Fe2+活化过硫酸钾降解2,4,6-三氯苯酚的研究*
2016-09-02刘姜裔钟美娥
刘姜裔,钟美娥,高 权
(1 湖南农业大学东方科技学院,湖南 长沙 410128;2 湖南农业大学理学院,湖南 长沙 410128)
Fe2+活化过硫酸钾降解2,4,6-三氯苯酚的研究*
刘姜裔1,钟美娥2,高权2
(1 湖南农业大学东方科技学院,湖南长沙410128;2 湖南农业大学理学院,湖南长沙410128)
以Fe2+为活化剂,K2S2O8为氧化剂,对水中2,4,6-三氯苯酚进行降解处理。首先研究K2S2O8浓度和FeSO4浓度等因素对2,4,6-三氯苯酚降解的影响,发现在K2S2O8浓度为3.75 mmol/L和FeSO4浓度为1.25 mmol/L,即K2S2O8/Fe2+=75:25的条件下,2,4,6-三氯苯酚的降解率达到最大值,为91%。动力学研究表明,Fe2+活化K2S2O8降解2,4,6-三氯苯酚的过程可分为两个阶段,其中第一阶段反应速度较快,第二阶段为慢速反应,并且第二阶段符合一级反应动力学规律。
2,4,6-三氯苯酚;硫酸根自由基;过硫酸钾;Fe2+
2,4,6-三氯苯酚作为染料中间体、杀菌剂、防腐剂,在染料、农药和化工等领域被广泛使用[1-2]。其化学性质稳定,自然条件下很难降解,具有脂溶性,可以通过食物链富集。据报道,2,4,6-三氯苯酚对人类神经系统、呼吸系统有不良影响,会带来许多健康问题[3]。因此,处理含2,4,6-三氯苯酚的废水和修复被其污染的土壤已成为环境领域的研究热点。
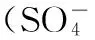
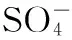
1 实 验
1.1主要试剂与仪器
试剂:2,4,6-三氯苯酚(2,4,6-TCP,江苏天容,96%),甲纯(色谱纯),过硫酸钾(K2S2O8,PDS)、七水合硫酸亚铁(FeSO4·7H2O)、冰乙酸均为分析纯。
仪器:HPLC高效液相色谱,Agilent1260);SPH-2102C恒温震荡器,上海世平实验设备有限公司;FA2400精密天平,上海民桥精密科学仪器有限公司。
1.2实验方法
向25 mL的比色管中依次加入一定浓度的2,4,6-三氯苯酚溶液和PDS溶液,最后加入一定量的FeSO4溶液开始反应并计时,实验中均未调节pH值。实验在150 r/min恒温振荡箱中进行,于25 ℃振荡反应,按照一定时间间隔取样1 mL,然后加入1 mL甲醇进行淬灭,摇匀后用高效液相色谱仪测试溶液中剩余的2,4,6-三氯苯酚,流动相为甲醇和水(1%冰乙酸)(V:V=8:2),检测波长290 nm,流动相速度为0.8 mL/min,进样量为20 μL。
2 结果与讨论
2.1Fe2+用量对2,4,6-三氯苯酚降解的影响
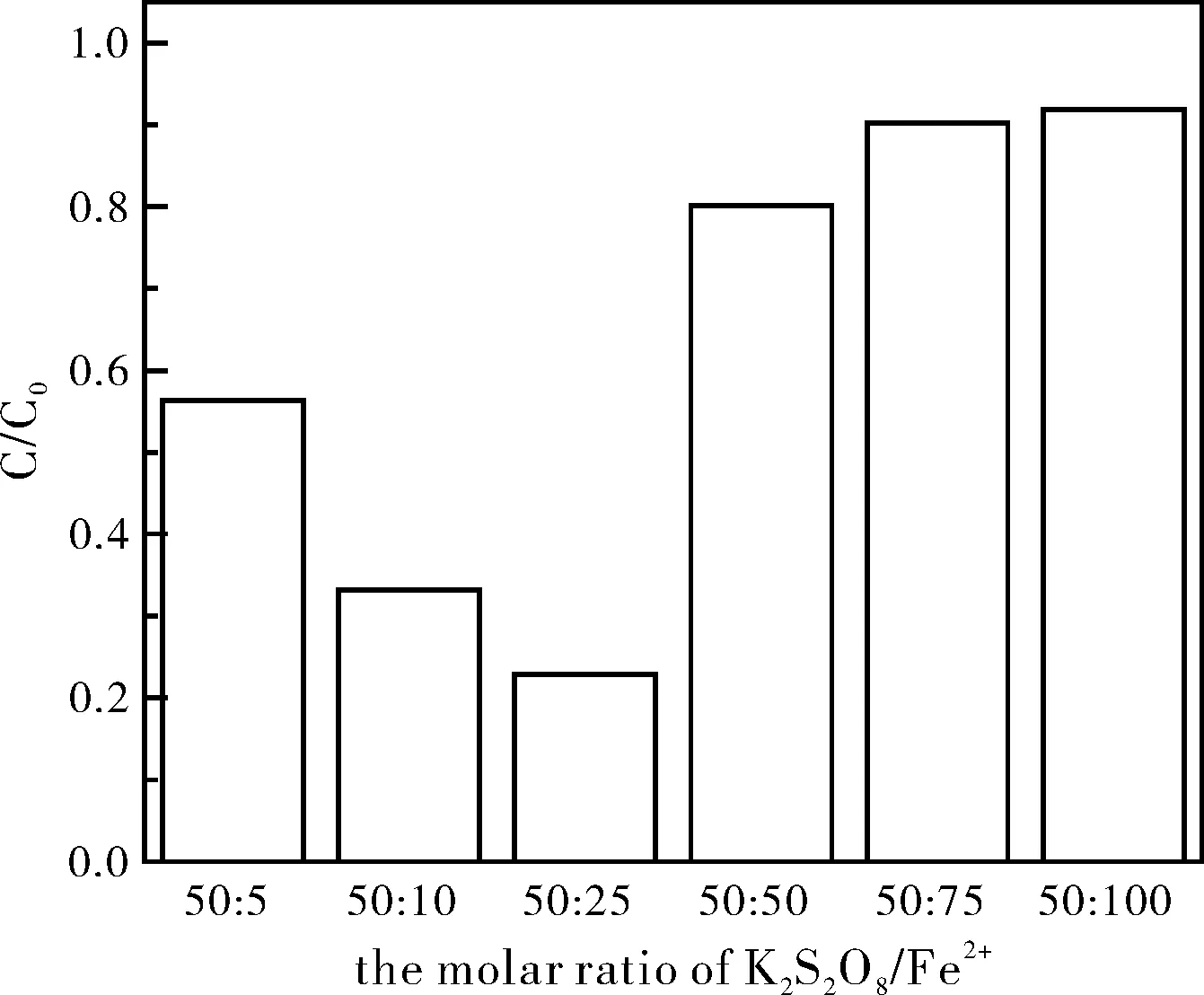
图1 Fe2+用量对2,4,6-三氯苯酚降解的影响
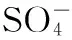
(1)
2.2K2S2O8用量对2,4,6-三氯苯酚降解的影响
氧化剂用量是影响污染物降解的重要因素,同时也是评估修复技术经济性的重要指标。使2,4,6-三氯苯酚初始浓度为0.05 mmol/L、FeSO4初始浓度为1.25 mmol/L,改变K2S2O8的用量,使溶液中K2S2O8/Fe2+的摩尔比为5:25、10:25、25:25、50:25、75:25、100:25、150:25,研究K2S2O8用量对2,4,6-三氯苯酚降解的影响,所得结果如图2所示。
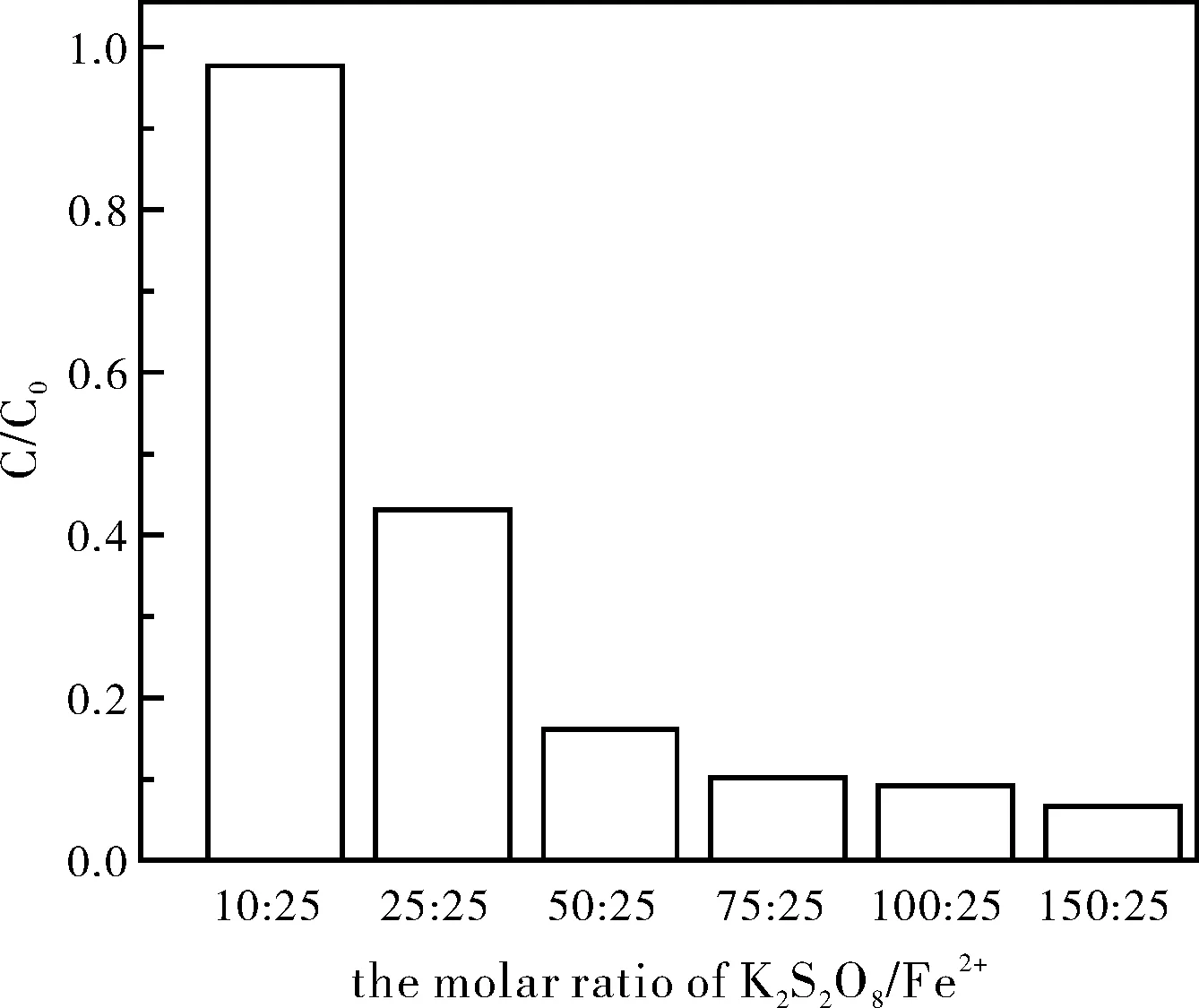
图2 K2S2O8用量对2,4,6-三氯苯酚降解的影响
由图2可知,随着K2S2O8用量的增加,2,4,6-三氯苯酚的降解率也随之升高,当K2S2O8/Fe2+的摩尔比为75:25时,反应24 h后,2,4,6-三氯苯酚的降解率达到了91%。当继续增加K2S2O8用量时,2,4,6-三氯苯酚的降解率增幅较小。表明过高浓度的K2S2O8并不能显著提高2,4,6-三氯苯酚的降解率。陈晓旸等[20]认为,K2S2O8浓度过高时,反应体系中产生大量的硫酸根自由基来不及消耗,过多的硫酸根自由基之间相互反应生成过硫酸盐,从而造成K2S2O8的利用率下降。
(2)
2.3K2S2O8/Fe2+体系中2,4,6-三氯苯酚降解的动力学过程
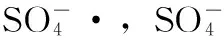
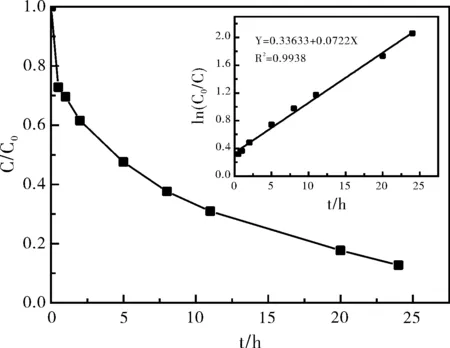
图3 K2S2O8/Fe2+体系中2,4,6-三氯苯酚降解动力学过程
3 结 论
(1)Fe2+活化K2S2O8能够在一定程度上降解2,4,6-三氯苯酚,适当提高Fe2+和K2S2O8浓度均能促进2,4,6-三氯苯酚的降解。
(2)FeSO4活化K2S2O8降解2,4,6-三氯苯酚的过程可分为两个阶段,其中第一阶段反应速度较快,第二阶段为慢速反应,并且第二阶段符合一级反应动力学规律。
[1]吴金钢, 戴友芝, 郭丽丽, 等. pH 值对“FeO-厌氧微生物”体系去除2,4,6-三氯酚过程的影响[J]. 环境工程学报, 2013, 7(4): 1273-1278.
[2]杨静, 崔世海, 练鸿振. 磁载光催化剂Fe3O4/C/TiO2的制备及对三氯苯酚的降解[J]. 无机化学学报, 2013, 29(10): 2043-2048.
[3]Farshid Ghanbaria, Mahsa Moradi, Fariba Gohari. Degradation of 2,4,6-trichlorophenol in aqueous solutions usingperoxymonosulfate/activated carbon/UV process via sulfate and hydroxyl radicals [J]. Journal of Water Process Engineering, 2016, 9: 22-28.
[4]A Go′mez-De Jesu′s, F J Romano-Baez, L Leyva-Amezcua, et al. Biodegradation of 2,4,6-trichlorophenol in a packed-bed biofilm reactor equipped with an internal net draft tube riser for aeration and liquid circulation Journal of Hazardous Materials[J]. 2009, 161: 1140-1149.
[5]Jeong-Hak Choi, Young-Hun Kim. Reduction of 2,4,6-trichlorophenol with zero-valent zinc and catalyzed zinc[J]. Journal of Hazardous Materials, 2009, 166: 984-991.
[6]Renchao Li, Xiaoying Jin, Mallavarapu Megharaj, et al. Heterogeneous Fenton oxidation of 2,4-dichlorophenol using iron-based nanoparticles and persulfate system[J]. Chemical Engineering Journal, 2015, 264: 587-594.
[7]Lei Xu, Ruixia Yuan, Yaoguang Guo, et al. Sulfate radical-induced degradation of 2,4,6-trichlorophenol: A de novo formation of chlorinated compounds[J]. Chemical Engineering Journal, 2013, 217: 169-173.
[8]Peidong Hu, Mingce Long. Cobalt-catalyzed sulfate radical-based advanced oxidation: a review on heterogeneous catalysts and applications [J]. Applied Catalysis B: Environmental, 2016, 181: 103-117.
[9]Minhui Xu, Xiaogang Gu, Shuguang Lu, et al. Degradation of carbon tetrachloride in aqueous solution in the thermally activated persulfate system [J]. Journal of Hazardous Materials, 2015, 286: 7-14.
[10]Jun Zhang, Xueting Shao, Chao Shi, et al. Decolorization of Acid Orange 7 with peroxymonosulfate oxidation catalyzed by granular activated carbon [J]. Chemical Engineering Journal, 2013, 232: 259-265.
[11]Y R Wang, W Chu. Photo-assisted degradation of 2,4,5-trichlorophenol by Electro-Fe(II)/Oxone process using a sacrificial iron anode: Performance optimization and reaction mechanism [J]. Chemical Engineering Journal, 2013, 215-216: 643-650.
[12]Danna Zhou, Long Chen, Changbo Zhang, et al. A novel photochemical system of ferrous sulfite complex: Kinetics and mechanisms of rapid decolorization of Acid Orange 7 in aqueous solutions [J]. water research, 2014, 57: 87-95.
[13]Yan Fan, Yuefei Jia, Deyang Kong, et al. Kinetic and mechanistic investigations of the degradation of sulfamethazine in heat-activated persulfate oxidation process [J]. Journal of Hazardous Materials, 2015, 300: 39-47.
[14]Xiaofang Xie, Yongqing Zhang, Weilin Huang, et al. Degradation kinetics and mechanism of aniline by heat-assisted persulfate oxidation [J]. Journal of Environmental Sciences, 2012, 24(5): 821-826.
[15]Yuefei Ji, Changxun Dong, Deyang Kong, et al. New insights into atrazine degradation by cobalt catalyzed peroxymonosulfate oxidation: Kinetics, reaction products and transformation mechanisms [J]. Journal of Hazardous Materials, 2015, 285: 491-500.
[16]Yingying Sha, Iswarya Mathewa, Qingzhou Cui, et al. Rapid degradation of azo dye methyl orange using hollow cobalt nanoparticles [J]. Chemosphere, 2016, 144: 1530-1535.
[17]Yuefei Ji, Changxun Dong, Deyang Kong, et al. New insights into atrazine degradation by cobalt catalyzed peroxymonosulfate oxidation: Kinetics, reaction products and transformation mechanisms [J]. Journal of Hazardous Materials, 2015, 285: 491-500.
[18]邓靖, 冯善方, 马晓雁, 等. 均相活化过硫酸氢盐高级氧化技术研究进展[J]. 水处理技术, 2015, 41(4): 13-19.
[19]王继鹏, 胡林潮, 杨彦, 等. Fe2+活化过硫酸钠降解1,2-二氯苯[J]. 环境工程学报, 2014, 8(9): 3767-3772.
[20]陈晓旸, 王卫平, 朱凤香, 等. UV/K2S2O8降解偶氮染料AO7 的研究: 动力学及反应途径[J]. 环境科学, 2010, 31(7): 1433-1537.
Study on Potassium Persulfate Activated by Fe2+for Degradation of 2,4,6-Trichlorophenol*
LIU Jiang-yi1, ZHONG Mei-e2, GAO Quan2
(1 College of Orient Science & Technology, Hunan Agricultural University, Hunan Changsha 410128;2CollegeofScience,HunanAgricultureUniversity,HunanChangsha410128,China)
To investigate the degradation of 2,4,6-trichlorophenol in water, the activating agent of Fe2+and the oxidant of K2S2O8were used. The effects of the concentrations of K2S2O8and FeSO4on the degradation of 2,4,6-trichlorophenol were studied. The optimal operating conditions were obtained as follows: 3.75 mmol/L of K2S2O8and 1.25 mmol/L of FeSO4, namely, the optimal molar ratio of oxidant K2S2O8to activating agent Fe2+was 75:25. Under these conditions, the degradation rate of 2,4,6-trichlorophenol was 91%. The degradation kinetics of 2,4,6-trichloro-phenol showed that the reaction included an initial fast stage and a final slow stage. The reaction was very fast at the first stage, and became slow at the second stage which followed a first-order kinetic.
2,4,6-trichlorophenol; sulfate radical; potassium persulfate; Fe2+
湖南农业大学东方科技学院大学生研究性学习和创新实验计划项目(No.DFCXY201324);湖南省教育厅科学研究项目(15C0653)。
钟美娥(1979-),女,博士,讲师,主要从事环境污染物的修复治理研究。
X703.1
A
1001-9677(2016)013-0073-03
