Who should be checked for hepatitis C virus infection in endemic areas?
2016-10-20WattanaSukeepaisalncharoenUdomlackPeansukwechKittisakSawanyawisuth
Wattana Sukeepaisalncharoen, Udomlack Peansukwech, Kittisak Sawanyawisuth,3,4✉
1Department of Medicine, Khon Kean University, Khon Kaen, 40002, Thailand
2Liver Disease Research group, Faculty of Medicine, Khon Kean University, Khon Kaen, 40002, Thailand
3Research Center in Back, Neck Other Joint Pain and Human Performance (BNOJPH), Khon Kaen University, Khon Kaen, 40002, Thailand
4Internal Medicine Research group, Faculty of Medicine, Khon Kean University, Khon Kaen, 40002, Thailand
Who should be checked for hepatitis C virus infection in endemic areas?
Wattana Sukeepaisalncharoen1,2, Udomlack Peansukwech1, Kittisak Sawanyawisuth1,3,4✉
1Department of Medicine, Khon Kean University, Khon Kaen, 40002, Thailand
2Liver Disease Research group, Faculty of Medicine, Khon Kean University, Khon Kaen, 40002, Thailand
3Research Center in Back, Neck Other Joint Pain and Human Performance (BNOJPH), Khon Kaen University, Khon Kaen, 40002, Thailand
4Internal Medicine Research group, Faculty of Medicine, Khon Kean University, Khon Kaen, 40002, Thailand
ARTICLE INFO
Article history:
in revised form 11 June 2016
Accepted 11 July 2016
Available online 20 October 2016
Hepatitis C virus
Risk factors
Family history
Sex
Age
Objective: To find additional factors suggestive of hepatitis C virus (HCV) infection in the general population by using data from a hepatitis virus survey. Methods: This study collected data of HCV infection from a hepatitis virus survey. The survey was conducted in 13 provinces in the northeast Thailand in 2014 and 2015. During the survey, a blood test was performed to screen for HCV. A questionnaire was also distributed to all participants asking about baseline characteristics, risk factors for HCV infection, and daily life activities. Risk factors for HCV infection were executed. Results: There were 2 112 participants for the survey. Of those, 110 participants (5.21%) tested positive for HCV infection. After adjustment by multivariate logistic regression, three factors were significantly associated with HCV infection,namely male gender, age, and family history of liver cancer. The adjusted ORs and 95% CI of these factors were 3.14 (1.50, 6.56), 3.78 (1.12, 12.76), and 2.28 (1.08, 4.80), respectively.
Conclusions: Male gender, increasing age, and family history of liver cancer are predictors of HCV infection in endemic areas. Males with a family history of liver cancer in their firstdegree relatives should be tested for HCV infection regardless of symptoms.
Document heading doi: 10.1016/j.apjtm.2016.07.031
1. Introduction
Chronic hepatitis C virus (HCV) is an emerging infection worldwide. The HCV infection rate increased globally from 2.3% in 1990 to 2.8% in 2005[1]. In that year, there were an estimated of 185 million people suffering from HCV infection, which may cause more than 350 000 deaths annually[1,2]. The HCV prevalence ratevaries among countries. It is more prevalent in developing countries such as those in East Asia or Southeast Asia. Similar to HBV,HCV is a cause of both cirrhosis and hepatocellular carcinoma. Approximately one-fourth of cirrhosis or hepatocellular carcinoma cases are caused by HCV[2]. In Japan, HCV was highly associated with hepatocellular carcinoma at 90%[2].
Unlike HBV infection, there is no effective vaccine against HCV and most people infected with HCV develop chronic HCV infections (80%)[3]. HCV seems to be more problematic for public health than HBV. Additionally, people infected with chronic HCV may remain asymptomatic for years before development of cirrhosis and hepatocellular carcinoma[4]. People may not be aware of this kind of HCV infection, particularly in developing countries where people are not well informed about health matters. HCV is mainly transmitted to humans via blood components. In Thailand,the main risk factors for HCV infection among blood donors are intravenous drug use or history of blood transfusion[5]. This studyaimed to find additional factors suggestive of HCV infection in the general population by using data from a hepatitis virus survey. Early detection and treatment may reduce risk of cirrhosis and hepatocellular carcinoma. Additionally, these data may apply to populations in other Asian countries.
2. Materials and methods
This study collected data of HCV infection from a hepatitis virus survey. The survey was conducted in 13 provinces in the northeastern Thailand in 2014 and 2015. Each survey was announced via local media such as radio or advertisement. Persons who registered in advance and participated in the program were enrolled in the study. During the survey, a blood test was performed to screen for HCV. A questionnaire was also distributed to all participants asking about baseline characteristics, risk factors for HCV infection, and daily life activities.
Details of the questionnaire were as follows. Participants were asked about family history of liver cancer, history of HBV vaccination over their lifetimes, alcohol consumption, smoking habits, herb/supplement use, and exercise habits. Family history of liver cancer was defined by the history of liver cancer in firstdegree relatives. History of HBV vaccination in their lifetime was asked. Alcohol consumption was categorized as 1) never 2) used to or currently consumed. Similarly, smoking history was defined as current/previous smoker vs. non-smoker. History of current supplements/herb use was asked. Exercise activities were categorized as whether or not the participant engaged in regular exercise for more than 30 min/d three times a week.
Participants were divided into two groups according to the HCV test, anti-HCV positive and negative. Data were compared between the groups using descriptive statistics. Univariate logistic regression analysis was applied to calculate the crude odds ratios (ORs) of individual variables for HCV infection. All variables were included in subsequent multivariate logistic regression analyses. A backward stepwise method was used to determine the final model. Factors with P value of more than 0.25 were excluded. Analytical results were presented as adjusted ORs, and 95% confidence intervals (CIs). All analyses were performed using STATA software (College station,Texas, USA).
3. Results
There were 2 112 participants for the survey. Of those, 110 participants (5.21%) tested positive for HCV infection. There were six significant factors between those with and without HCV infection including age, sex, family history of liver cancer, alcohol consumption, history of smoking, and history supplement/herb use(Table 1). Those with HCV infection had significantly higher mean age (50 vs. 48 years; P < 0.001) and lower proportion of supplement/ herb use (25% vs. 36%; P=0.034) than those without HCV infection. The HCV infection group had higher proportions than the non-HCV infection group in other categories. For example, the HCV infection group had a higher proportion of males than non-HCV infection group (63% vs. 32%; P < 0.001).
After adjustment by multivariate logistic regression, the final model comprised of six factors (Table 2). Only three factors were significantly associated with HCV infection, namely male gender,age, and family history of liver cancer. The adjusted ORs and 95% CI of these factors were 3.14 (1.50, 6.56), 3.78 (1.12, 12.76), and 2.28(1.08, 4.80), respectively.
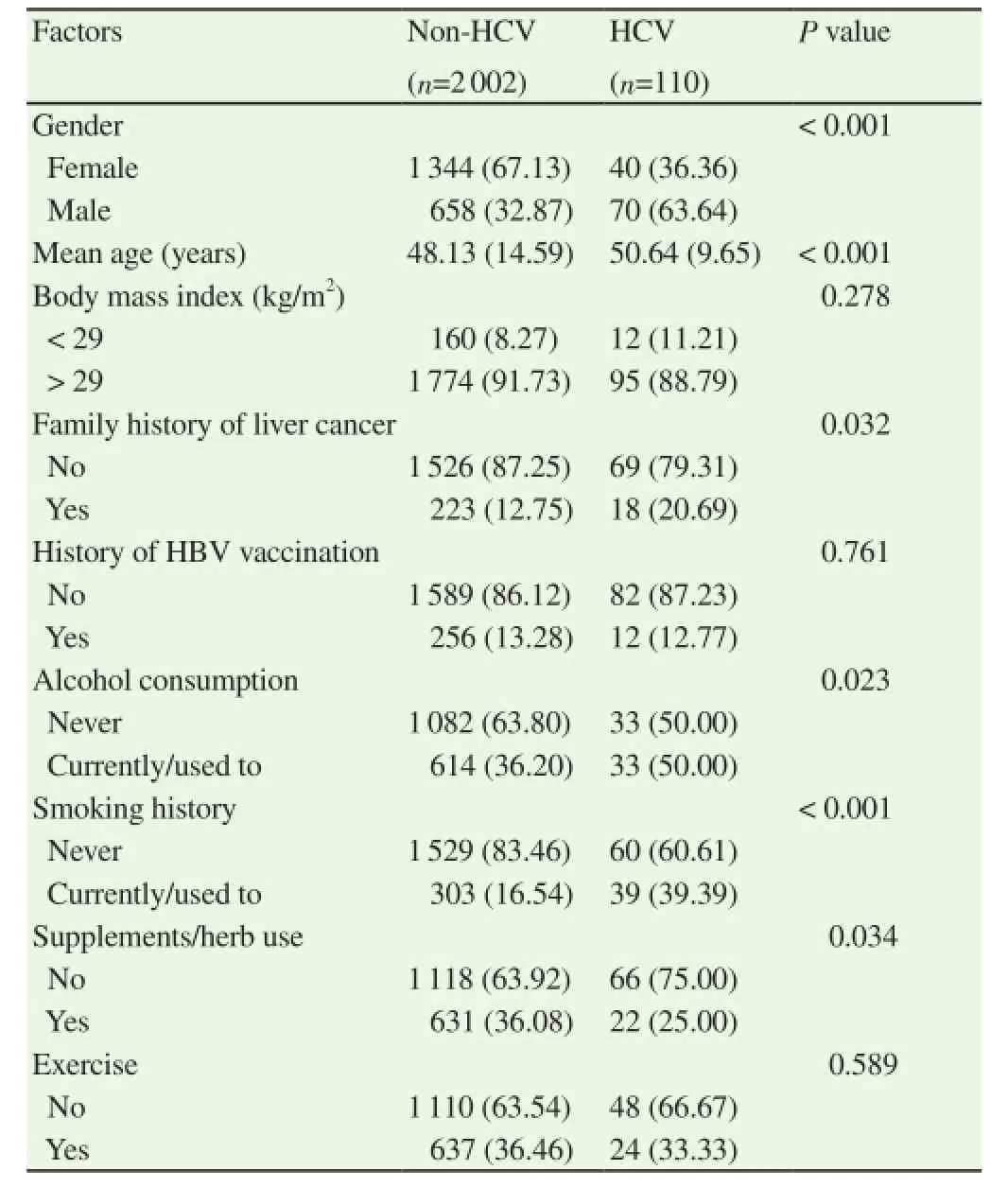
Table 1Baseline characteristics of subjects with and without HCV infection.
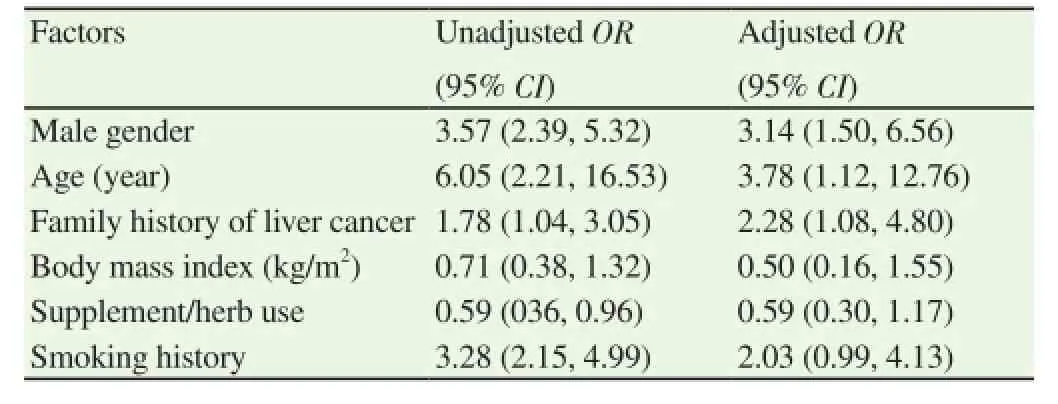
Table 2Univariate and multivariate logistic regression analysis of factors associated with HCV infection.
4. Discussion
The prevalence of HCV infection in this study was 5.21%, which is higher than that in other reports and in the general population[6,7]. The estimated prevalence of HCV infection in the published literature was 2.7%[6], while another report from Thailand showed the prevalence of HCV infection to be 0.94%[7]. The reason for low prevalence rate of the latter report was that blood samples were collected primarily from pediatric health checkups. The mean age of the participants was quite young, resulting in a low rate of HCV infection. Further extensive studies should be conducted to confirm the national HCV infection rate in Thailand. The high HCV infection rate in this study may be due to two factors. First, participants in the survey may be at higher-than-normal risk for HCV infection; second,participants had an average age of 48-50 years, which meant that they were at higher risk than younger people.
There are three independent factors associated with HCV infection,including male gender, age, and family history of liver cancer. These results suggested that males with a family history of liver cancer in their first-degree relatives should be tested for HCV infection,regardless of symptoms. For every one-year increase in age, risk of being found to be HCV positive increased by 3.78 times. This recommendation may apply to endemic countries for HCV infection,particularly developing countries[8].
There are two explanations for why men had a higher risk for HCV infection than women. These are gender risk and HCV clearance ability. HCV is primarily transmitted by through blood, putting intravenous drug users, tattoo recipients, and men who engage in sexual intercourse with other men particularly at risk[9-11]. Men are at higher risk of contracting an infection in these ways than are women. A report from Egypt[12] found that females also had a higher ability to clear the HCV virus than men (44.6% vs. 33.7%; P=0.001). Increased age is also associated with increased risk for HCV infection. A systematic review found that three out of six articles indicated that age was an independent factor for HCV infection[13]. A recent study from China also found that the most common age group was between 41-60 years and accounted for 60% of all HCV infected patients[14].
A family history of liver cancer increases the risk for HCV infection by 2.28 times. This finding may indicate that HCV may be transmitted among family members, vertically or other routes. Theoretically, the rate of HCV vertical transmission is much lower than HBV[15,16]. The transmission rate is 70%-90% for HBe positive and 10%-40% for HBs positive HBV pregnant carriers[15], while HCV transmission rate is 3%-10%[16]. HCV may spread to spouse for five times in HCV group than non-HCV group (OR= 5.75, 95% CI: 1.94-17.07)[14]. Parents with the HCV gene have also been reported to pass HCV to their children via sperm[17]. This evidence suggests that it is possible that HCV may be vertically transmitted or transmitted among family members and cause liver cancer in families. Those people with a family history of liver cancer, HBV or HCV should be tested. A study from China[14] also indicated that a family history of HCV infection is associated with HCV infection with adjusted odds ratio of 4.68 (95% CI of 2.67, 8.75). It may,therefore, be worthwhile to screen for HCV infection in patients with a family history of HCV infection. Other routes of transmission such as oral route as in Campylobacter pylori require further investigation. There are several limitations to this study. First, although it included participants from almost every province in the northeast Thailand, it may not be representative of the general population in the northeast or Thailand as a whole. Second, some other factors are not included in the study such as the presence of fatty liver disease,history of tattooing, or HCV genotypes. Body mass index, smoking,and alcohol consumption were included in the analysis, but they were not independent factors for HCV infection in our results. Third,the results of this study may not be universal particularly for areas that are not endemic for HBV infection. Further studies are needed to confirm the results of this study both in endemic and non-endemic countries.
In conclusion, male gender, increasing age, and family history of liver cancer are predictors of HCV infection in endemic areas. Males with a family history of liver cancer in their first-degree relatives should be tested for HCV infection regardless of symptoms. Early HCV screening in younger age groups is more beneficial than HCV.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
The authors would like to thank Mr. Dylan Southard for his kind manuscript English editing via Research Affair, Faculty of Medicine,Khon Kaen University, Thailand; the Thailand Research Fund(TRF): IRG 5780016; the Higher Education Research Promotion National Research University Project of Thailand; Office of the Higher Education Commission through the Health Cluster (SHePGMS), Thailand; the Faculty of Medicine, Khon Kaen University with grant number TR57201; the TRF Senior Research ScholarGrant; Thailand Research Fund with grant number RTA5880001.
[1] Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013; 57(4): 1333-1342.
[2] Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol 2006; 45(4): 529-538.
[3] Chitapanarux T, Phornphutkul K. Risk Factors for the development of hepatocellular carcinoma in Thailand. J Clin Transl Hepatol 2015; 3(3): 182-188.
[4] Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med 1995; 332(22): 1463-1466.
[5] Tanwandee T, Piratvisuth T, Phornphutkul K, Mairiang P, Permpikul P,Poovorawan Y. Risk factors of hepatitis C virus infection in blood donors in Thailand: a multicenter case-control study. J Med Assoc Thai 2006;89(S5): S79-S83.
[6] Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol 2014; 61(S1): S45-S57.
[7] Wasitthankasem R, Posuwan N, Vichaiwattana P, Theamboonlers A,Klinfueng S, Vuthitanachot V, et al. Decreasing hepatitis C virus infection in Thailand in the past decade: evidence from the 2014 National Survey. PLoS One 2016; 11(2): e0149362.
[8] Averhoff FM, Glass N, Holtzman D. Global burden of hepatitis C: considerations for healthcare providers in the United States. Clin Infect Dis 2012; 55(S1): S10-S15.
[9] Nadol P, O'Connor S, Duong H, Mixson-Hayden T, Tram TH, Xia GL,et al. High hepatitis C virus (HCV) prevalence among men who have sex with men (MSM) in Vietnam and associated risk factors: 2010 Vietnam Integrated Behavioural and Biologic Cross-Sectional Survey. Sex Transm Infect 2016. doi: 10.1136/sextrans-2015-052518.
[10] Puri P, Anand AC, Saraswat VA, Acharya SK, Dhiman RK, Aggarwal R, et al. Consensus statement of HCV Task Force of the Indian National Association for Study of the Liver (INASL). PartⅠ: status report of HCV infection in India. J Clin Exp Hepatol 2014; 4(2): 106-116.
[11] Umer M, Iqbal M. Hepatitis C virus prevalence and genotype distribution in Pakistan: comprehensive review of recent data. World J Gastroenterol 2016; 22(4): 1684-1700.
[12] Bakr I, Rekacewicz C, El Hosseiny M, Ismail S, El Daly M, El-Kafrawy S, et al. Higher clearance of hepatitis C virus infection in females compared with males. Gut 2006; 55(8): 1183-1187.
[13] Alvarez KJ, Smaldone A, Larson EL. Burden of hepatitis C virus infection among older adults in long-term care settings: a systematic review of the literature and meta-analysis. Curr Infect Dis Rep 2016;18(4): 13.
[14] Piao HX, Yang AT, Sun YM, Kong YY, Wu XN, Zhang YZ, et al. Increasing newly diagnosed rate and changing risk factors of HCV in Yanbian Prefecture, a high endemic area in China. PLoS One 2014; 9(1): e86190.
[15] Gentile I, Borgia G. Vertical transmission of hepatitis B virus: challenges and solutions. Int J Womens Health 2014; 6: 605-611.
[16] Pawlowska M, Domagalski K, Pniewska A, Smok B, Halota W, Tretyn A. What's new in hepatitis C virus infections in children? World J Gastroentero 2015; 21(38): 10783-10789.
[17] Ma M, Zhu Y, Wang D, Hou Z, Huang J, Zhang D, et al. Research on the vertical transmission of hepatitis C gene from father-to-child via human Sperm. Clin Lab 2016; 62(1-2): 1-6.
11 May 2016
Wattana Sukeepaisalncharoen, Associate Professor, Department of Medicine, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand. Email: wattanasukee@gmail.com
Tel: 66 43 363664
Fax: 66 43 347542
Kittisak Sawanyawisuth, MD, PhD, Department of Medicine, Faculty of Medicine, Khon Kean University, Khon Kaen, 40002, Thailand. Email: kittisak@kku.ac.th
Foundation project: This study was supported by the Thailand Research Fund(TRF): IRG 5780016; the Higher Education Research Promotion National Research University Project of Thailand; Office of the Higher Education Commission through the Health Cluster (SHeP-GMS), Thailand; the Faculty of Medicine, Khon Kaen University with grant number TR57201; the TRF Senior Research Scholar Grant;Thailand Research Fund with grant number RTA5880001.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Arginine kinase in Toxocara canis: Exon-intron organization, functional analysis of site-directed mutants and evaluation of putative enzyme inhibitors
- Identification and analysis of a processed cytochrome P450 pseudogene of the disease vector Aedes aegypti
- Melaleuca quinquinervia (Cav.) S.T. Blake (Myrtales: Myrtaceae): Natural alternative for mosquito control
- Epidemiology of the outbreak, vectors and reservoirs of cutaneous leishmaniasis in Mali: A systematic review and meta-analysis
- Skin whitening and anti-corrugation activities of glycoprotein fractions from liquid extracts of boiled sea cucumber
- Effect of emodin on mobility signal transduction system of gallbladder smooth muscle in Guinea pig with cholelithiasis
