Expression of miRNA-140 in Chondrocytes and Synovial Fluid of Knee Joints in Patients with Osteoarthritis△
2016-10-13HaiboSiYiZengZongkeZhouFuxingPeiYanrongLuJingqiuChengandBinShen
Hai-bo Si, Yi Zeng, Zong-ke Zhou, Fu-xing Pei, Yan-rong Lu, Jing-qiu Cheng, and Bin Shen,*
Expression of miRNA-140 in Chondrocytes and Synovial Fluid of Knee Joints in Patients with Osteoarthritis△
Hai-bo Si1,2,#, Yi Zeng1,#, Zong-ke Zhou1, Fu-xing Pei1, Yan-rong Lu2, Jing-qiu Cheng2, and Bin Shen1,*
1Department of Orthopedics,2Key Laboratory of Transplant Engineering and Immunology, Regenerative Medicine Research Centre, West China Hospital, Sichuan University, Chengdu 610041, China
microRNA-140; osteoarthritis; chondrocytes; synovial fluid
Objective To investigate the expression of miRNA-140 in chondrocytes and synovial fluid of osteoarthritis (OA) patients, and explore the relationship between the miRNA-140 expression and OA severity.
Methods This study enrolled 30 OA patients who underwent total knee arthroplasty for chondrocytes sampling and 30 OA patients who underwent intra-articular injection for synovial fluid sampling. All OA patients were grouped intomild [Kellgren and Lawrence (KL) grade 1-2], moderate (KL grade 3) and severe (KL grade 4), with 10 in each subgroups for each sampling purposes. 7 non-OA patients and 10 patients with knee injury were collected for cartilage and synovial fluid sampling respectively as control groups. Chondrocytes were isolated from the cartilage tissue and cultured. Quantitative real time PCR for miRNA-140 in chondrocytes and synovial fluid were performed, and the U6 snRNA was used as internal control. The expression difference of miRNA-140 among groups and correlation between the expression and the KL grade of OA were analysed using one-way ANOVA and Spearman test respectively.
Results The expression of miRNA-140 in chondrocytes of knees in OA patients was reduced than that in normal knees, and the between-group difference was statistically significant (=305.464,<0.001). miRNA-140 could be detected in synovial fluid of both normal knees and OA knees, its relative expression level was reduced in synovial fluid of OA group compared with normal group, and the between-group difference was statistically significant as well (=314.245,<0.001). The relative expression level of miRNA-140 in both chondrocytes and synovial fluid were negatively correlated with the KL grade of OA(=-0.969,<0.001;=-0.970,<0.001).
Conclusion miRNA-140 could be detected in chondrocytes and synovial fluid of OA patients, and its expression was negatively correlated with the severity of OA.
Chin Med Sci J 2016; 31(4):207-212
STEOARTHRITIS (OA) is a chronic and highly prevalent degenerative joint disease, chara- cterized by progressive destruction of articular cartilage, alterations of subchondral bone, formation of osteophytes and synovitis.1Current clinical treatment of OA is limited to pain management, and there is no effective treatment for the damaged articular cartilage besides the joint replacement.2Although various factors are known to participate in the pathogenesis of OA, such as heredity, age, obesity, inflammation and mechanical factors, the molecular mechanisms underlying OA develop- ment remain unclear.3
microRNAs (miRNAs) are a class of endogenous and non-coding single-strand RNAs with a length of about 22 nucleotides, and many of them exhibit tissue-specific or developmental stage-specific expression patterns associated with several human diseases.4,5miRNA-140 was shown to be specially expressed in cartilage tissue, and play an important role in cartilage homeostasis and OA patho- genesis.6However, the results on expression of miRNA- 140 in cartilage as reported in previous studies were not always identical,4and few study had investigated the expression of miRNA-140 in OA synovial fluid. Therefore, this study examine the expression of miRNA-140 in chondrocytes and synovial fluid of OA patients, in order to investigate the relationship between the relative expression level of miRNA-140 and the severity of OA.
PATIENTS AND METHODS
Patientsand grouping
From January 2015 to February 2016, 7 consecutive normal donors who underwent amputation because of non-knee disease, such as thigh destructive injury, and 30 consecutive OA patients (10 patients in each subgroup) undergoing unicompartmental or total knee arthroplasty in the In-patientDepartment of Orthopedics of West China Hospital, Sichuan University were enrolled in this study for cartilage sampling. 10 patients who underwent intra- articular paracentesis for ligament or meniscus injury, and 30 OA patients (10 patients in each subgroup) who underwent intra-articular injection of hyaluronan or corticosteroid in the Out-patient Department of Orthopedics of West China Hospital, Sichuan University were enrolled in the study for synovial fluid sam- pling.
OA patients were classified by the severity according to the X-ray diagnosis criteria of the Kellgren and Lawrence (KL),7and assigned into three subgroups with 10 in each for cartilage or synovial fluid study: mild (KL grade 1-2; doubtful narrow of the joint, doubtful or minimal osteophytes), moderate (KL grade 3; definite narrow of the joint, osteophytes, and sclerosis or abrasion of subchondral bone) and severe (KL grade 4; severe narrow of the joint, massive osteophytes, severe sclerosis and abrasion of subchondral bone, and obvious joint deformity) OA.
Collection of cartilage tissue and synovial fluid samples was approved by the Institutional Ethics Committee of West China Hospital, Sichuan University, and conformed to the tenets of the Declaration of Helsinki. Written information consents were obtained from all subjects.
Cartilage tissue and synovial fluid sampling
After disinfection, draping and exposure of the knee, cartilage samples without subchondral bone at the distal area of the medial femoral condyle were whistled with scalpel and placed in 50 ml sterile centrifuge tube con- taining Dulbecco’s modified Eagle medium-High Glucose (DMEM-HG; Hyclone, USA), and transported immediately to the laboratory on ice for further chondrocytes isolation. The synovial fluid werecollected using syringe after piercing into the joint cavity and before first injection of the medicine, and then transported immediately to the laboratory on ice. All the synovial fluid samples were cen- trifuged for 10 min, then the supernatant were collected and stored at -80°C for further RNA extraction.
Chondrocytes isolation and culture
Cartilage samples were first washed twice in a phosphate buffer solution (PBS) and then minced with a scalpel blade. Cartilage samples were incubated with 0.25% trypsin (Hyclone, USA) for 15-30 minutes, and then incubated overnight with 2 mg/ml type Ⅱ collagenase (Sigma, USA) in DMEM-HG (Hyclone, USA) containing 5% fetal bovine serum (FBS, Hyclone, USA) at 37°C. Isolated chondrocytes were resuspended by pipetting several times and filtered through 100μm filter to remove undigested cartilage frag- ments and extracellular matrix (ECM) remnants. After centrifugation the cells were resuspended in DMEM-HG supplemented with 15% FBS. Chondrocytes were seeded as primary cell culture in 75 cm2culture flasks (BD, Germany) at low density (5×103cells/cm2), cultured in a humidified antibiotics-free atmosphere at 37°C containing 5% CO2, and the medium was replaced every 2-3 days. At 80%-90% confluence, the primary chondrocytes were trypsinized and passaged, and the experiments were per- formed in passage one chondrocytes.
Quantitative real-time polymerase chain reaction (qPCR)
Total small RNAs were extracted from chondrocytes and synovial fluid, using a microRNA Purification Kit (Norgen, CA) in accordance with the manufacturer’s directions. Quantitative real time PCR (qPCR) for miRNAs was performed using the TaqMan miRNA RT kit and Megaplex RT primers (Applied Biosystems, CA) according to the manufacturer’s protocol. qPCR was carried out for miRNA- 140 on a PCR thermocycler (Applied Biosystems, CA) using the manufacturer’s recommended cycling conditions. The U6 small nucleolar RNA (snRNA) was used as an internal control to normalize differences in each sample, and fold changes for miRNA-140 was normalized to the U6 snRNA. Assays that had a threshold cycle (> 35 were removed from the analysis to exclude the non-specific gene expression, the delta(△) and △△values were calculated by using U6 as the endogenous control (△Ct,miRNA-140−Ct,U6; △△=△,OA group−△,normal group), and the 2-△△Ctmethod was used to calculate the expression of miRNA-140 in OA group relative to normal group.8
Statistical analysis
Data analysis was performed using SPSS software version 22.0 (IBM, USA). The one-way analysis of vaviance and Chi-square (2) test were used to compare the differences among normal and OA subgroups, and the Spearman test was used to investigate the correlation between miRNA- 140 relative expression and KL grade of OA. Avalue of<0.05 was considered statistically significant.
RESULTS
The expression of miRNA-140 in normal and OA articular chondrocytes
The cartilage tissues were collected from 7 normal and 30 OA knees. The typical X-rays findings of the each OA group are shown in Fig. 1 and general information of the selected patients are shown in Table 1. The average ages of OA patients in each OA groups were higher than that of normal group, and significant difference among groups was found (=47.23,<0.001), although there was no signi- ficant difference between OA subgroups. Furthermore, male/ female ratio of patients was significantly higher in normal group than those in OA groups (2=8.97,=0.03), while females overwhelmed males in OA patients without signi- ficant difference among subgroups (2=0.48,=1.00). Additionally, there was no between-group differences of suffering side in OA patients (2=1.88,=0.60).
miRNA-140 expression was detected in human articular chondrocytes. The relative expression level in normal group, mild, moderate and severe OA groups were 1.003 ± 0.083, 0.332 ± 0.123, 0.041 ± 0.017 and 0.004 ±0.002 respectively, the between-group difference was statistically significant (=305.464,<0.001), which exhibited a decreasing trend with increase of severity (Fig. 2). The miRNA-140 expression in severe and moderate OA groups were significantly lower than those in mild OA and normal groups, and mild OA group was significantly lower than normal group, while no significant difference was found between severe and moderate OA group. Spearman tests revealed that the relative expression level of miRNA-140 in human articular chondrocytes was negatively correlated with the KL grade of OA (=-0.969,<0.001).

Figure 1. Anteroposterior X-ray views of a normal knee (A) and osteoarthritic (OA) knees: the typical radiological findings of mild OA (B), moderate OA (C) and severe OA (D).
The expression of miRNA-140 in normal and OA synovial fluid
The synovial fluid samples were collected from 10 normal knees and 30 OA knees, and the characteristics of the selected patients are shown in Table 2. Significant between- group differences in the age were found (=61.93,<0.001). The average age of severe and moderate OA groups were significantly higher than that of mild OA and normal groups, and mild OA group was significantly higher than normal group, while no significant difference was found between severe and moderate OA groups. There was no between- group difference in the sex or side (>0.05).
miRNA-140 expression could be detected in normal and OA synovial fluid, and the expression profile was similar to that in chondrocytes. The relative expression level of miRNA-140 in synovial fluid were 1.056 ± 0.139, 0.589 ± 0.109, 0.041 ± 0.020 and 0.007 ± 0.003 in normal, mild, moderate and severe group respectively, the between- group difference was statistically significant (=314.245,<0.001) (Fig. 3). Compared with normal group, the expression of miRNA-140 showed a decreasing trend with increase of severity of OA. Spearman test revealed that the relative expression level of miRNA-140 in synovial fluid was negatively correlated with the KL grade of OA (=-0.970,<0.001).
DISCUSSION
In recent years, studies have shown that miRNA-140, located between exons 16 and 17 of the Wwp2 in the small arm of chromosome 16 in humans and on murine chromo- some 8, is specially expressed in cartilage tissues, and plays an important role in the pathogenesis of OA.9,10However, results about the expression of miRNA-140 in cartilage were not always identical in previous studies, and few study investigated the expression of miRNA-140 in OA synovial fluid. In this study, we confirmed that the expression of miRNA-140 was statistically reduced in OA chondrocytes and synovial fluid, comparing with normal group, and negatively correlated with the severity of OA.
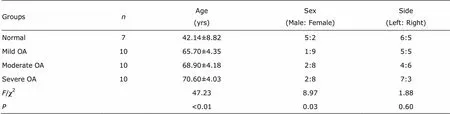
Table 1. Characteristics of the patients for cartilage study
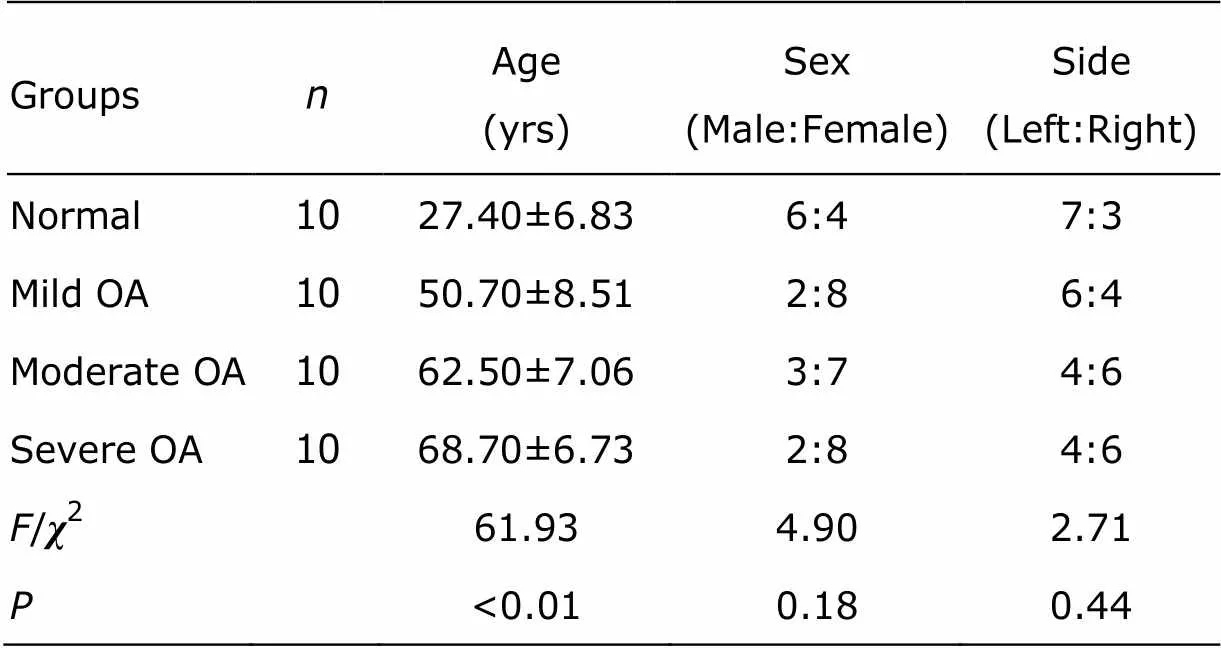
Table 2. Characteristics of the patientsfor synovial fluid study

Figure 2. The relative expression level of miRNA-140 in humanarticular chondrocytes.*<0.05 compared with normal group;#<0.05 compared with mild OA group.

Figure 3. The relative expression level of miRNA-140 in human synovial fluid.*<0.05 compared with normal group;#<0.05 compared with mild OA group.
Nakamura11first reported that miRNA-140 was encoded in an intronic region of the ubiquitin E3 ligase,. They also found that in zebrafishwas strongly expressed in cartilage tissue, which was consistent with the notion that Wwp2 and miRNA-140 were derived from a common transcript. Furthermore, Yang12reported that the miRNA-140 primary transcript was intron-retained RNA of, the precursor miRNA-140 was located in intron 16 of this gene, and mature miRNA-140 was co-expressed with theisoform in chondrogenesis. However, knock down ofcaused a phenotype different from that of miRNA-140 deficiency, suggesting that miRNA-140 and its host genehad unique roles in regulating cartilage homeostasis.11
In previously published study, Asahara and his collea- gues reported that normal human articular cartilage expressed miRNA-140, and this expression was significantly reduced in OA tissue;treatment of chondrocytes with IL-1β, a cytokine classically involved in the patho- genesis of OA, could suppress miRNA-140 expression; Conversely, transfection of chondrocytes with miRNA-140 could down-regulate IL-1β-induced OA-like changes.6,9,13In this study, we detected the expression of miRNA-140 in normal and OA chondrocytes obtained from human femoral condyle, and found that the expression of miRNA-140 was statistically reduced in OA chondrocytes when comparing with normal chondrocytes. However, Swingler14 reported that the expression of miRNA-140 was increased in cartilage of OA patients, comparing with cartilage of non-OA patients who suffered from femoral neck fracture. This contradiction about miRNA-140 expression in OA cartilage might be due to the differences of the human cartilage samplingbetweeneach study.
This study found that the miRNA-140 was detected in normal and OA synovial fluid. Similarly, its expression level was reduced in OA synovial fluid when comparing with that in normal synovial fluid, and was negatively correlated with the KL grade of OA as well. No study had reported the biogenesis of the miRNA-140 in synovial fluid. As miRNA-140 was specially synthesized in chondrocytes, we speculated that miRNA-140 in synovial fluid might be released from the chondrocytes. Therefore, we believe that the down-regulated expression of miRNA-140 in OA chondrocytes and synovial fluid of knee joint suggested that miRNA-140 might be used as a novel target for the treatment of knee OA.
The chondrocytes constitute the unique cellular component of articular cartilage and synthesize the compo- nents of the extracellular matrix (ECM), including collagens, proteoglycans and non-collagen proteins.15In normal cartilage, there is a balance between the synthetic and degenerative activity, and the chondrocytes exhibit a good viability. In OA pathogenesis, the chondrocytes are terminaldifferentiated and eventually apoptosis, causing a reduction in the number of chondrocytes; as the OA worsening, the terminal differentiation and apoptosis will be accelerated and the chondrocytes number will be further reduced.16It had beenwell known that miRNA-140 was specifically synthesised by chondrocytes, and the progressively reduction of the number of chondrocytes in OA cartilagemight explain our findings that the miRNA-140 expression was negatively correlated with the KL grade of OA.
Synovial fluid, secreted by the synovial tissue, can penetrate into ECM and provide nutrition for chondrocytes.2,17Base on our results, we hypothesized that intra-articular injection of exogenous miRNA-140 might be a feasible and effective approach for OA treatment. Shoji18reported that intra-articular injection of miRNA-210 could promote the healing of partially torn anterior cruciate ligament through enhancement of angiogenesis via up-regulating vascular endothelial growth factor (VEGF) and fibroblast growth factor 2 (FGF 2) expression. Furthermore, Kawanishi19found that an intra-articular injection of ds miRNA-210 was effective in healing of damaged white zone meniscus through promoting the production of collagen type Ⅱ from meniscus cells. Nevertheless, no study on using intra-articularinjection of miRNAs in the treatment of OA has been reported. In order to verify the hypothesis we proposed above, anstudy regarding intra-articular injection of miRNA-140 in rat OA model is currently conducted by our team, and we hope it will provide more evidence on this topic.
Although this study have clarified the relationship between miRNA-140 expression and OA severity in primary chondrocytes and synovial fluid, it also have several limitations. Firstly, the study included a relatively small number of patients, mainly because of the difficulty in collecting normal knee cartilage and synovial fluid samples. Secondly, the patients of normal group was significantly younger than those in OA group, because it was difficult to match the age of donors with partients in OA groups for the fact that the donor who underwent amputation in normal group was usuallyyoung. Furthermore, no significant difference in age was found among the OA subgroups, so the relationship between age and miRNA-140 expression could not be determined in this study. There was few study reporting the relationship between age and miRNA-140 expression. Large-sample studies with different ages and OA severity are necessary in further experiments.
In conclusion, miRNA-140 could be detected in synovial fluid, as well as in chondrocytes. Compared with normal chondrocytes or synovial fluid, the expression of miRNA- 140 was reduced remarkably in cartilage and synovial fluid of OA patients, and was negatively correlated with the severity of OA.
1. Sharma L. Osteoarthritis year in review 2015: clinical. Osteoarthritis Cartil 2016; 24: 36-48.
2. Poulet B, Staines KA. New developments in osteoarthritis and cartilage biology. Curr Opin Pharmacol 2016; 28: 8-13.
3. Xia B, Di Chen, Zhang J, et al. Osteoarthritis pathogenesis: a review of molecular mechanisms. Calcif Tissue Int 2014; 95: 495-505.
4. Zhang R, Ma J, Yao J. Molecular mechanisms of the cartilage-specific microRNA-140 in osteoarthritis. Inflamm Res 2013; 62: 871-7.
5. Trzeciak T, Czarny-Ratajczak M. MicroRNAs: Important epigenetic regulators in osteoarthritis. Curr Genomics 2014; 15: 481-4.
6. Miyaki S, Nakasa T, Otsuki S, et al. MicroRNA-140 is expressed in differentiated human articular chondrocytes and modulates interleukin-1 responses. Arthritis Rheum 2009; 60: 2723-30.
7. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957; 16: 494-502.
8. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402-8.
9. Araldi E, Schipani E. MicroRNA-140 and the silencing of osteoarthritis. Genes Dev 2010; 24: 1075-80.
10. Nugent M. MicroRNAs: exploring new horizons in osteoar- thritis. Osteoarthr Cartil 2016; 24: 573-80.
11. Nakamura Y, He X, Kobayashi T, et al. Unique roles of microRNA140 and its host gene WWP2 in cartilage biology. J Musculoskelet Neuronal Interact 2008; 8: 321-2.
12. Yang J, Qin S, Yi C, et al. MiR-140 is co-expressed with Wwp2-C transcript and activated by Sox9 to target Sp1 in maintaining the chondrocyte proliferation. FEBS Lett 2011; 585: 2992-7.
13. Asahara H. miRNAs in cartilage development. Clin Calcium 2012; 22: 653-7.
14. Swingler TE, Wheeler G, Carmont V, et al. The expression and function of microRNAs in chondrogenesis and osteoar- thritis. Arthritis Rheum 2012; 64: 1909-19.
15. Goldring MB. Update on the biology of the chondrocyte and new approaches to treating cartilage diseases. Best Pract Res Clin Rheumatol 2006; 20: 1003-25.
16. Blasioli DJ, Kaplan DL. The roles of catabolic factors in the development of osteoarthritis. Tissue Eng Part B Rev 2014; 20: 355-63.
17. Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone 2012; 51: 249-57.
18. Shoji T, Nakasa T, Yamasaki K, et al. The effect of intra- articular injection of microRNA-210 on ligament healing in a rat model. Am J Sports Med 2012; 40: 2470-8.
19. Kawanishi Y, Nakasa T, Shoji T, et al. Intra-articular injection of synthetic microRNA-210 accelerates avascular meniscal healing in rat medial meniscal injured model. Arthritis Res Ther 2014; 16: 488.
for publication April 6, 2016.
, Tel: 86-13881878767, E-mail: shenbin_1971@163.com
#These authors contributed equally to this paper.
△Supported by the National Natural Science Foundation of China (No. 81672219; No. 81601936), and the Science and Technology Support Program of Sichuan province (No. 2014SZ0023-2).
杂志排行
Chinese Medical Sciences Journal的其它文章
- Frontal Absence Seizures: Clinical and EEG Analysis of Four Cases
- Clinical Observation of Bevacizumab Combined with S-1 in the Treatment of Pretreated Advanced Esophageal Carcinoma△
- Early Enteral Combined with Parenteral Nutrition Treatment for Severe Traumatic Brain Injury: Effects on Immune Function, Nutritional Status and Outcomes△
- A Case Report of Acute Arterial Embolization of Right Lower Extremity As the Initial Presentation of Nephrotic Syndrome with Minimal Changes△
- Pseudohyperkalemia with Myelofibrosis after Splenectomy
- Meta-analysis of aspirin-heparin therapy for un-explained recurrent miscarriage
