New Edges of RNA Adenosine Methylation Modifications
2016-09-27YeWngGuifngJi
Ye WngGuifng Ji*b
Synthetic and Functional Biomolecules Center,Beijing National Laboratory for Molecular Sciences,Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education,College of Chemistry and Molecular Engineering,Peking University,Beijing 100871,China
RESEARCH HIGHLIGHT
New Edges of RNA Adenosine Methylation Modifications
Ye Wanga,Guifang Jia*,b
Synthetic and Functional Biomolecules Center,Beijing National Laboratory for Molecular Sciences,Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education,College of Chemistry and Molecular Engineering,Peking University,Beijing 100871,China
Available online 30 May 2016
Handled by Yi Xing
http://dx.doi.org/10.1016/j.gpb.2016.05.003
1672-0229ⓒ2016 The Authors.Production and hosting by Elsevier B.V.on behalf of Beijing Institute of Genomics,Chinese Academy of Sciences and Genetics Society of China.
This is an open access article under the CC BY license(http://creativecommons.org/licenses/by/4.0/).
Recently an article published in Molecular Cell reveals the mechanism of a nuclear N6-methyladenosine(m6A)reader,the YTH domain-containing protein 1(YTHDC1),in regulating pre-mRNA splicing[1].Meanwhile,two additional articles published in Nature and Nature Chemical Biology report the first transcriptome-wide maps of N1-methyladenosine(m1A)at high resolution,suggesting a functional role for m1A in translation regulation[2,3].
m6A reader YTHDC1 in pre-mRNA alternative splicing
m6A is the most abundant endogenous mRNA modification,which is conserved across archea,bacteria,and eukaryotes[4].Nonetheless,the importance of m6A in mammals had been underappreciated for about 40 years until the discovery of its reversibility by an m6A demethylase—fat mass and obesityassociated protein(FTO)[5]in 2011.Ever since,the widespread regulatory roles of m6A have been unraveled through the transcriptome-wide mapping of m6A modification[6,7],the characterizationofthesecondm6AdemethylaseAlkB homolog 5(ALKBH5)[8]and three subunits of m6A methyltransferase complex(methyltransferase like 3,METTL3;METTL14;and Wilms tumor 1 associated protein,WTAP)[9,10],and the functional studies of m6A readers YTH domain family protein 1(YTHDF1)and YTHDF2 in humans,which regulates m6A methylated RNA stability[11]and translational efficiency[12],respectively.In addition,m6A in primary micro-RNAs can be recognized by another m6A reader,the heterogeneous nuclear ribonucleoproteins A2/B1(HNRNPA2B1),which consequently recruits DiGeorge syndrome critical region 8(DGCR8)and DROSHA complex and promotes the maturation of microRNAs[13,14].
YTHDC1,as reflected by its name,contains the YTH domain that selectively binds to m6A[15].Unlike the other twocytoplasmicm6AbindingproteinsYTHDF1and YTHDF2,YTHDC1 is localized in YT bodies near the nuclear speckles[16],supporting its association with pre-mRNA splicing.Xiao and colleagues[1]identified several YTHDC1 partnersincludingfivetrans-actingsplicingfactors(serine/ arginine-rich splicing factors;SRSF1/3/9/7/10)by tandemaffinity purification following by mass spectrometric analysis,suggesting the potential regulatory role of YTHDC1 in premRNA splicing.To test such possibility,they measured the alternative splicing(AS)events using RNA-seq data upon knockdown of YTHDC1 and its potential SRSF partners in HeLa cells,respectively.Their findings indicate that YTHDC1and SRSF3 facilitate exon inclusion,while SRSF10 promotes exon skipping;however,silencing of other SRSF proteins(SRSF1,SRSF7,and SRSF9)has no significant effect on AS events.Photoactivatableribonucleosidecrosslinkingand immunoprecipitation(PAR-CLIP)sequencing shows that the targeted regions of YTHDC1,SRSF3,and SRSF10 are enriched in the coding sequences(CDS)and the 3′untranslated regions(UTR).Through analyzing the targeted exons,they further confirmed the opposite roles of YTHDC1/SRSF3 and SRSF10 in AS regulation.The change of AS events on the transcripts targeted by both YTHDC1 and SRSF3 in HeLa cells with YTHDC1 or SRSF3 silenced shows similar features with that in METTL3-silenced HeLa cells,suggesting that YTHDC1 and SRSF3 co-regulates AS events in an m6A-dependent manner.
Next,the authors set out to validate the interaction of YTHDC1 with either SRSF3 or SRSF10.PAR-CLIP data show that the YTHDC1 target regions are located closer to the binding sites of SRSF3 than those of SRSF10.In vivo andinvitroco-immunoprecipitationassayverifiesthat YTHDC1 directly interacts with SRSF3 and SRSF10 through the N-terminal of YTHDC1 and C-terminal of SRSF3 or SRSF10.The different AS events affected by YTHDC1/ SRSF3 and SRSF10 prompts them to speculate that SRSF3 and SRSF10 might competitively bind to YTHDC1.Indeed they confirm the hypothesis using competing pull-down assays. The authors then examine whether YTHDC1 regulates localization of SRSF3 and SRSF10.Immunostaining assays show that silencing of YTHDC1 reduces SRSF3 but increases SRSF10 in nuclear speckle.Interestingly,this phenomenon can be rescued by complementation of wild-type YTHDC1,but not YTHDC1 mutant without m6A binding ability,indicating that YTHDC1 regulates the subcellular localization of SRSF3 and SRSF10 in an m6A-dependent manner.Further RNA binding assay shows that YTHDC1 deficiency disrupts the RNA binding of SRSF3 but enhances that of SRSF10,which can be complemented by wild-type YTHDC1,but not an m6A-binding-defective variant.These results indicate that the impact of YTHDC1 on AS events relies on the presence of m6A and the binding ability of YTHDC1 to methylated RNA.
Clearly,the comprehensive analysis presented by Xiao et al. reveals that m6A reader YTHDC1 facilitates exon inclusion by recruiting RNA splicing factor SRSF3 but blocking SRSF10 for its access to the binding regions of its target mRNAs(Figure1).Indeed,apartfromYTHDC1,m6Areader HNRNPA2B1[14]and indirect m6A reader HNRNPC[17]are both involved in RNA splicing.What roles do these proteins play in AS?Are there any other splicing factors regulated by m6A?Does YTHDC1 play other regulatory role apart from splicing?These questions warrant further investigations.
The reversible and dynamic m1A methylome in eukaryotic mRNA
m1A,another RNA adenosine methylation modification,has been identified in total RNA[18],rRNA[19],and tRNA[20]for decades.m1A modification contains a methyl group on N1(hydrogen bond receptor)to form the positive charge and disturbs Watson-Crick base pairs.Unlike m6A,m1A can cause both reverse transcription stops and read-throughs accompanied by mismatches.m1A has been shown to affect the structure and function of tRNA and rRNA[21,22].However,the presence and functions of m1A in mRNA remain unknown.

Figure 1 A proposed model of pre-mRNA splicing regulated by YTHDC1
In the two recently-released papers,Dominissini et al.[2]and Li et al.[3]reported two transcriptome-wide sequencing methods(termed m1A-seq and m1A-ID-seq,respectively)to map m1A in mRNA at high resolution(Figure 2).Their work reveals that m1A is the second reversible and dynamic modificationineukaryoticmRNA.Theyfirstlyenrich m1A-containing mRNA fragments from human or mouse cell lines by m1A-specific antibody immunoprecipitation,and then take advantage of m1A property in reverse transcription toimprove the sequencing resolution,albeit later on the two groups employ different approaches for locating m1A sites(Figure 2).As m1A modification can be converted to m6A in alkaline conditions(Dimroth rearrangement),Dominissini et al.treated a portion of precipitated m1A-containing mRNA fragments with alkaline buffer to chemically rearrange m1A to m6A prior to cDNA synthesis.By comparing mismatch rates between treated and untreated samples,they located m1A position within m1A peaks,in which mutation rates are high in the treated sample but low in the untreated sample.In this way,they can achieve m1A sequencing peaks at the resolution of 5-15 nucleotides(conserved m1A sites in rRNA can be mapped at the resolution of one nucleotide)[2](Figure 2). Different from Dominissini et al.,Li et al.used Escherichia coli AlkB protein to demethylate m1A to regular adenosine and performed cDNA synthesis with AMV reverse transcriptase to maximally confer cDNA truncations near m1A sites.In this way,they achieved the m1A map at the resolution of 55 nucleotides by comparing the m1A peak features between the untreated and treated samples[3](Figure 2).In fact,both strategies,based on mutations or truncations,sacrifice the sequencing signal and lose some sequence information near the modified sites,which make it difficult to obtain single-base resolution m1A maps of high quality.
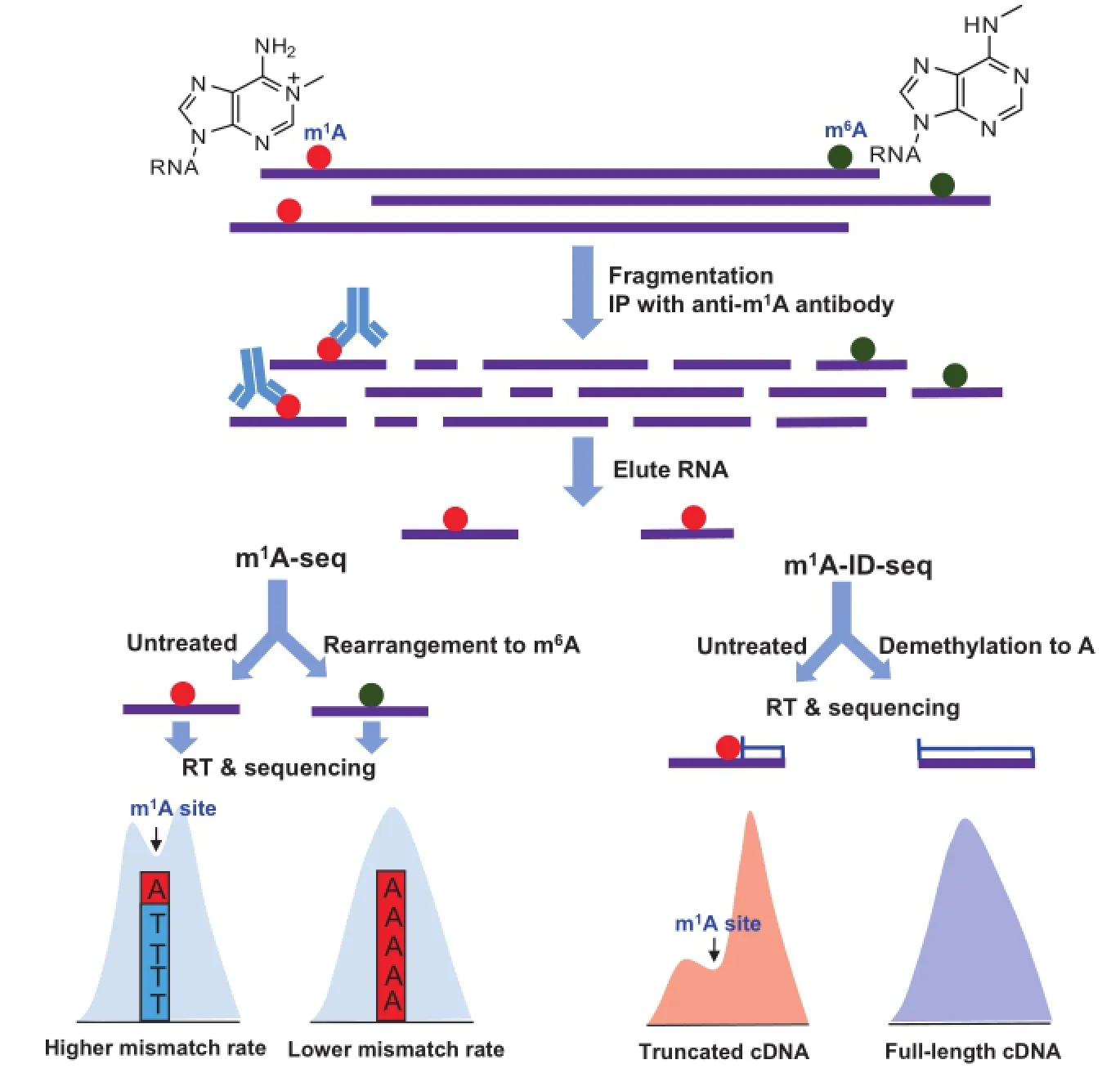
Figure 2 Schematic outline of m1A-seq and m1A-ID-seq
The relative abundance of m1A in mammalian mRNA is much lower(m1A/A:0.015%-0.054%in cell lines and upto0.16%intissues)thanthatofm6A(m6A/A: 0.4%-0.6%)[2].m1A-seq identified 7154 m1A peaks covering 4151 coding and 63 non-coding genes in humans[2],whereas m1A-ID-seq detected 901 m1A peaks with high confidence in 600 human genes[3].Both studies show that most of the identified transcripts contain only one m1A peak.Unlike m6A peaks that are enriched in the last transcribed exon[6,7,23,24],m1A peaks are highly enriched within 5’UTR and near start codons.
According to the estimation of Dominissini and colleagues[2],~20%genes contain a single m1A.Through the deep analysis,they find that m1A is associated with canonical and alternative translation initiation sites,as well as the first splice site. Therefore they presume that the first spicing reaction might guide m1A deposition.m1A prefers more structured regions with high GC content and low minimum free energy.It is of note that m1A level and distribution pattern in mouse embryonic fibroblasts(MEFs)and mouse embryonic stem cells(mESCs)are comparable to those in human cell lines,suggesting an evolutionarily-conserved pattern of m1A methylome. They also survey the influence of different stress conditions on m1A,and find that the total level and peak number of m1A can be reduced by glucose starvation but enhanced by heat shock,indicating the dynamic feature of m1A under different physiological conditions.Given the close association of m1A with the translation initiation sites,Dominissini and colleagues examine whether m1A affects mRNA translation by using published ribosome profiling and proteomics data.Notably,m1A-containing genes have higher translation efficiency and protein levels compared to non-m1A-containing genes,implying that m1A modification is correlated with elevated translation.
Meanwhile,Li and colleagues[3]studied the m1A dynamics induced by H2O2treatment and serum starvation.They propose that m1A may reside in a prominent motif with a GA-rich consensus.Similar with the aforementioned Nature paper,they state that m1A prefers structured sequences with high GC content.It is notable that ALKBH3(human ortholog of E.coli AlkB)is found to be able to demethylate m1A in human mRNA,indicating that m1A is a reversible modification and may play an important regulatory role on mRNA.
Collectively,the two studies by Dominissini and his colleagues[2]and Li and his colleagues[3]provide the first map of transcriptome-wide m1A methylome and suggest new roles for m1A:this reversible modification is enriched around start codon,dynamically regulated by stress conditions,and correlated with elevated translation.Although the two m1A-seq techniques discussed here provide m1A maps with relatively-high resolution compared to m6A-seq method(at the resolution of~200 nucleotides),a big challenge is to develop single-base resolution methods for m6A and for m1A as well.Another challenge is to uncover the broader biological functions of m6A and m1A modifications. Future studies will focus on the identification and characterization of writer and reader proteins and functional roles of these two modifications.Given that m6A as an RNA structure switch affects RNA-protein interaction[17],the RNA structure changed by m1A modification might also play certain functions.We expect more investigations to draw a more comprehensive picture of RNA modification story.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
This work was supported by the National Basic Research Program of China(973 Program;Grant No.2014CB964900)and the National Natural Science Foundation of China(Grant Nos.21432002,21372022,and 21210003).
References
[1]Xiao W,Adhikari S,Dahal U,Chen YS,Hao YJ,Sun BF,et al. Nuclear m6A reader YTHDC1 regulates mRNA splicing.Mol Cell 2016;61:507-19.
[2]Dominissini D,Nachtergaele S,Moshitch-Moshkovitz S,Peer E,Kol N,Ben-Haim MS,et al.The dynamic N1-methyladenosine methylome in eukaryotic messenger RNA.Nature 2016;530: 441-6.
[3]Li X,Xiong X,Wang K,Wang L,Shu X,Ma S,et al. Transcriptome-wide mapping reveals reversible and dynamic N1-methyladenosine methylome.Nat Chem Biol 2016;12:311-6.
[4]Yue Y,Liu J,He C.RNA N6-methyladenosine methylation in post-transcriptionalgeneexpressionregulation.GenesDev 2015;29:1343-55.
[5]Jia G,Fu Y,Zhao X,Dai Q,Zheng G,Yang Y,et al.N6-Methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO.Nat Chem Biol 2011;7:885-7.
[6]Dominissini D,Moshitch-Moshkovitz S,Schwartz S,Salmon-Divon M,Ungar L,Osenberg S,et al.Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq.Nature 2012;485:201-6.
[7]Meyer KD,Saletore Y,Zumbo P,Elemento O,Mason CE,Jaffrey SR.Comprehensive analysis of mRNA methylation reveals enrichment in 3′UTRs and near stop codons.Cell 2012;149:1635-46.
[8]Zheng G,Dahl JA,Niu Y,Fedorcsak P,Huang C-M,Li CJ,et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility.Mol Cell 2013;49:18-29.
[9]Liu J,Yue Y,Han D,Wang X,Fu Y,Zhang L,et al.A METTL3-METTL14complexmediatesmammaliannuclear RNA N6-adenosine methylation.Nat Chem Biol 2014;10:93-5.
[10]Ping XL,Sun BF,Wang L,Xiao W,Yang X,Wang WJ,et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase.Cell Res 2014;24:177-89.
[11]Wang X,Lu Z,Gomez A,Hon GC,Yue Y,Han D,et al.N6-methyladenosine-dependent regulation of messenger RNA stability.Nature 2014;505:117-20.
[12]Wang X,Zhao BS,Roundtree IA,Lu Z,Han D,Ma H,et al.N6-Methyladenosine modulates messenger RNA translation efficiency.Cell 2015;161:1388-99.
[13]Alarco´n CR,Lee H,Goodarzi H,Halberg N,Tavazoie SF.N6-Methyladenosine marks primary microRNAs for processing. Nature 2015;519:482-5.
[14]Alarco´n CR,Goodarzi H,Lee H,Liu X,Tavazoie S,Tavazoie SF.HNRNPA2B1 is a mediator of m6A-dependent nuclear RNA processing events.Cell 2015;162:1299-308.
[15]Xu C,Wang X,Liu K,Roundtree IA,Tempel W,Li Y,et al. Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain.Nat Chem Biol 2014;10:927-9.
[16]Nayler O,Hartmann AM,Stamm S.The ER repeat protein YT521-B localizes to a novel subnuclear compartment.J Cell Biol 2000;150:949-62.
[17]Liu N,Dai Q,Zheng G,He C,Parisien M,Pan T.N6-Methyladenosine-dependent RNA structural switches regulate RNA-protein interactions.Nature 2015;518:560-4.
[18]Dunn D.The occurence of 1-methyladenine in ribonucleic acid. Biochim Biophys Acta 1961;46:198-200.
[19]Srivastava R,Gopinathan KP.Ribosomal-RNA methylation in Mycobacterium smegmatis SN2.Biochem Int 1987;15:1179-88.
[20]El Yacoubi B,Bailly M,de Cre´cy-Lagard V.Biosynthesis and function of posttranscriptional modifications of transfer RNAs. Annu Rev Genet 2012;46:69-95.
[21]Helm M,Brule´H,Degoul F,Cepanec C,Leroux J-P,Giege´R,et al.The presence of modified nucleotides is required for cloverleaf folding of a human mitochondrial tRNA.Nucleic Acids Res 1998;26:1636-43.
[22]Peifer C,Sharma S,Watzinger P,Lamberth S,Ko¨tter P,Entian KD.Yeast Rrp8p,a novel methyltransferase responsible for m1A 645basemodificationof25SrRNA.NucleicAcidsRes 2013;41:1151-63.
[23]Batista PJ,Molinie B,Wang J,Qu K,Zhang J,Li L,et al.m6A RNA modification controls cell fate transition in mammalian embryonic stem cells.Cell Stem Cell 2014;15:707-19.
[24]Ke S,Alemu EA,Mertens C,Gantman EC,Fak JJ,Mele A,et al. A majority of m6A residues are in the last exons,allowing the potential for 3′UTR regulation.Genes Dev 2015;29:2037-53.
1 April 2016;revised 27 May 2016;accepted 27 May 2016
*Corresponding author.
E-mail:guifangjia@pku.edu.cn(Jia G).aOCRID:0000-0001-6823-9040.bOCRID:0000-0002-4186-6922.
Peer review under responsibility of Beijing Institute of Genomics,Chinese Academy of Sciences and Genetics Society of China.
杂志排行
Genomics,Proteomics & Bioinformatics的其它文章
- Maintenance of Genome Stability
- Endogenous DNA Damage and Repair Enzymes—A short summary of the scientific achievements of Tomas Lindahl,Nobel Laureate in Chemistry 2015
- DNA Damage Response in Hematopoietic Stem Cell Ageing
- Connecting Malfunctioning Glial Cells and Brain Degenerative Disorders
- Topoisomerase I in Human Disease Pathogenesis and Treatments
