夹竹桃麻素治疗大鼠急性坏死性胰腺炎的疗效
2016-09-08何文华夏亮谢川祝荫刘丕朱勇曾皓朱萱吕农华
何文华 夏亮 谢川 祝荫 刘丕 朱勇 曾皓 朱萱 吕农华
夹竹桃麻素治疗大鼠急性坏死性胰腺炎的疗效
何文华夏亮谢川祝荫刘丕朱勇曾皓朱萱吕农华
目的观察NADPH氧化酶(NOX)抑制剂夹竹桃麻素治疗急性坏死性胰腺炎(ANP)大鼠的疗效,探讨其作用机制。方法60只SD大鼠按数字表法随机分为对照组、ANP组、夹竹桃麻素组。采用胰胆管逆行注射5%牛磺胆酸钠方法制备ANP模型。夹竹桃麻素组在制模同时腹腔注射夹竹桃麻素10 mg·kg-1·d-1。对照组仅开腹及关腹。术后12、24 h分批处死大鼠。观察24 h内各组大鼠存活率及呼吸、肾脏衰竭情况。取动脉血检测PaO2、淀粉酶和肌酐,采用ELISA法检测血TNF-α、IL-6水平,蛋白质印迹法检测胰腺组织NOX亚基NOX2、p22phox、p67phox及p-NF-κB p65表达。 结果ANP组大鼠24 h的急性呼吸衰竭发生率为71.4%(5/7),急性肾衰竭发生率为100%(7/7);夹竹桃麻素组发生率分别为22.2%(2/9)和0。两组差异均有统计学意义(P值均<0.01)。24 h时夹竹桃麻素组血PaO2显著高于ANP组[(68.4±6.8)mmHg比(56.5±6.1)mmHg,1 mmHg=0.133 kPa],血淀粉酶、肌酐、TNF-α、IL-6水平显著低于ANP组[(2 907±849)U/L比(6 421±690)U/L,(122.3±19.4)μmol/L比(213.0±39.2)μmol/L,(120.0±11.9)ng/L比(302.5±41.6)ng/L,(174.4±19.3)ng/L比(378.6±13.6)ng/L],胰腺病理评分显著低于ANP组[(3.16±0.91)分比(7.95±1.23)分],胰腺组织的p22phox、p67phox和p-NF-κB p65表达显著低于ANP组[0.79(0.75,0.84)比1.36(1.18,1.51),0.82(0.80,0.87)比1.70(1.47,1.80),0.82(0.80,0.84)比1.50(1.31.1.61)] ,NOX2表达完全被抑制[0比0.93(0.87,1.06)] ,差异均有统计学意义(P值均<0.01)。结论夹竹桃麻素对ANP大鼠有治疗作用,其机制可能是通过抑制NOX介导的NF-κB活化和TNF-α、IL-6释放实现的。
胰腺炎,急性坏死性;NADP氧化酶;夹竹桃麻素;治疗结果
Fund Program:Natural Science Youth Foundation of Jiangxi Province (20142BAB215010); Science and Technology Research Project of Education Department of Jiangxi Province(GJJ14019)
急性胰腺炎(AP)是世界范围内的常见疾病,发病率高,总体病死率达5%~10%[1-2]。最新的指南将AP的严重程度分为轻度AP(MAP)、中度AP(MSAP)和重度AP(SAP);根据病程分为早期(1周内)和后期(1周至数月)[2-3]。临床发现早期全身炎症反应综合征(SIRS)与SAP的发生密切相关[4-5],早期阻断 SIRS可望阻止病情进展。烟酰胺腺嘌呤二核苷酸磷酸(NADPH)氧化酶(NADPH oxidase,NOX)在胰腺炎的发病中起重要作用,它通过产生活性氧自由基(ROS)不仅直接引起氧化应激,参与胰蛋白酶的激活和胰腺自我消化,还介导促炎信号通路的激活[6-10]。但大多数研究是在细胞水平上进行的,体内研究较少。本研究以NOX抑制剂夹竹桃麻素干预急性坏死性胰腺炎(ANP)大鼠,观察其疗效,并探讨其机制。
材料与方法
一、实验材料与主要试剂
清洁级健康雄性SD大鼠60只,4~6周龄,体重160~200 g,由南昌大学实验动物中心提供,在标准环境下饲养,自由进食、进水,实验过程中对动物的处理严格遵循《实验动物管理条例》。 牛磺胆酸钠、夹竹桃麻素购自美国Sigma-Aldrich公司,抗NOX2、p22phox抗体购自英国Abcam公司,抗p67phox、p-NF-κB p65 (Ser536)抗体购自美国Cell Signaling Technology公司,大鼠细胞因子试剂盒(TNF-α、IL-6)为美国R&D 公司产品,免疫组化检测试剂盒(PV-6002)及DAB 试剂盒为北京中杉金桥生物技术有限公司产品。
二、方法
1.动物模型制备及分组:60只SD大鼠入室适应1周后按数字表法随机分为对照组、ANP组、夹竹桃麻素组,每组20只。采用逆行胰胆管内加压注射5%牛磺胆酸钠0.1 ml/100 g体重方法制备ANP模型[11];夹竹桃麻素组在制模同时腹腔注射夹竹桃麻素10 mg·kg-1·d-1;对照组仅行开、关腹手术。术后12、24 h分批处死大鼠。
2.观察大鼠存活及脏器衰竭情况:观察24 h内3组大鼠存活情况。采用改良Mashall标准判定3组大鼠呼吸衰竭和肾衰竭发生率。
3.血生物化学指标检测:腹部动脉取血8~10 ml,应用血气分析仪检测动脉血氧分压(PaO2)、全自动血生物化学分析仪检测血淀粉酶和肌酐水平,采用ELISA法检测血清TNF-α、IL-6水平。
4.胰腺组织病理学检查:取部分胰腺组织,置10%甲醛中固定过夜,常规石蜡包埋、切片,HE染色,光镜下观察胰腺组织病理学改变,参照Schmidt等[12]标准进行胰腺病理损伤程度评分。
5.胰腺组织NOX2、p22phox、p67phox和p-NF-κB p65蛋白表达检测:取液氮保存的胰腺组织,研磨成粉末后用蛋白提取液提取蛋白,常规行蛋白质印迹法检测NOX2、p22phox、p67phox和p-NF-κB p65蛋白表达,以GAPDH为内参。抗NOX2、p67phox、p22phox、p-NF-κB p65抗体工作浓度均为1∶1 000,HRP标记的羊抗兔或羊抗鼠二抗工作浓度1∶5 000。最后用SuperSignal West Femto敏感曝光试剂盒曝光底片,采用Gel-Proanalyzer 4软件分析蛋白条带灰度值,以目的条带与内参条带的灰度值比表示蛋白的相对表达量。
三、统计学处理
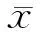
结 果
一、大鼠存活情况、脏器衰竭发生率及血PaO2、淀粉酶、肌酐水平
对照组大鼠全部存活, ANP组大鼠24 h内死亡3只,夹竹桃麻素组大鼠24 h内死亡1只,各组间差异无统计学意义(P=0.13)。ANP组24 h的急性呼吸衰竭发生率为71.4%(5/7),急性肾衰竭发生率为100%;夹竹桃麻素组发生率分别为22.2%(2/9)和0,均显著低于ANP组(P值分别为0.004、0.000)。
ANP组PaO2显著低于对照组,夹竹桃麻素组显著高于ANP组;ANP组血淀粉酶、肌酐水平均显著高于对照组,夹竹桃麻素组又显著低于ANP组。差异均有统计学意义(P值均<0.001,表1)。
二、大鼠胰腺组织病理改变
24 h时对照组大鼠胰腺组织结构清晰,细胞形态正常(图1A);ANP组胰腺的腺小叶排列紊乱,片状坏死,坏死区腺泡结构消失,有炎症细胞浸润(图1B);夹竹桃麻素组胰腺组织水肿,无明显坏死,腺泡细胞颗粒样或空泡变性,间质有炎症细胞浸润(图1C)。对照组、ANP组、夹竹桃麻素组胰腺组织病理评分分别为(0.18±0.23)、(7.95±1.23)和(3.16±0.91)分,组间差异均有统计学意义(P值均<0.01)。
三、大鼠血清TNF-α、IL-6水平
ANP组血清TNF-α、IL-6水平显著高于对照组,夹竹桃麻素组显著低于ANP组,但仍显著高于对照组,差异均有统计学意义(P值均<0.001,表2)。
四、胰腺组织p22phox、p67phox、NOX2和p-NF-κB p65蛋白表达
对照组胰腺组织NOX2未能检出,p22phox、p67phox和p-NF-κB p65低表达;ANP组胰腺组织NOX2、p22phox、p67phox和p-NF-κB p65表达均显著高于对照组;夹竹桃麻素组NOX2未能检出,p22phox、p67phox和p-NF-κB p65表达显著低于ANP组。3组间差异均有统计学意义(P值均<0.001,表3、图2)。

表1 各组大鼠动脉血氧分压、血清淀粉酶、血清肌酐的变化±s)
注:与对照组比较,aP<0.001;与ANP组比较,bP<0.001;1 mmHg=0.133 kPa

图1 对照组(1A)、ANP组(1B)、夹竹桃麻素组(1C)胰腺病理改变(HE ×200)

组别只数12hTNF-αIL-624hTNF-αIL-6对照组2070.8±8.2117.5±10.474.2±7.7125.2±9.6ANP组20321.8±25.4a332.7±27.7a302.5±41.6a378.6±13.6a夹竹桃麻素组20109.2±12.8b149.9±12.0b120.0±11.9b174.4±19.3bF值613.70388.20218.16662.34P值<0.001<0.001<0.001<0.001
注:与对照组比较,aP<0.001;与ANP组比较,bP<0.001
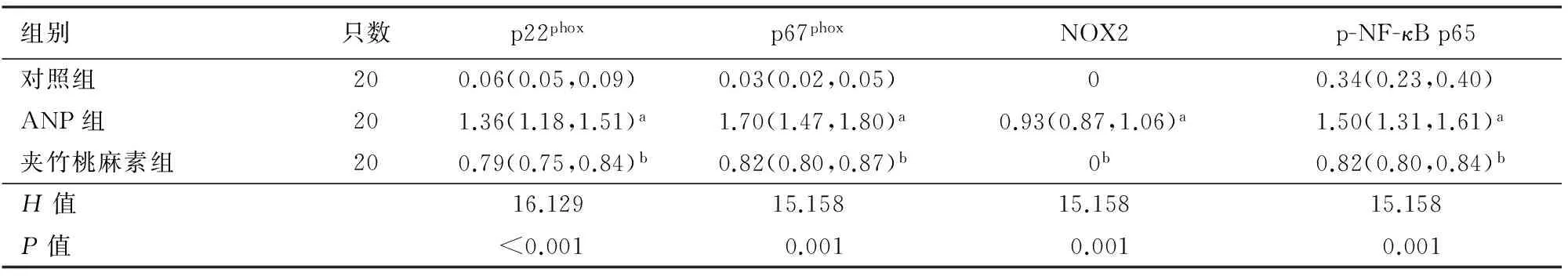
表3 各组胰腺组织NOX亚基和p-NF-κB p65表达[M(P25,P75)]
注:与对照组比较,aP<0.001;与ANP组比较,bP<0.001
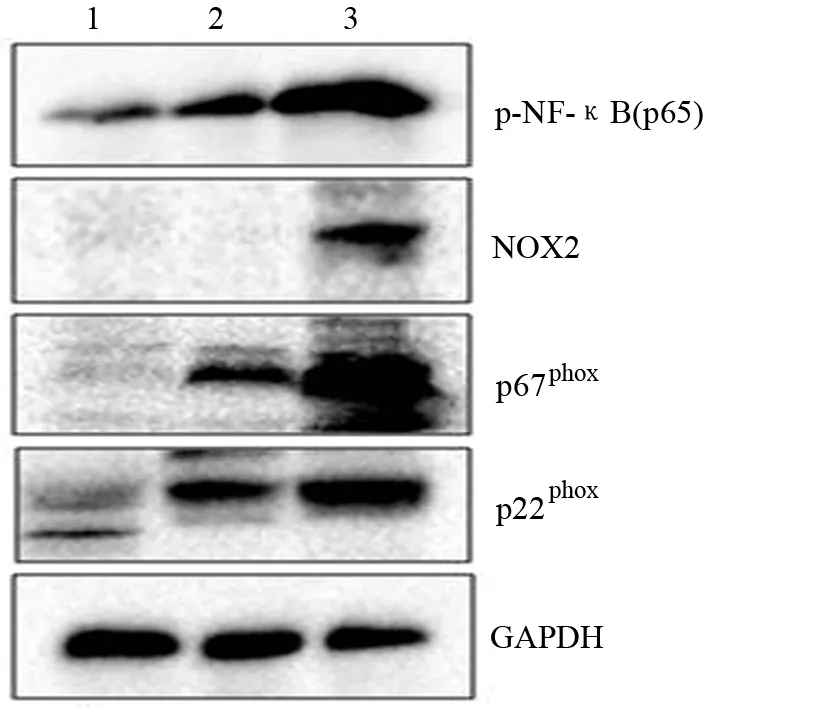
图2 对照组(1)、夹竹桃麻素组(2)、ANP组(3)胰腺组织p22phox、p67phox 、NOX2和p-NF-κB p65蛋白表达
讨 论
最近的研究发现胰腺腺泡细胞内早期触发且持续激活的炎症信号通路是AP发病的关键[13]。胰腺腺泡内胰蛋白酶原的激活虽然引起细胞损伤,但并不引起AP的炎症反应[14]。而胰腺腺泡细胞早期NF-κB被活化,它与胰蛋白酶原的激活是相互独立的事件[14-15]。 NF-κB的激活可引起局部损伤和全身炎症反应,导致SAP的发生[16]。
近年研究发现,NOX在AP发病中起重要作用。吞噬细胞型NOX由细胞膜上的催化亚基NOX2 (gp91phox)、调节亚基p22phox和胞质的调节亚基p47phox、p40phox、p67phox、Rac组成,目前发现有6种NOX2的同源蛋白(NOX1、NOX3、NOX4、NOX5、DUOX1和DUOX2),NOX是专职产生ROS的多蛋白复合体,它们产生的ROS介导炎症信号通路的激活[6,8-9],参与多种生理活动,也与疾病的发生密切相关[17-19]。有研究在胰腺腺泡细胞(AR42J)检测到NOX1的表达,给予雨蛙素可激活NOX产生ROS,引起NF-κB活化和IL-6表达,应用NOX抑制剂DPI可抑制NF-κB活化和IL-6表达[8]。另有研究发现,胰腺腺泡细胞NOX产生的ROS介导JAK2/STAT3和MAPKs信号通路的激活,DPI同样可抑制这些信号通路的活化[9]。
本研究结果显示,NOX抑制剂夹竹桃麻素干预ANP大鼠后,大鼠血淀粉酶、TNF-α、IL-6水平下降,胰腺组织损伤减轻,急性呼吸衰竭和急性肾衰竭的发生率降低。此外胰腺组织NOX2的表达完全被抑制,p22phox、p67phox、p-NF-κB p65表达也显著低于ANP大鼠,表明夹竹桃麻素对大鼠ANP有治疗作用,其作用机制可能是通过抑制NOX2的表达,进而抑制NF-κB的活化,减少炎症细胞因子TNF-α、IL-6的表达,阻断SIRS的发生,从而阻止器官功能损伤。
[1]Tenner S, Baillie J, Dewitt J, et al. American college of gastroenterology guidelines: management of acute pancreatitis[J]. Am J Gastroenterol, 2013, 108(9): 1400-1415. DOI: 10.1038/ajg.2013.218
[2]中华医学会消化病学分会胰腺疾病学组, 《中华胰腺病杂志》编辑委员会, 《中华消化杂志》编辑员会. 中国急性胰腺炎诊治指南(2013,上海)[J].中华胰腺病杂志, 2013, 13(2): 73-78. DOI:10.3760/cma.j.issn.1674-1935.2013.02.001.
[3]何文华, 吕农华. 亚特兰大急性胰腺炎分类国际共识2012年修订解读[J]. 中国实用内科杂志, 2013,33(9): 708-711.
[4]Singh VK, Wu BU, Bollen TL, et al. Early systemic inflammatory response syndrome is associated with severe acute pancreatitis[J]. Clin Gastroenterol Hepatol, 2009, 7(11): 1247-1251. DOI: 10.1016/j.cgh.2009.08.012.
[5]Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis-2012: revision of the Atlanta classification and definitions by international consensus[J]. Gut, 2013, 62(1): 102-111. DOI: 10.1136/gutjnl-2012-302779.
[6]Leung PS, Chan YC. Role of oxidative stress in pancreatic inflammation[J]. Antioxidants Redox Signal, 2009, 11(1): 135-165. DOI: 10.1089/ars.2008.2109.
[7]Gukovskaya AS, Vaquero E, Zaninovic V, et al. Neutrophils and NADPH oxidase mediate intrapancreatic trypsin activation in murine experimental acute pancreatitis[J]. Gastroenterology, 2002, 122(4): 974-984.
[8]Yu JH, Lim JW, Kim H, et al. NADPH oxidase mediates interleukin-6 expression in cerulein-stimulated pancreatic acinar cells[J]. Int J Biochem Cell Biol, 2005, 37(7): 1458-1469. DOI: 10.1016/j.biocel.2005.02.004.
[9]Ju KD, Lim JW, Kim KH, et al. Potential role of NADPH oxidase-mediated activation of Jak2/Stat3 and mitogen-activated protein kinases and expression of TGF-beta1 in the pathophysiology of acute pancreatitis[J]. Inflamm Res, 2011, 60(8): 791-800. DOI: 10.1007/s00011-011-0335-4.
[10]Wu Y, Lu J, Antony S, et al. Activation of TLR4 is required for the synergistic induction of dual oxidase 2 and dual oxidase A2 by IFN-gamma and lipopolysaccharide in human pancreatic cancer cell lines[J]. J Immunol, 2013, 190(4): 1859-1872. DOI: 10.4049/jimmunol.1201725.
[11] 夏亮, 陈江, 刘丕, 等. 蛋白酶激活受体-2在急性坏死性胰腺炎大鼠肠黏膜组织中的表达[J]. 中华消化杂志, 2012, 32(9): 598-601.Doi:10.3760/cma.j.issn.0254-1432.2012.09.008.
[12]Schmidt J, Lewandrowsi K, Warshaw AL, et al. Morphometric characteristics and homogeneity of a new model of acute pancreatitis in the rat[J]. Int J Pancreatol, 1992, 12(1): 41-51.
[13]Sah RP, Dawra RK, Saluja AK. New insights into the pathogenesis of pancreatitis[J]. Curr Opin Gastroenterol, 2013, 29(5): 523-530. DOI: 10.1097/MOG.0b013e328363e399.
[14]Dawra R, Sah RP, Dudeja V, et al. Intra-acinar trypsinogen activation mediates early stages of pancreatic injury but not inflammation in mice with acute pancreatitis[J]. Gastroen-terology, 2011, 141(6): 2210-2217 e2212. DOI: 10.1053/j.gastro.2011.08.033.
[15]Ji B, Gaiser S, Chen X, et al. Intracellular trypsin induces pancreatic acinar cell death but not NF-kappaB activation[J]. J Biol Chem, 2009, 284(26): 17488-17498. DOI: 10.1074/jbc.M109.005520.
[16]Huang H, Liu Y, Daniluk J, et al. Activation of nuclear factor-kappaB in acinar cells increases the severity of pancreatitis in mice[J]. Gastroenterology, 2013, 144(1): 202-210. DOI: 10.1053/j.gastro.2012.09.059.
[17]Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology[J]. Physiol Rev, 2007, 87(1): 245-313. DOI: 87/1/245 [pii]10.1152/physrev.00044.2005.
[18]Kleniewska P, Piechota A, Skibska B, et al. The NADPH oxidase family and its inhibitors[J]. Arch immunol Ther Exp(Warsz), 2012, 60(4): 277-294.DOI: 10.1007/s00005-012-0176-z.
[19]何文华, 朱萱. NADPH氧化酶产生的活性氧簇对肝星状细胞内信号转导的调控[J]. 世界华人消化杂志, 2008, 16(17): 1897-1903. DOI:10.3969/j.issn.1009-3079.2008.17.012.
(本文编辑:吕芳萍)
Therapeutic efficacy of apocynin on acute necrotizing pancreatitis rats
HeWenhua,XiaLiang,XieChuan,ZhuYin,LiuPi,ZhuYong,ZengHao,ZhuXuan,LyuNonghua.
DepartmentofGastroenterology,FirstAffiliatedHospitalofNanchangUniversity,Nanchang330006,China
Correspondingauthor:LyuNonghua,Email:lunonghua@163.com
ObjectiveTo explore the effect and mechanism of NADPH oxidase (NOX) inhibitor apocynin in treating acute necrotizing pancreatitis (ANP) rat. MethodsSixty SD rats were randomly divided into three groups: control group, ANP group and apocynin treated group. ANP rat model was established by retrograde injection of 5% sodium taurocholate into pancreatic duct. ANP rats in apocynin group were treated by intraperitoneal injection of 10 mg·kg-1·d-1apocynin. Rats in control group underwent sham surgery of opening and closing abdominal cavity. Rats were killed at 12 h and 24 h, respectively. Survival, respiration and renal failure were observed within 24 h. PaO2, amylase and creatinine in arterial blood specimen were detected. Serum TNF-α and IL-6 level was detected by enzyme-linked immunosorbent assay (ELISA). NOX2, p22phox, p67phoxand p-NF-κB p65 expression in pancreatic tissue were detected by Western blot. ResultsIn ANP group, the incidence of acute respiratory failure and acute renal failure was 71.42%(5/7) and 100% (7/7)at 24 h, respectively. The incidence of acute respiratory failure and acute renal failure was 22.2%(2/9) and 0(0/9) in apocynin treated group, and the difference was statistically significant (bothP<0.01). In Apocynin treated group, PaO2level was higher than that in ANP group at 24 h [(68.4±6.8)vs(56.5±6.1)mmHg, 1 mmHg=0.133 kPa]. Serum amylase, creatinine, TNF-α and IL-6 level was obviously lower than those in ANP group at 24 h [(2 907±849)vs(6 421±690)U/L,(122.3±19.4)vs(213.0±39.2)μmol/L,(120.0±11.9)vs(302.5±41.6)ng/L,(174.4±19.3)vs(378.6±13.6)ng/L. Pancreatic pathological score was significantly lower than that in ANP group [(3.16±0.91)vs(7.95±1.23); p22phox, p67phox, and p-NF-κB p65 expression in pancreatic tissue was also significantly lower than those in ANP model group[0.79(0.75, 0.84)vs1.36(1.18,1.51),0.82(0.80, 0.87)vs1.7(1.47,1.8), 0.82(0.80, 0.84)vs1.50(1.31, 1.61)]; NOX2 expression was completely inhibited by apocynin[0vs0.93(0.87,1.06)], which were statistically different (allP<0.001). ConclusionsApocynin could exert a therapeutic effect in ANP rats, and the potential mechanism may be associated with NOX mediated activation of NF-κB and TNF-α, IL-6 release.
Pancreatitis, acute necrotizing;NADP oxidase;Apocynin;Treatment outcome
10.3760/cma.j.issn.1674-1935.2016.04.009
330006南昌,南昌大学第一附属医院消化内科
吕农华,Email: lunonghua@163.com
江西省青年自然基金(20142BAB215010);江西省教育厅科学技术研究项目(GJJ14019)
2015-11-26)
