Epidemiology of the outbreak, vectors and reservoirs of cutaneous leishmaniasis in Mali: A systematic review and meta-analysis
2016-10-20AbdoulayeKassoumKoneDoumboSafiatouNiareMahamadouAliTheraKassoumKayentaoAbdoulayeDjimdePascalDelaunayBouremaKouribaPascaldelGiudiceArezkiIzriPierreMartyOgobaraDoumbo
Abdoulaye Kassoum Kone, Doumbo Safiatou Niare, Mahamadou Ali Thera, Kassoum Kayentao, Abdoulaye Djimde, Pascal Delaunay, Bourema Kouriba, Pascal del Giudice, Arezki Izri, Pierre Marty, Ogobara K Doumbo✉
1Malaria Research and Training Center, Department of Epidemiology of Parasitic Diseases/ Faculty of Medicine, and Dentistry/ UMI-3189/ University of Science, Technique and Technology of Bamako, BP 1805, Bamako, Mali
2Inserm U1065, Centre Méditerranéen de Médecine Moléculaire, C3M, Université de Nice-Sophia Antipolis, 151, route St Antoine de Ginestière, BP 2 3194, 06204 Nice Cedex, France AND Parasitologie-Mycologie, Hôpital de l'Archet, Centre Hospitalier Universitaire de Nice, France
3Unit of Infectious et Tropical Diseases, Hospital Bonnet 83700 Fréjus, France
4Parasitology-Mycology, Hospital Avicenne, Paris 13 University, UMR 190, Aix-Marseille University, France
Epidemiology of the outbreak, vectors and reservoirs of cutaneous leishmaniasis in Mali: A systematic review and meta-analysis
Abdoulaye Kassoum Kone1, Doumbo Safiatou Niare1, Mahamadou Ali Thera1, Kassoum Kayentao1, Abdoulaye Djimde1, Pascal Delaunay2, Bourema Kouriba1, Pascal del Giudice3, Arezki Izri4, Pierre Marty2, Ogobara K Doumbo1✉
1Malaria Research and Training Center, Department of Epidemiology of Parasitic Diseases/ Faculty of Medicine, and Dentistry/ UMI-3189/ University of Science, Technique and Technology of Bamako, BP 1805, Bamako, Mali
2Inserm U1065, Centre Méditerranéen de Médecine Moléculaire, C3M, Université de Nice-Sophia Antipolis, 151, route St Antoine de Ginestière, BP 2 3194, 06204 Nice Cedex, France AND Parasitologie-Mycologie, Hôpital de l'Archet, Centre Hospitalier Universitaire de Nice, France
3Unit of Infectious et Tropical Diseases, Hospital Bonnet 83700 Fréjus, France
4Parasitology-Mycology, Hospital Avicenne, Paris 13 University, UMR 190, Aix-Marseille University, France
ARTICLE INFO
Article history:
in revised form 3 July 2016
Accepted 10 July 2016
Available online 20 October 2016
Epidemiology
Leishmaniasis
Cutaneous
Risk Factors Vectors
Reservoirs
Mali
Objective: To compile available data and to estimate the burden, characteristics and risks factors of cutaneous leishmaniasis (CL) in Mali. Methods: Articles in English and French were searched in Hinari, Google scholar and PubMed. Unpublished studies were identified by searching in Google.com. Terms used were Cutaneous leishmaniasis Mali; Leishmaniasis Mali, Leishmania major Mali; or Phlebotomus Mali or Sergentomyia Mali. We select descriptive studies on CL and sandflies in Mali. Data were extracted and checked by the author, then analyzed by region, by study population and type of biological tests, meta-analysis approach with STATA software was used. Results: Nineteen published (n=19) and three unpublished were included. CL epidemiology was characterized by occurrence of clinical cases in different areas of Mali, outbreaks restricted to known areas of transmission and isolated cases diagnosed in travelers. In endemic areas,population at risk are young age persons, farmers, ranchers, housewives, teachers and military personnel. The annual incidence ranged from 290 to 580 cases of CL. Leishmania major is the main species encountered throughout the country (North Savanna, Sahel and Sub-Saharan areas),and Phlebotomus duboscqi has been identified as the vector and Sergentomyia (Spelaeomyia)darlingi as possible vector. The overall estimated prevalence of positive LST (Leishmanin Skin Test) was 22.1%. The overall frequency of CL disease among suspected cases was 40.3%.
Conclusions: Although descriptive, hospital-based and cross-sectional studies are robust enough to determine the extent of CL in Mali; future well-designed eco-epidemiological studies at a nationwide scale are needed to fully characterize CL epidemiology and risk factors in Mali.
Document heading doi: 10.1016/j.apjtm.2016.07.025
1. Introduction
Leishmaniases are zooanthroponoses common in animalsand human being. Globally, leishmaniases are endemic in 98 countries, with an annual incidence of 0.7 to 1.2 million cases of cutaneous leishmaniasis (CL) and 0.2 to 0.4 million cases of visceral leishmaniasis (VL) causing 20 000 to 40 000 deaths each year[1]. In Sub-Saharan Africa, the estimated annual incidence of CL is between 770 and 1 500 cases. The annual incidence of CL is between 40 and 80 cases in Senegal, and 30 to 50 in Nigeria[1].
In Mali, CL was described for the first time in 1948 by Lefrou[2]. Izri et al. reported for the first time the zymodeme MON-26 of Leishmania major (L. major) in a Malian patient in 1989[3]. Otherauthors described zymodemes MON- 25, MON- 17 and MON- 117 of L. major in Mali[4,5]. Little data on CL have been reported by the national demographic and health survey[6]; given the difficulty of CL diagnosis, health facilities are not well equipped to diagnose CL,leading to under-notification. Research studies conducted on CL have used different diagnostic methods in different study populations within different regions of Mali. However, CL variation and its burden at the national level remain poor known.
2. Materials and methods
2.1. Search
A literature search was performed to identify records on CL in Mali. The search included original research done in Mali and publications in both French and English. Searches were performed on Hinari, Google scholar, and PubMed using the following terms:“Cutaneous leishmaniasis Mali”; “Leishmaniasis Mali”; “Leishmania major Mali”; or “Phlebotomus Mali”. Unpublished data on LC were searched for in Google.com using the same search terms.
2.2. Study selection
Papers were screened manually. Descriptive studies (cross sectional and cohort) conducted in communities or in health facilities were selected. Articles with full text available were preferred. When full text was not available, the published abstracts were included. Data from unpublished literature (Master's, MD dissertation defended at the Faculty of Medicine, Pharmacy and Dentistry of Bamako) have also been searched, checked and included in our review. Participants of any age were included. Citations were excluded when the full text or abstract were not available. Published papers on visceral leishmaniasis were not included.
2.3. Data collection process
The first author did the literature review, read the documents and checked citation eligibility. Data included in the meta-analysis were checked by the author and the statistician.
2.4. Data items
The data extracted from each study included: study location(community- or hospital-based study, geographic region),participants (tools of diagnosis, frequency of CL, age), and sandfly fauna (description of fauna, frequency).
2.5. Planned methods of analysis
Dates, type of study, diagnosis tools and localization were also included to place data in context. Data from each study (LST prevalence or frequency of confirmed CL, community or health facility-based studies) were considered separately. Data were analyzed by region and study population. A meta-analysis approach using STATA software was performed to compare frequencies and to compute the mean prevalence rate of LST-positive reactions in communities and the mean proportion of CL in suspected cases in heath facilities.
3. Results
A total of 39 studies were identified, including 30 peer reviewed papers and nine from the unpublished literature. Five were removed mainly because of duplicating results, and 14 records were excluded after checking of eligibility criteria. Twenty studies have been included, among them six records (four full-text articles, an abstract article and one MD dissertation) that have been included in metaanalysis (Figure 1).

Table 1Porporttion of positive skin test by study population and proportion of LC among suspected cases in clinic.
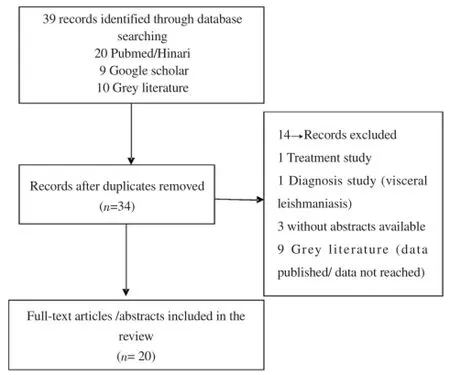
Figure 1. Study flow chart.
The mean prevalence of positive LST in the general population was 22.1%; however, it varied by region and by study population(Table 1). The frequency of CL in patients where there was clinical suspicion of CL was 78.4% (251/320) in Bamako (Mali) between 1997 and 2001[7]. The prevalence of positive reactions to the LST in 1969 in school aged children in Bamako city was 12.7 % (90/705)[8],and in the city of Mopti in the general population was 4.8% in 1974(12/249)[9]. In the general population in Kemena, Segou region, the prevalence rate of LST positive reactions was 45.4% (301/663) in 2008[10] and 30.4% (34/112) frequency of LST positive reactions observed between 1957 and 1966 in residents of Bamako from the Segou region (P<0.01)[8].
A recent survey conducted in two villages of Segou region shows an annual incidence rate of 18.5% (53/287) in Kemena and 5.7%(32/366) in Sougoula in 2008[10] (P<0.01).
Sand flies collected in rural and suburban areas in Mali have shown a variability of sand fly fauna. To date four species of Phlebotomus sp.: Phlebotomus duboscqi, Phlebotomus rodhaini, Phlebotomus sergenti and Phlebotomus kazeruni have been identified in Mali. Phlebotomus sergenti has been cached in Mopti and Bamako areas. P. kazeruni has been identified in Mopti area[11-14].
Twenty two species of Sergentomyia sp. have been identified: Sergentomyia schwetzi, Sergentomyia antennenta, Sergentomyia dubia, Sergentomyia cleydi, Sergentomyia africana, Sergentomyia squamipleuris, Sergentomyia affinis affinis, Sergentomyia affinis vorax, Sergentomyia balmicola, Sergentomyia bedfordi, Sergentomyia fallax, Sergentomyia buxtoni, Sergentomyia (Spelaeomyia) darlingi,Sergentomyia christophersi, Sergentomyia wansoni, Sergentomyia magna, Sergentomyia davidsoni, Sergentomyia freetownensis,Sergentmoyia herollandi, Sergentomyia congolensis, Sergentomyia ghesquierei and Sergentomyia (Schoutedeni) schoutedeni, Sergentomyia wansoni, Sergentomyia magna, Sergentomyia davidsoni, Sergentomyia affinis affinis and Sergentomyia balmicola were traped in Mopti and Bamako areas. Sergentomyia freetownensis, Sergentomyia herollandi,Sergentomyia congolensis, Sergentomyia ghesquierei and Sergentomyia(S) schoutedeni have been identified in suburban area of Bamako[11-16].
砂糖橘(Citrus reticulata Blanco)是目前我国华南地区最畅销的柑橘品种,在广东、广西广泛栽培。但由于南方气温高、雨水多,砂糖橘园杂草种类多、生长速度快,平均每年须要治理3~4次,尤其是施肥前,但砂糖橘园杂草化学防治研究尚未见报道。本研究以草铵膦和45%丙炔氟草胺悬浮剂及它们的混剂作为供试除草剂,比较它们对砂糖橘园杂草的防治效果,进一步利用叶绿素荧光技术评价2种除草剂及其混剂对砂糖橘的安全性,以期为南方砂糖橘园杂草治理及除草剂安全使用提供一定的参考和依据。
4. Discussion
4.1. Burden
The mean prevalence of positive LST in the general population was 22.1%; however, it varied by region and by study population. The frequency of CL in patients in Bamako was higher than that reported in Niamey (Niger): 66.7% (64/96) between 1985 and 1987[17].
The differences observed in disease estimates between the general population (community-based surveys) and suspected cases(hospitals and outbreaks in Dogon villages) could be explained by the study populations, area and the tools used for Leishmania infection detection. In the suspected cases group, the CL cases were diagnosed using PCR or microscopy, while in the general population,the LST (Leishmanin Skin Test) was used for detecting cases contact with Leishmania.
4.1.1. CL in urban areas
In addition to the scarcity of studies on L. major transmission in urban areas of Mali, data on CL in urban areas are old, in Bamako,the city study population consisted of primary and secondary schools, while in Mopti city, it was the general population (no cases of CL have been detected where the LST has been used). The study in Bamako city with school-aged children from different regions gives the range of the frequency of positive LST in Mali[8].
At the Centre National de Lutte contre la Maladie (CNAM, the dermatology reference center in Bamako), suspected cases were ill patients carrying chronic skin wounds that were diagnosed as CL on Leishmania positive thin smears at microscopy. The higher proportion of confirmed CL within suspected patients in CNAM could be explained by the fact that this center is the country-wide reference facility for the diagnosis and the treatment skin diseases[7]. This result differs from that of the Center of Dermatology and Venereology in Segou, where the frequency of 14,06% LC was reported[18].
4.1.2. CL in rural areas
Descriptive studies on L. major transmission in rural areas indicated that populations older than one year from two villages of Segou region have positive reactions to leishmanin[10]. Outbreaks of CL may occur frequently in rural foci. In 2010, an outbreak of cutaneous wounds in Dogon villages was investigated. Suspected cases of CL were examined by a dermatologist, and samples were collected. PCR of scrapings from wounds edges was performed, and Western blot of sera was used to detect antibodies against Leishmania. Out of 50 patients examined, 28% (14/50) were diagnosed as CL cases.Microscopy examination was negative and frequency of antibody detection by IFA was lower than that of Western blot[19].
Data collected in the Segou region in 2008[10] and in the Mopti region in 2010[19] suggest an increasing contact with L. major when the results of a positive LST in Segou and a positive Western blot in Bandiagara are compared to previous results on reactivity to LST in 1967[8,9]. However these data show that transmission of CL remains significant over time in the Segou region.
Besides the possibility that the prevalence rate of LST-positive reaction observed in these studies could be less than the rate of Leishmania infection in the population, as individuals harboring L. major infection are often LST negative, the rate may also be higher because more than a 5% LST false positive rate is expected [8]. In addition, a recent analysis of the geographic distribution of PCR-confirmed cases of CL in the data collected by the CNAM reveals that the disease is endemic in the north Sudan savanna, Sahelian areas, and the Sub-Saharan region spanning parts of the Kayes,Koulikoro, Segou, Mopti and Tombouctou regions[20]. These findings support previous results on geographic distribution of positive LST in Mali[20,21].
Among CL cases reported, 70.1 % (413/589) are from Kayes region (Nioro, Kayes, Bafoulabe, and Yelimane) from 1957-1966[9], showing that western rural areas of Mali could be more endemic compared to others areas of Mali. Recent findings support these previous data and show also that CL cases come from all the country[22].
Although the accurate incidence rate of CL in Mali is not known,Alvar and colleagues reported an annual incidence of 290 to 580 cases from 2004 to 2008. The same report suggested an incidence of 2-5 times the number of reported cases[1]. An incidence rate of 6.7 per 1 000 was reported in CNAM in 2003[7].
Data reviewed suggest a lower transmission of CL in urban areas[8]compared to rural area of Segou[13]. The transmission may vary considerably between villages in rural area. A discrepant prevalence and incidence rates[10] and discrepant frequency[19] of CL within villages in rural area have been observed.
4.1.3. Limitations
We did a meta-analysis to measure the burden of CL; few studies have been conducted on CL in Mali. We analyzed studies conducted in different areas in Mali; these studies may have different designs and may use different diagnosis tools conducted at different times. The differences in the estimates and risk of CL in Mali could be explained by the heterogeneity of the studies and design effect. In spite of these differences, this review shows that CL is a rural disease and that transmission occurs mainly in rural areas of the North savanna and Sahel regions of Mali.
4.1.4. Risk factors
The risk of CL is likely to be important in the rural areas of Kayes,Koulikoro, Segou, and Mopti where higher LST-positive reaction has been reported[8]. CL cases recorded (n=251) between 1997 and 2001 show that CL is endemic in the eight regions of Mali;few cases (n=4) were from Tombouctou-Gao-Kidal in the north of Mali compared to Bamako-Kayes-Segou, with (n=233) cases at the center-west of Mali. Infected people were between 20 and 40 years old; men were more infected than women[19], CL cases occurred in travelers visiting endemic areas of Mali[23] where locals populations such as farmers, ranchers, and military personnel are mostly infected by L. major[7,10,19,23,24]. Although the disease affects all age groups,the risk of Leishmania infection increases with age[9]; children aged less than three years have lower infection rates compared to adults[10].
In West Africa, as in Dogon villages in Mali, outbreaks were also reported in Ouagadougou city, Burkina-Faso[25] and in Ghana within communities presenting significant population movements between endemic and non-endemic areas[26]. Mass migration of unimmunized people in endemic areas and domestication of zoonotic foci due to rapid urbanization and agro-industrial projects such as dams, wells,roads, trash deposits, irrigation systems, and deforestation may have contributed to increased risk of parasitic diseases such as CL[25-27].
4.1.5. Leishmania-HIV co-infection
Co-infection with Leishmania and HIV was reported in Mali[7,28];the risk factors associated with HIV-Leishmania co-infection are not well documented in Mali. Descriptive studies at CNAM show that in 261 skin diseases in HIV-positive, there were two (0.7 %) CL cases[28], and among 251 CL cases, six (2.3 %) were HIV-positive[7]. These results could be explained by the overall lower prevalence of HIV infection in Mali (1.1%)[6]. In Cameroon, 4.8% of CL (7/146)cases were HIV-positive; women were more co-infected than men,likely due to the higher prevalence of HIV in women[29]. However,in Burkina Faso, such differences were not observed[30]. HIV-positive patients were not more exposed than HIV-negative patients to Leishmania infection, and coinfected patients showed a lack of healing of skin lesions[31]. Severe and atypical clinical spectrum of CL has been observed in HIV-positive patients and children[7,31],co-infection with Leishmania and HIV has caused death (n=1) in CNAM[7].
4.1.6. Leishmania parasite
The enzyme electrophoresis analysis identified four strains of L. major in Mali. The most prevalent strain is MON- 26, (15 out of 30),followed by MON-74, which is the most prevalent strain described in Burkina Faso[5,31]. Attempts to culture parasites from samples collected in Dogon country for identification of L. major strains was unsuccessful, but Izri et al., were able to successfully grow L. major from a patient in Bamako and described the first case of CL due to L. major MON-26[3]. L. major DNA was detected in wound scrapings and sand flies in Dogon country and Segou[13,14,19]. PCR-confirmed CL cases demonstrated that L. major was the main species in Mali,with a large geographical distribution[19]. However in regions of Maliwhere LST-positive reaction has been reported without documented PCR-confirmed CL, Leishmania sp. screening should be done.
4.1.7. Vectors
The first collection of sandflies was done in Mali in 1943[11]. Ranque et al., in 1972 described sand fly fauna on the mountain of Point G around Bamako and have identified P. sergenti and several species of Sergentomyia sp.[12]. Other studies have shown an important variability of sandfly fauna within surveyed areas where the Phlebotomus duboscqi vector of L. major was frequently trapped in rural and suburban areas. Sergentomyia schwetzi was most abundant in the Segou region but very rare in the Mopti region;Sergentomyia (Spelaeomyia) darlingi was more frequently trapped in the Bandiagara area (Mopti) than the Segou region[13,14].
In Dogon country, four Phlebotomus species (P. duboscqi,P. rodhaini, P. kazeruni, and P. sergenti) and 16 Sergentomyia species were found, including S. (S) darlingi which contained L. major DNA and could play a role in endemic transmission of CL in Mali[14]. These data call for investigating the role of S. (S) darlingi in L. major transmission in Mali. It has been shown that S. schwetzi is refractory to L. major, Leishmania donovani and Leishmania infantum[32], but in Segou area, no Sergentomyia species were incriminated as a possible vector of L. major, compared to Bandiagara[14], Ghana[33] and Portugal[34] where respectively S. (S) darlingi , Sergentomyia ingrami and Sergentomyia hamoni and Sergentomyia minuta were naturally infected by L. major.
Few data were found on L. major transmission in urban and suburban areas of Mali. Bamako sandfly surveys showed the presence of two genera:d three Phlebotomus species (P. duboscqi, P. rodhaini and P. sergenti) and several Sergentomyia species[12,15].
A residual local transmission of L. major in Bamako and surrounding areas might be possible. CL cases reported in residents of Bamako[7], the presence of P. duboscqi, P. sergenti and P. rodhaini[12,15] in suburban area and the presence of P. duboscqi in surrounding villages[16] support the possible transmission of L. major in suburban areas of Bamako. P. rodhaini captured around Bamako has been described recently as a possible vector of Leishmania spp.[27,34]. So far, urban and suburban transmission of Leishmania spp. has not been established in Mali. P. duboscqi has been confirmed as the vector of L. major in rural areas of Mali[13,14].
4.1.8. Parasite reservoirs
The reservoirs of L. major in West Africa are rodents (Table 2). In Senegal Mastomys erythrolocus, Tatera gambiana and Arvicanthis niloticus have been found to be infected by L. major[35,36]. To our knowledge, there is no data found on identification of Leishmania parasites in mammals in Mali, and an attempt to identify Leishmania in rodents was unsuccessful[24]. However, in Kenya Mastomys natalensis, Tatera robusta and Arvicanthis niloticus are reservoirs of L. major[37]. Future investigations on Leishmania's reservoirs in Mali should cover rodents and other mammals such as hedgehogs, as L.
major has been isolated from the two species of hedgehog Atelerix algirus and Paraechinus aethiopicus in Algeria[38].
In Mali, CL is widely distributed, and the prevalence rates of positive reactions to LST are variable; age, residence and occupation are risk factors. Because of the wide geographic distribution, climate and ecological changes, and the risk of outbreak, heath workers and authorities should be aware of possible transmission of L. major and occurrence risk of CL outbreaks in Mali. Sparse data on geographic distribution of CL, population movement, HIV/AIDS co-infection and climate change call for implementation of epidemiological surveillance and more research, with the aim to better understand the epidemiology of CL in Africa. Identification of reservoirs and transmission paths in urban and suburban areas of Mali are required. This would help in the design of new and better adapted strategies for CL prevention and treatment.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
We thank Mark Travassos for revising the manuscript, the population of Bandiagara district for participating to the study. This work was supported by the UMI3189, FMERIEUX foundation, and Fogarty International Center[grant number D43TW001589].
[1] Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 2012 ; 7: e35671.
[2] Lefrou G. La Leishmaniose cutanee au Soudan francais. Frequence de la forme sèche papulo-tuberculeuse. Bull Soc Pathol Exot 1948; 41: 622-627.
[3] Izri MA, Doumbo O, Belazzoug S, Pratlong F. Presence of Leishmania major MON-26 in Mali. Ann Parasitol Hum Comp 1989; 64: 510-511.
[4] Garin JP, Peyramond D, Piens MA, Rioux JA, Godfrey DG, Lanotte G et al.:[Presence of Leishmania major Yakimoff and Schokhor, 1914 in Mali. Enzymatic identification of a strain of human origin. Ann Parasitol Hum Comp 1985; 60: 93-94.
[5] Pratlong F, Lami P, Ravel C, Balard Y, Dereure J, Serres G et al.: Geographical distribution and epidemiological features of Old World Leishmania infantum and Leishmania donovani foci, based on the isoenzyme analysis of 2277 strains. Parasitology 2013; 140: 423-434.
[6] ICF International Rockville. Maryland, USA, Mai 2014. Available at : http://www.dhsprogram.com/pubs/pdf/FR286/FR286.pdf. [Accessed on July, 16 2016].
[7] Keita AS, NDiaye HT, Faye O, Konare HD: Epidémiologie etpolymorphisme clinique de la leishmaniose cutanée observée au CNAM(ex-Institut Marchoux) Bamako (Mali). Mali Med 2003; 18: 29-31.
[8] Imperato PJ, Diakite S. Leishmaniasis in republic of Mali. Trans R Soc Trop Med Hyg 1969; 63: 236-241.
[9] Imperato PJ, Fofana B, Sow O, Diallo S. Leishmanin skin sensitivity in the inland delta of the Niger. Trop Geogr Med 1974; 26: 303-306.
[10] Oliveira F, Doumbia S, Anderson JM, Faye O, Diarra SS, Traore P, et al. Discrepant prevalence and incidence of Leishmania infection between two neighboring villages in Central Mali based on Leishmanin skin test surveys. PLoS Negl Trop Dis 2009; 3: e565.
[11] Parrot L, Cadenat J. Notes sur les phlébotomes. Les phlébotomes de l'Afrique occidentale française I. Senegal, Soudan et Niger. Arch Inst Pasteur Alg 1945; 23: 232-244.
[12] Ranque P, Sangare C, Abonnenc E, Leger N. Preliminary note on the Phlebotomus of the Bamako-Mali region. Presence of Phlebotomus sergenti, Parrot 1917. Acta Trop 1975; 32: 348.
[13] Anderson JM, Samake S, Jaramillo-Gutierrez G, Sissoko I, Coulibaly CA, Traore B, et al. Seasonality and prevalence of Leishmania major infection in Phlebotomus duboscqi Neveu-Lemaire from two neighboring villages in central Mali. PLoS Negl Trop Dis 2011; 5: e1139.
[14] Berdjane-Brouk Z, Kone AK, Djimde AA, Charrel RN, Ravel C, Delaunay P, et al. First detection of Leishmania major DNA in Sergentomyia (Spelaeomyia) darlingi from cutaneous leishmaniasis foci in Mali. PLoS One 2012; 7: e28266.
[15] Demba-Kodindo I, Cheick-Coulibaly A, Traore B, Sissoko I, Samake S,Doumbia S. Study of phlebotomines sand fly wildlife suburban location of Bamako (Mali) presence of Phlebotomus (Phlebotomus) duboscqi. Bull Soc Pathol Exot 2015;108: 130-132.
[16] Kone AK, Diarra AZ, Coulibaly C, Niaré S, Berthe M, Traoré A, et al. Distribution spatio-temporelle de la faune de phlébotomes en zones urbaine et périurbaine de Bamako, Mali. Annales de la Société entomologique de France (N.S.). doi=10.1080/00379271.2016.1194773[Online] Avalable at :http://dx.doi.org/10.1080/00379271.2016.1194773[Accessed on 1 April 2016]
[17] Develoux M, Blanc L, Garba S, Mamoudou HD, Warter A, Ravisse P. Cutaneous leishmaniasis in Niger. Am J Trop Med Hyg 1990; 43: 29-30.
[18] Kampo OM. Etude des motifs de consultation dermatologique dans un hôpital Régional: cas de l'hôpital Nianankoro Fomba de Ségou (Mali). Bamako: University of Bamako Médecine; 2009.
[19] Kone AK, Delaunay P, Djimde AA, Thera MA, Giudice PD, Coulibaly D, et al. Epidemiology of cutaneous leishmaniasis in five villages of Dogon country, Mali. Bull Soc Pathol Exot 2012; 105: 8-15.
[20] Paz C, Samake S, Anderson JM, Faye B, Traore P, Tall K et al. Leishmania major, the predominant Leishmania species responsible for cutaneous leishmaniasis in Mali. Am J Trop Med Hyg 2013 ; 88: 583-585.
[21] Dooko CLB. Evolution spatiale et temporelle de la leishmaniose cutanée au Mali. Dakar: Université Cheikh Anta Diop de Dakar; 2008.
[22] Tall K. Etude Epidemio-clinique et prise en charge de la leishmaniose cutanée à Bamako et dans les villages endémiques du Mali. Bamako: Université de Bamako; 2008.
[23] Kelly P, Baudry T, Peyron F. Imported cutaneous leishmaniasis in a shortterm traveler returning from Central Mali - The role of PCR. Travel Med Infect Dis 2012;10: 97-100.
[24] Paz C, Doumbia S, Keita S, Sethi A: Cutaneous leishmaniasis in Mali. Dermatol Clin 2011; 29: 75-78.
[25] Traore KS, Sawadogo NO, Traoré A, Ouedraogo JB, Traoré KL,Guiguemdé TR. Preliminary study of cutaneous leishmaniasis in the town of Ouagadougou from 1996 to 1998. Bull Soc Pathol Exot 2001; 94: 52-55.
[26] Kweku MA, Odoom S, Puplampu N, Desewu K, Nuako GK, Gyan B, et al. An outbreak of suspected cutaneous leishmaniasis in Ghana: lessons learnt and preparation for future outbreaks. Glob Health Action 2011; 4: doi: 10.3402/gha.v4i0.5527.
[27] Mott KE, Desjeux P, Moncayo A, Ranque P, de Raadt P. Parasitic diseases and urban development. Bull World Health Organ 1990; 68: 691-698.
[28] Mahé A, Bobin P, Coulibaly S, Tounkara A. Skin diseases disclosing human immunodeficiency virus infection in Mali. Ann Dermatol Venereol 1997; 124: 144-150.
[29] Ngouateu OB, Kollo P, Ravel C, Dereure J, Kamtchouing P, Same-Ekobo A, et al. Clinical features and epidemiology of cutaneous leishmaniasis and Leishmania major/HIV co-infection in Cameroon: results of a large cross-sectional study. Trans R Soc Trop Med Hyg 2012 ; 106: 137-142.
[30] Niamba P, Traoré A, Goumbri-Lompo O, Labrèze C, Traoré-Barro F,Bonkoungou M, et al.: Leishmaniose cutanée chez les malades infectés par le VIH. Ann Dermatol Venereol 2006;133: 537-542.
[31] Guiguemdé TR, Sawadogo NO, Botero S, Traore KL, Nezien D, Nikiema L, et al.: Leishmania major and HIV co-infection in Burkina Faso. Trans R Soc Trop Med Hyg 2003; 97: 168-169.
[32] Sadlova J, Dvorak V, Seblova V, Warburg A, Votypka J, Volf P. Sergentomyia schwetzi is not a competent vector for Leishmania donovani and other Leishmania species pathogenic to humans. Parasit Vectors 2013;6:186.
[33] Nzelu CO, Kato H, Puplampu N, Desewu K, Odoom S, Wilson MD, et al. First detection of Leishmania tropica DNA and Trypanosoma species in Sergentomyia sand flies (Diptera: Psychodidae) from an outbreak area of cutaneous leishmaniasis in Ghana. PLoS Negl Trop Dis 2014;8(2):e2630.
[34] Campino L, Cortes S, Dionísio L, Neto L, Afonso MO, Maia C: The first detection of Leishmania major in naturally infected Sergentomyia minuta in Portugal. Mem Inst Oswaldo Cruz 2013; 108: 516-518.
[35] Dedet JP, Derouin F. Isolation of Leishmania major from Mastomys erythroleucus and Tatera gambiana in Senegal (West Africa). Ann Trop Med Parasitol 1979; 73: 433-437.
[36] Camerlynck P, Ranque P, Quilici M. Importance of systematic cultures and subcultures in research on natural viral reservoirs in cutaneous leishmaniasis. Apropos of the isolation of 5 strains of Leishmania in Arvicanthis niloticus. Med Trop (Mars) 1967; 27: 89-92.
[37] Githure JI, Ngumbi PM, Anjili CO, Lugalia R, Mwanyumba PM, Kinoti GK, et al.: Animal reservoirs of leishmaniasis in Marigat, Baringo District, Kenya. East Afr Med J 1996; 73: 44-47.
[38] Tomás-Pérez M, Khaldi M, Riera C, Mozo-León D, Ribas A, Hide M, et al.: First report of natural infection in hedgehogs with Leishmania major,a possible reservoir of zoonotic cutaneous leishmaniasis in Algeria. Acta Trop 2014; 135: 44-49.
3 June 2016
Abdoulaye Kassoum Kone, Malaria Research and Training Center,Department of Epidemiology of Parasitic Diseases/ Faculty of Medicine, and Dentistry/ UMI-3189/ University of Science, Technique and Technology of Bamako,BP 1805, Bamako, Mali.
E-mail : fankone@icermali.org
Ogobara K Doumbo, Malaria Research and Training Center, Department of Epidemiology of Parasitic Diseases/ Faculty of Medicine, and Dentistry/ UMI-3189/ University of Science, Technique and Technology of Bamako,BP 1805, Bamako, Mali.
Tel: (223) 20 22 81 09
E-mail: okd@icermali.org
猜你喜欢
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Arginine kinase in Toxocara canis: Exon-intron organization, functional analysis of site-directed mutants and evaluation of putative enzyme inhibitors
- Identification and analysis of a processed cytochrome P450 pseudogene of the disease vector Aedes aegypti
- Melaleuca quinquinervia (Cav.) S.T. Blake (Myrtales: Myrtaceae): Natural alternative for mosquito control
- Who should be checked for hepatitis C virus infection in endemic areas?
- Skin whitening and anti-corrugation activities of glycoprotein fractions from liquid extracts of boiled sea cucumber
- Effect of emodin on mobility signal transduction system of gallbladder smooth muscle in Guinea pig with cholelithiasis
