新型1-取代-3-(4-甲氧基苯基)脲衍生物的合成及其生物活性
2016-09-08严晓阳赵胜贤孔黎春郑绍成
严晓阳, 赵胜贤, 孔黎春, 郑绍成*
(1. 浙江师范大学 行知学院,浙江 金华 321004; 2. 浙江普洛得得邦制药有限公司,浙江 东阳 322118;3. 浙江师范大学 化学与生命科学学院,浙江 金华 321004)
·快递论文·
新型1-取代-3-(4-甲氧基苯基)脲衍生物的合成及其生物活性
严晓阳1, 赵胜贤2, 孔黎春3, 郑绍成1*
(1. 浙江师范大学 行知学院,浙江 金华321004; 2. 浙江普洛得得邦制药有限公司,浙江 东阳322118;3. 浙江师范大学 化学与生命科学学院,浙江 金华321004)
以对甲氧基苯胺和固体三光气为起始原料,制得4-甲氧基苯异氰酸酯(1); 1与取代胺(2a~2i)反应合成了9个新型的1-取代-3-(4-甲氧基苯基)脲衍生物(3a~3i),其结构经1H NMR,13C NMR和HR-MS表征。用黄瓜子叶扩张法和小麦芽鞘法研究了3a~3i的生物活性。结果表明:1-(4-甲氧基苯基)-3-[3-(三氟甲基)苯基]脲(3c)的生长素活性最好。在用药浓度为10 mg·L-1时,3c的生长素活性为29.8%,优于β-吲哚乙酸(29.3%)。
对甲氧基苯胺; 三光气; 4-甲氧基苯异氰酸酯; 脲衍生物; 合成; 生物活性
取代脲类化合物因其良好的细胞分裂素活性而备受关注[1]。部分化合物已经商品化,广泛应用于农业,如N-苯基-N′-(1,2,3-噻二唑-5-基)脲(TDZ),N,N′-二苯基脲(DPU)等。
1-取代-3-(4-甲氧基苯基)脲衍生物具有较好的植物生长调节活性,可促进细胞分裂、生长[2-3],

Scheme 1
在农业和林业领域有潜在的应用前景[4-6]。
本文以对甲氧基苯胺和固体三光气为起始原料,制得4-甲氧基苯异氰酸酯(1); 1与取代胺(2a~2i)反应合成了9个新型的1-取代-3-(4-甲氧基苯基)脲衍生物(3a~3i, Scheme 1),其结构经1H NMR,13C NMR和HR-MS表征。用黄瓜子叶扩张法和小麦芽鞘法研究了3a~3i的生物活性。
1 实验部分
1.1仪器与试剂
RY-2型熔点仪(温度未校正);Varian-Mercury 600型超导核磁共振仪(CDCl3为溶剂,TMS 为内标);Thermo Scientific Q Exactive型高分辨质谱仪。
所用试剂均为分析纯。
1.2合成
(1) 1的合成[7-8]
搅拌下,在干燥的反应瓶中依次加入三光气2.96 g(10 mmol)和二氯甲烷30 mL,于0~5 ℃缓慢滴加对甲氧基苯胺1.23 g(10 mmol)的二氯甲烷(20 mL)溶液,滴毕,滴加三乙胺3 mL,滴毕,于室温反应3~4 h(TLC检测)。过滤,滤液减压浓缩,残余物用硅胶柱层析[洗脱剂:V(石油醚) ∶V(乙酸乙酯)=10 ∶1]纯化得无色液体1 1.12 g,产率75.17%;1H NMRδ: 7.01(d,J=8.80 Hz, 2H), 6.83(d,J=8.80 Hz, 2H), 3.79(s, 3H)。
(2) 3a~3i的合成通法
搅拌下,在干燥的反应瓶中依次加入1 149 mg(10 mmol),甲苯10 mL和取代胺(2a~2i),于70~80 ℃反应8~10 h(TLC检测)。冷却至室温,过滤,滤饼用甲苯洗涤2次,于60 ℃真空干燥得白色固体3a~3i。
1-(4-甲氧基-2-甲基苯基)-3-(4-甲氧基苯基)脲(3a): 产率81.2%, m.p.220~222 ℃;1H NMRδ: 8.61(s, 1H), 7.67(s, 1H), 7.54(d,J=8.80 Hz, 1H), 7.27~7.44(m, 2H), 6.84~6.8(m, 2H), 6.78(d,J=2.93 Hz, 1H), 6.72(dd,J=2.93 Hz, 8.80 Hz, 1H), 3.72(s, 6H), 2.21(s, 3H);13C NMRδ: 155.7, 154.7, 153.8, 133.6, 131.2, 130.9, 124.4, 120.1, 115.9, 114.4, 111.7, 55.6, 55.6, 18.5; HR-MSm/z: Calcd for C16H18N2O3{[M+H]+}287.139 0, found 287.139 1; C16H18N2O3Na{[M+Na]+}390.121 0, found 390.121 0。
1-丁基-3-(4-甲氧基苯基)脲(3b): 产率84.2%, m.p.120~122 ℃;1H NMRδ: 8.15(s, 1H), 7.26 ~7.31(m, 2H), 6.75~6.86(m, 2H), 5.97(t,J=5.69 Hz, 1H), 3.69(s, 3H), 3.01~3.13(m, 2H), 1.37~1.43(m, 2H), 1.31(m,J=7.37 Hz, 2H), 0.89(t,J=7.34 Hz, 3H);13C NMRδ: 155.9, 154.3, 134.2, 119.8, 114.3, 55.6, 39.2, 32.4, 20.0, 14.1; HR-MSm/z: Calcd for C12H18N2O2{[M+H]+} 223.144 1, found 223.144 1; C12H18N2O2Na{[M+Na]+}245.126 0, found 245.126 2。
1-(4-甲氧基苯基)-3-[3-(三氟甲基)苯基)脲(3c): 产率81.9%, m.p.189~191 ℃;1H NMRδ: 8.95(s, 1H), 8.58(s, 1H), 8.01(s, 1H), 7.54~7.59(m, 1H), 7.46~7.52(m, 1H), 7.37(d,J=8.99 Hz, 2H), 7.29(d,J=7.70 Hz, 1H), 6.88(d,J=8.99 Hz, 2H), 3.73(s, 3H);13C NMRδ: 155.2, 153.2, 141.3, 132.8, 130.3, 130.0(q,J=30.8 Hz), 125.6(q,J=272.9 Hz), 122.1, 120.9, 118.3(q,J=3.3 Hz), 114.5, 55.6; HR-MSm/z: Calcd for C15H13N2O2F3{[M+H]+}311.100 2, found 311.100 0; Calcd for C15H13N2O2F3Na{[M+Na]+}333.082 1, found 333.082 0。
1-(4-甲氧基苯基)-3-甲苯基脲(3d): 产率86.8%, m.p.202~204 ℃;1H NMRδ: 8.82(s, 1H), 7.84(d,J=7.89 Hz, 1H), 7.81(s, 1H), 7.37(d,J=8.99 Hz, 2H), 7.17(d,J=7.34 Hz, 1H), 7.13(t,J=7.70 Hz, 1H), 6.93(dt,J=0.83 Hz, 7.38 Hz, 1H), 6.83~6.89(m, 2H), 3.72(s, 3H), 2.24(s, 3H);13C NMRδ: 154.8, 153.3, 138.1, 133.4, 130.6, 127.7, 126.6, 122.9, 121.3, 120.2, 114.5, 55.6, 18.3; HR-MSm/z: Calcd for C15H16N2O2{[M+H]+}257.128 5, found 257.128 6; Calcd for C15H16N2O2Na{[M+Na]+}279.110 4, found 279.110 6。
1-(2,4-二甲苯基)-3-(4-甲氧基苯基)脲(3e):产率82.6%, m.p.232~234 ℃;1H NMRδ: 8.73(s, 1H), 7.73(s, 1H), 7.66(d,J=8.07 Hz, 1H), 7.36(d,J=8.99 Hz, 2H), 6.98(s, 1H), 6.94(d,J=8.25 Hz, 1H), 6.84~6.88(m, 2H), 3.72(s, 3H), 2.23(s, 3H), 2.20(s, 3H);13C NMRδ: 154.8, 152.8, 135.4, 133.5, 131.8, 131.2, 128.1, 127.0, 121.8, 120.1, 114.5, 55.6, 20.8, 18.3; HR-MSm/z: Calcd for C16H18N2O2{[M+H]+}271.144 1, found 271.144 4; Calcd for C16H18N2O2Na{[M+Na]+}293.126 0, found 293.126 2。
1-(4-甲氧基苯基)-3-(嘧啶-2-基)脲(3f): 产率84.6%, m.p.245~247 ℃;1H NMRδ: 8.90(s, 3H), 8.80(s, 1H), 8.78(s, 1H), 7.37(d,J=8.62 Hz, 2H), 6.89(d,J=8.80 Hz, 2H), 3.73(s, 3H);13C NMRδ: 155.4, 153.0, 152.2, 146.7, 135.8, 132.5, 121.1, 114.5, 55.7; HR-MSm/z: Calcd for C12H12N4O2{[M+H]+}245.103 3, found 245.103 5; Calcd for C12H12N4O2{[M+Na]+}267.085 2, found 267.085 4。
1-(3,4-二甲氧基苯基)-3-(4-甲氧基苯基)脲 (3g) : 产率84.6%, m.p.184~186 ℃;1H NMRδ: 8.39(s, 1H), 8.36(s, 1H), 7.34(d,J=8.99 Hz, 2H), 7.20(s, 1H), 6.87(s, 1H), 6.86(d,J=0.73 Hz, 2H), 6.85(brs, 1H), 3.74(s, 3H), 3.72(s, 3H), 3.71(s, 3H);13C NMRδ: 154.8, 153.4, 149.3, 144.4, 134.0, 133.3, 120.5, 114.4, 113.0, 110.5, 104.4, 56.4, 55.8, 55.6; HR-MSm/z: Calcd for C16H18N2O4{[M+H]+}303.133 9, found 303.134 0; calcd for C16H18N2O4Na{[M+Na]+}325.115 9, found 325.115 8。
1-(4-氯-3-甲氧基苯基)-3-(4-甲氧基苯基)脲(3h): 产率85.9%, m.p.234~236 ℃;1H NMRδ: 8.74(s, 1H), 8.49(s, 1H), 7.42(d,J=2.38 Hz, 1H), 7.34~7.38(m, 2H), 7.27(d,J=8.62 Hz, 1H), 6.94(dd,J=2.20 Hz, 8.62 Hz, 1H), 6.86~6.89(m, 2H), 3.83(s, 3H), 3.72(s, 3H);13C NMRδ: 155.1, 155.0, 153.1, 140.7, 132.9, 130.1, 120.7, 114.5, 113.6, 111.2, 103.2, 56.2, 55.6; HR-MSm/z: Calcd for C15H15N2O3Cl{[M+H]+}307.084 4, found 307.084 1; Calcd for C16H18N2O4Na{[M+Na]+}329.066 3, found 329.066 3。
3-(3-(4-甲氧基苯基)脲)-4-甲基苯甲酸甲酯(3i): 产率82.6%, m.p.236~238 ℃;1H NMRδ: 9.08(s, 1H), 8.20(d,J=8.62 Hz, 1H), 8.08(s, 1H), 7.78(s, 1H), 7.76(dd,J=1.83 Hz, 8.62 Hz, 1H), 7.35~7.42(m, 2H), 6.90(d,J=8.99 Hz, 2H), 3.82(s, 3H), 3.73(s, 3H), 2.31(s, 3H);13C NMRδ: 166.5, 155.1, 152.7, 143.1, 132.9, 131.7, 128.3, 126.1, 122.8, 120.4, 118.8, 114.6, 55.6, 52.2, 18.3; HR-MSm/z: Calcd for C17H18N2O4{[M+H]+}315.133 9, found 315.133 9; Calcd for C17H18N2O4Na{[M+Na]+}337.115 9, found 337.115 8。
2 结果与讨论
2.1合成
1非常活泼,极容易与水反应,因此在合成1时应保持干燥。在1与2a~2i的加成反应中,如果2过量,后处理时分离纯化的难度较大。因此控制1过量,后处理时可直接用甲苯将其洗除。
2.2生物活性[9]
分别用黄瓜子叶法和小麦芽鞘法测定3a~3i的细胞分裂素活性[β-吲哚乙酸(IAA)为对照样]和生长素活性[激动素(KT)为对照样],结果见表1。
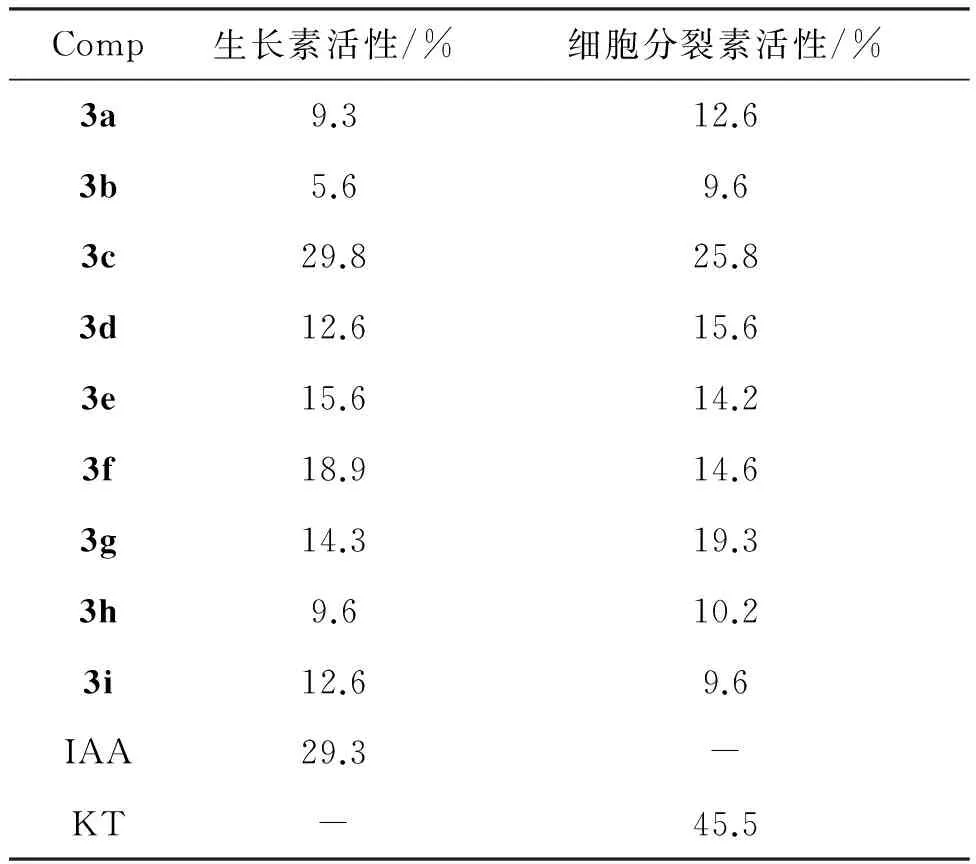
表1 3a~3i的生物活性
由表1可知,3a~3i均有一定的生物活性,其中3c生长素活性最好(29.8%),高于IAA(29.3%)。
合成了9个新型的1-取代-3-(4-甲氧基苯基)脲衍生物(3a~3i)。用黄瓜子叶扩张法和小麦芽鞘法研究了3a~3i的生物活性。结果表明:1-(4-甲氧基苯基)-3-[3-(三氟甲基)苯基]脲(3c)的生长素活性最好。在用药浓度为10 mg·L-1时,3c的生长素活性为29.8%,优于β-吲哚乙酸(29.3%)。
[1]Yonova P A, Stoilkova G M. Synthesis and biological activity of urea and thiourea derivatives from 2-aminoheterocyclic compounds[J].Journal of Plant Growth Regulation,2004,23(4):280-291.
[2]Nielsen A T, Norris W P. Gem-diferrocenylalkane derivatives:Acid-catalyzed condensation of ferrocene with methyl levulinate and 5-hydroxy-2-pentanone[J].The Journal of Organic Chemistry,1976,41(4):655-659.
[3]Keith J M, Apodaca R, Xiao W,etal. Thiadiazolopiperazinyl ureas as inhibitors of fatty acid amide hydrolase[J].Bioorganic & Medicinal Chemistry Letters,2008,18(17):4838-4843.
[4]Danci M, Cretu I, Velicevici G,etal. Influence of plant growth regulator uponinvitropropagation ofPaulowniasp[J].Journal of Biotechnology,2015,208:S113.
[5]Aggarwal G, Gaur A, Srivastava D K. Establishment of high frequency shoot regeneration system in Himalayan poplar(Populus ciliata Wall.ex Royle) from petiole explants using thidiazuron cytokinin as plant growth regulator[J].Journal of Forestry Research,2015,26(3):651-656.
[6]Carlson A S, Dole J M, Whipker B E. Plant growth regulator drenches suppress foliage and inflorescence height of ‘Leia’pineapple Lily[J].Horttechnology,2015,25(1):105-109.
[7]Yang L L, Li G B, Ma S,etal. Structure-activity relationship studies of pyrazolo[3,4-d]pyrimidine drivatives leading to the discovery of a novel multikinase inhibitor that potently inhibits FLT3 and VEGFR2 and evaluation of its activity againstacutemyeloidleukemiainvitroandinvivo[J].Journal of Medicinal Chemistry,2013,56(4):1641-1655.
[8]钱宇,田静,常霄巍,等. 新型N-取代苯基-9-烷基-3-咔唑磺酰脲类化合物的合成及其抗肿瘤活性[J].合成化学,2015,23(5):369-375.
[9]汪焱钢,赵新筠,龚银香.N-5-1H-1,2,4-三唑基-N-芳甲酰基脲的合成与生物活性[J].有机化学,2003,23(10):1165-1168.
Synthesis and Biological Activities of 1-Substituted-3-(4-methoxyphenyl)urea Derivatives
YAN Xiao-yang1,ZHAO Sheng-xian2,KONG Li-chun3,ZHENG Shao-cheng1
(1. Xingzhi College, Zhejiang Normal University, Jinhua 321004, China;2. Zhejiang Apeloa Tospo Pharmaceutical Co., Ltd., Dongyang 322118, China;3. College of Chemistry and Life Science, Zhejiang Normal University, Jinhua 321004, China)
1-Isocyanato-4-methoxybenzene(1) was prepared by the reaction of methoxyaniline with triphosgene. Nine novel 1-substituted-3-(4-methoxy-phenyl)urea derivatives(3a~3i) were synthesized by the reaction of 1 wtih substituted amines(2a~2i). The structures were characterized by1H NMR,13C NMR and HR-MS. The biological activities of 3a~3i were investigated by cucumber cotyledon expansion method and wheat sprout and sheath method. The results showed that 1-(4-methoxyphenyl)-3-[3-(trifluoromethyl)phenyl]urea(3c) exhibited best biological activities with auxin activity of 29.8% at dosage of 10 mg·L-1, better thanβ-indoleacetic acid(29.3%).
methoxyaniline; triphosgene; 1-isocyanato-4-methoxybenzene; urea derivative; synthesis; biological activity
2015-10-10;
2016-05-23
严晓阳(1976-),男,汉族,浙江东阳人,高级工程师,主要从事精细化工的研究。 E-mail: eastmorningsun@163.com
通信联系人: 郑绍成,教授级高级工程师, E-mail: jhzsc@zjnu.cn
O623.76; O625.63
A
10.15952/j.cnki.cjsc.1005-1511.2016.08.15346
