 2.13 (d,J=16.0 Hz, 1H, CHAHB), 2.19 (d,J=16.0 Hz, 1H, CHAHB), 2.50-2.60 (m, 2H, CH2), 7.13 (d,J= 7.2 Hz, 1H, ArH), 7.52 (d,J= 7.2 Hz, 1H, ArH), 7.99 (d,J= 8.4 Hz, 1H, ArH), 7.47 (s, 2H, NH2), 11.27 (s, 1H, NH).13C NMR δ: 27.7, 27.8, 32.5, 46.5, 50.2, 56.4, 110.4, 117.4, 122.6, 123.7, 130.0, 130.9, 138.4, 139.1, 159.5, 165.6, 179.0, 195.7. 4j.1H NMR δ: 0.99 (s, 3H, CH3), 1.03 (s, 3H, CH3), 2.07 (d,J=16.0 Hz, 1H, CHAHB), 2.18 (d,J= 16.0 Hz, 1H, CHAHB), 2.21 (s, 3H, CH3), 2.50-2.61 (m, 2H, CH2), 6.79 (t,J= 6.8 Hz, 1H, ArH), 6.82 (s, 1H, ArH), 6.95 (d,J= 6.8 Hz, 1H, ArH), 7.21 (s, 2H, NH2), 10.44 (s, 1H, NH).13C NMR δ: 16.8, 27.4, 28.1, 32.4, 47.5, 50.5, 58.3, 111.4, 117.8, 118.7, 120.8, 122.0, 130.0, 134.6, 141.1, 159.2, 164.4, 178.9, 195.2. 4k.1H NMR δ: 0.79 (t,J= 7.2 Hz, 3H, CH3), 0.94 (s, 3H, CH3), 1.02 (s, 3H, CH3), 2.00 (d,J= 15.6 Hz, 1H, CHAHB), 2.15 (d,J=15.0 Hz, 1H, CHAHB), 2.17 (s, 3H, CH3), 2.45-2.61 (m, 2H, CH2), 3.63-3.74 (m, 2H, CH2), 6.65 (d,J= 6.4 Hz, 1H, ArH), 6.68 (t,J= 7.2 Hz, 1H, ArH), 6.86 (d,J= 6.8 Hz, 1H, ArH), 7.84 (s, 2H, NH2), 10.17 (s, 1H, NH).13C NMR δ: 13.3, 16.8, 27.0, 28.3, 32.0, 47.3, 51.1, 59.3, 77.0, 113.7, 117.5, 120.2, 121.0, 128.9, 136.0, 142.9, 159.5, 162.7, 168.2, 180.7, 195.0. 4l.1H NMR δ: 1.92 (t,J= 6.4 Hz, 2H, CH2), 2.21-2.25 (m, 2H, CH2), 2.23 (s, 3H, CH3), 2.65 (t,J= 6.0 Hz, 2H, CH2), 6.66 (d,J= 8.0 Hz, 1H, ArH), 6.81 (s, 1H, ArH), 6.93 (d,J= 7.6 Hz, 1H, ArH), 7.17 (s, 2H, NH2), 10.26 (s, 1H, NH).13C NMR δ: 20.2, 21.1, 27.2, 36.9, 47.4, 58.2, 109.3, 112.4, 117.9, 124.2, 128.9, 130.9, 135.1, 140.0, 159.0, 166.4, 178.5, 195.4. 4m.1H NMR δ: 1.93 (t,J= 6.0 Hz, 2H, CH2), 2.17-2.29 (m, 2H, CH2), 2.62-2.68 (m, 2H, CH2), 6.92 (t,J= 8.0 Hz, 1H, ArH), 7.01 (d,J= 7.2 Hz, 1H, ArH), 7.21 (d,J= 8.0 Hz, 1H, ArH), 7.31 (s, 2H, NH2), 10.83 (s, 1H, NH).13C NMR δ: 20.2, 27.2, 36.7, 48.2, 57.4, 112.0, 113.9, 117.0, 122.4, 123.4, 128.7, 136.7, 140.2, 159.2, 166.8, 178.6, 195.6. 4n.1H NMR δ: 1.92 (t,J= 6.0 Hz, 2H, CH2), 2.16-2.29 (m, 2H, CH2), 2.50-2.67 (m, 2H, CH2), 6.77 (d,J= 7.6 Hz, 1H, ArH), 6.88 (t,J= 7.6 Hz, 1H, ArH), 7.00 (d,J= 7.2 Hz, 1H, ArH), 7.13 (t,J= 7.6 Hz, 1H, ArH),, 7.18 (s, 2H, NH2), 10.37 (s, 1H, NH).13C NMR δ: 20.2, 27.2, 36.8, 47.3, 58.0, 109.6, 112.3, 117.8, 122.1, 123.6, 128.6, 135.0, 142.4, 159.1, 166.5, 178.6, 195.5.
(责任编辑王海雷)
Synthesis of Spiro[4H-pyran-oxindole] Derivatives Catalyzed by Ytterbium Triflate
Wang Mengjia Xu Huiting Chen Fei Xie Jiahui Wu Xiaoya Qu Xisheng Wu Chunlei
(School of Chemistry and Chemical Engineering, Shaoxing University, Shaoxing, Zhejiang 312000)
A series of spiro[4H-pyran-oxindole] derivatives in yield of 87% ~ 95% was synthesized by the reaction of isatins, malononitrile or ethyl cyano-acetate, and 1,3-dicarbonyl compounds in the presence of catalytic amount of Yb(OTf)3using PEG-400 as the solvent at 60℃ for 30 min.The structures were confirmed by1H NMR and13C NMR. It is proven that Yb(OTf)3/ PEG-400 can be recycled for five times.
isatins; spiro[4H-pyran-oxindole]; PEG-400; Yb(OTf)3
2016-03-31
浙江省教育厅科研项目(编号:Y201431839);绍兴文理学院学生科技创新重点项目。
王梦佳(1994-),女,浙江杭州人,主要研究方向为药物及中间体合成.
10.16169/j.issn.1008-293x.k.2016.08.17
O626.13
A
1008-293X(2016)08-0093-05
