移植肾急性排斥血氧水平依赖性成像价值初步研究
2016-08-18黄海波黄桂雄刘旭阳管俊覃明李大创杨建均
黄海波,黄桂雄*,刘旭阳,管俊,覃明,李大创,杨建均
移植肾急性排斥血氧水平依赖性成像价值初步研究
黄海波1,黄桂雄1*,刘旭阳2,管俊1,覃明1,李大创1,杨建均3

目的 探讨血氧水平依赖功能磁共振成像(blood oxygen level dependent functional magnetic resonance imaging, BOLD-fMRI)移植肾急性排斥早期诊断价值。材料与方法 应用3.0 T BOLD-fMRI序列,扫描专用水模(含氯化锰盐酸混合液小瓶15只)及临床志愿者,临床志愿者扫描包括原位肾51例(A组)、正常移植肾34例(B组)和急性排斥移植肾15例(C组)。应用软件计算水模、肾皮质、髓质值,统计分析水模3次扫描间差异、肾皮质和肾髓质3组间差异、原位肾左右差异、3组肾皮质与髓质间差异。通过受试者工作特征曲线(receiver operating characteristic curve, ROC曲线)评价BOLD-fMRI成像急性排斥移植肾早期诊断效能及确定最佳阈值。结果 水模3次扫描间无统计学差异(P> 0.05);急性排斥肾髓质为(19.36±3.94) Hz,显著低于原位肾(29.73±2.92) Hz和正常移植肾(29.80±2.75) Hz,P<0.05,但A与B组髓质间及3组肾皮质间无统计学意义(P>0.05),以病理为标准,髓质=24.67 Hz为界值,BOLD-fMRI诊断急性排斥移植肾ROC曲线下面积为0.975,敏感性和特异度分别为86.7%和98.5%;原位肾左右侧无统计学意义(P>0.05);非急性排斥肾皮髓质间有统计学意义(P<0.05),髓质明显高于皮质,而急性排斥肾皮髓质间未显示统计学差异(P>0.05)。结论 BOLD-fMRI在肾移植急性排斥早期诊断中有重要价值。
肾移植;移植物排斥;血氧水平依赖;功能磁共振成像;诊断显像
1Department of Medical Imaging, 303rdHospital of PLA, Nanning 530021, China
2Department of Transplantation, 303rdHospital of PLA, Nanning 530021, China
3Department of Pathology, 303rdHospital of PLA, Nanning 530021, China
*Correspondence to: Huang GX, E-mail: 303hgx@163.com
ACKNOWLEDGMENTS This work was part of Guangxi scientific research and technology development project (No. GUIKEGONG1298003-8-6).
广西科学研究与技术开发计划项目(编号:桂科攻1298003-8-6)
黄海波, 黄桂雄, 刘旭阳, 等. 移植肾急性排斥血氧水平依赖性成像价值初步研究. 磁共振成像, 2016, 7(6): 443-448.
移植肾急性排斥指供肾携带的异体抗原所导致的受体内发生的免疫反应,细胞免疫类型为临床上最常见的急性排斥,常常发生在术后4天到2周,大量单核和淋巴细胞浸润为其病理组织学特征,但可通过大剂量激素冲击逆转大多数病例。急性排斥为术后肾损害最常见并发症,同时还是慢性排斥和肾功能丧失、影响患者生存及生活质量的重要因素[1],因此急性排斥早期评估具有重要临床意义。目前穿刺活检组织学是移植肾急性排斥诊断的金标准,然而穿刺活检本身为有创性检查,存在穿刺感染、出血、破裂及难于为受检者耐受等潜在危险及不足[2-3]。临床依靠血清肌酐、尿素氮水平亦难于满足早期、准确诊断肾急性排斥之目的,因为血清肌酐在发生组织明显损伤才可能升高[4]。故而长期以来,移植肾及相关医学研究一直希望找到一种高敏感和特异的技术,以实现急性排斥早期诊断,但这方面研究至今尚未取得突破及公认标准。笔者旨在通过血氧水平依赖功能磁共振成像(blood oxygen level dependent functional magnetic resonance imaging, BOLD-fMRI)序列扫描标准水模及原位肾、移植肾和急性排斥移植肾3组志愿者,应用受试者工作特征曲线(receiver operating characteristic curve, ROC曲线),探讨弛豫率成像移植肾急性排斥的早期诊断价值及最佳阈值。
1 材料与方法
1.2 临床志愿者
随机选取我院2012年4月至2014年10月申请扫描原位肾51例(A组),男35例、女16例,年龄18~55岁,平均(34.9±10.9)岁;正常移植肾34例(B组),男20例、女14例,年龄16~57岁,平均(35.9±11.4)岁;急性排斥移植肾15例(C组),男10例、女5例,年龄25~53岁,平均(35.5±5.6)岁,13例为T细胞免疫和2例体液免疫类型)纳入研究。入组标准:原位肾无临床症状,血肌酐、尿素氮指标阴性及超声未提示弥漫肾病;移植正常肾满足术后3个月至5年,余标准同原位肾;急性排斥移植肾为术后1~4周,出现低热、全身不适及尿量进行性减少,血肌酐>186.0 μmol/L、尿素氮>7.14 mmol/L,并经穿刺组织学活检符合2013年Banff会议移植肾急性排斥病理诊断标准[5]。剔除标准:原发或继发性血色病、严重伪影及已知改变肾氧合状态药物如襟利尿剂、乙酰唑胺、碘对比剂等近期使用。研究实验获我院伦理委员会批准,志愿者知情并签署同意书。
1.3 设备与方法
Philips 3.0 T MR扫描仪,水模静置磁体室2小时及应用SENSE HEAD 8 coils扫描,试验开始及每两个月重复水模扫描。肾扫描使用SENSE XL TORSO 16 coils配合呼吸门控、头先进仰卧位并以目标肾为中心定位,BOLD-fMRI序列:TR=200 ms,FA=20°,层数=1,水模序列TE(ms)=1.2/2.1/3.1/ 4.0/5.0/5.9/6.9/ 7.8/8.6/9.7/10.6/11.6,肾序列TE(ms)=9.2/13.2/ 17.2/21.2/25.2/29.2/33.2/37.2/ 41.2/45.2/49.2/53.2,且志愿者常规横断与冠状位T2WI及冠状位T1WI扫描并参考后者确定肾门,采用呼气末屏气扫描经肾门区冠状位序列,余
1.1 材料
专用标准水模(商品名:FerriScan,批号:FPP 20120406-B005),由澳大利亚Ferriscan公司提供,内含浓度0~3.2 mM氯化锰盐酸溶液小瓶15只。详细参数设置见表1,完成扫描保存原始数据。
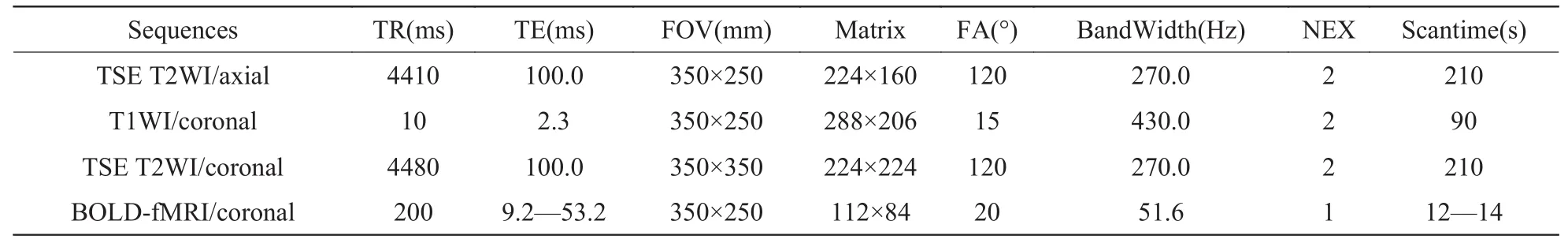
表1 肾脏扫描序列参数设置Tab. 1 Protocol parameters for renal coronal & transverse scanning
1.4 数据处理扫描DICOM数据,由受过良好培训的医师完成水模、肾皮质和髓质R∗2(1000/T∗2)测量,水模测量感兴趣区(region of interest, ROI)位于小瓶内,肾脏测量选择冠状位并参照T1WI、T2WI界定皮质、髓质,取5~10个适形ROI(10~20 mm2)且避开伪影、皮髓质重叠,取3次测量平均值。
1.5 统计学处理
2 结果
2.1 水模
水模小瓶Mncl2浓度为(1.41±0.95) mM,初始、1年及2年时刻扫描水模驰豫率R∗2分别为(183.09±118.63) Hz、(187.22±119.84) Hz、(188.66± 117.27) Hz,组间差异无统计学意义(F=0.451,P=0.769),见图1~3,提示主磁场高度稳定,这对确保不同时间扫描的临床志愿者间弛豫参数的比较有重要意义。
2.2 志愿者
扫描原位肾51例,102个肾脏中因左肾2个和右肾1个出现严重磁敏感性伪影被剔除外共99个原位肾纳入3组比较研究,总成功率约为98.0% (148/151),3组肾皮质和髓质驰豫率R∗2值见表2、3。

图1~3 水模初始(A)、1年(B)、2年(C)时刻扫描CMRtools处理图:3次扫描R∗2值分别为150.38 Hz、145.99 Hz、145.99 Hz,拟合曲线R2均超过0.99,由此可见,水模波动性均小于5%,重复扫描间无统计学差异(P=0.769),提示磁体稳定性相当好Fig. 1—3 The model images scanned at beginning (A), one (B) and two years (C) later respectively and processed by CMRtools:R∗2were 150.38 Hz, 145.99 Hz, 145.99 Hz for three time scanning respectively, all of R2>0.99. It follows that modelR∗2fluctuation was below 5% and no statistic difference was found forR∗2value among groups(P=0.769), it illustrated a high magnetic stability.
表2 原位肾左右侧皮髓质Tab. 2 Cortex and medulla of in-situ kidneys both left and right

表2 原位肾左右侧皮髓质Tab. 2 Cortex and medulla of in-situ kidneys both left and right
Medullary Left renal 49 17.54±0.67(14.98—19.82)29.56±2.67(24.91—41.19)Right renal50 17.71±0.75(15.59—19.26)29.90±3.17(22.34—38.26)t -0.213-0.568 P 0.7370.571 Variables n CorticalR∗2R∗2
表3 三组肾皮质和髓质(单位:Hz)Tab. 3 Renalon cortex and medulla among three groups (Hz)

表3 三组肾皮质和髓质(单位:Hz)Tab. 3 Renalon cortex and medulla among three groups (Hz)
Note: A: In-situ kidneys group; B: Transplanted renal group; C: Kidney with acute rejection group. ※: There were statistically significant between medulla and cortex both group A and B (P=0.000). ☆: There wasn’t statistically significant between medulla and cortex on group C(P=0.108).
Medullary A9917.65±0.85(14.98—19.82)29.73±2.92(22.34—41.19)B3417.84±0.99(16.44—20.67)29.80±2.75(25.77—40.15)※C1517.68±1.07(15.57—18.90)19.36±3.94(14.63—27.59)☆F 0.79380.903 P 0.4540.000 PC_A0.6670.000 PC_B0.2820.000 PA_B0.2800.916 Group N CorticalR∗2 R∗2
3组间性别、年龄无统计学差异(χ2性别= 0.218,F年龄=1.694,P性别/年龄=0.625/0.569),说明3组肾脏具有科学性与可比性。原位肾皮质、髓质左右侧R∗2值经两独立样本t检验无统计学差异(P>0.05),见表2。3组肾弛豫率值符合正态分布且方差齐同,经One-way ANOVA检验,髓质组间有统计学差异(P<0.05),两两比较发现急性排斥肾髓质明显低于原位肾、移植正常肾(P< 0.05),而原位肾、正常移植肾髓质间及肾皮质3组间均无统计学差异(P>0.05),见表3和图4~6。原位肾、正常移植肾皮质明显高于髓质,其间差异有统计学意义(t原位肾=-40.179,t正常移植肾=-38.235, P=0.000),急性排斥移植肾皮质与髓质值趋于一致,皮-髓质间比较尚不能认为有统计学差异(t=-1.701,P=0.108),见表3。
组织学穿刺活检15例急性排斥移植肾中,13例(86.7%)为T细胞免疫型,2例(13.3%)为体液免疫型,以病理为标准,髓质=24.67 Hz为最佳阈值,BOLD-fMRI诊断急性排斥肾ROC曲线下面积(AUC)为0.975,敏感性(Se)和特异度(Sp)分别为86.7%和98.5%(图6C和图7)。
3 讨论
BOLD-fMRI[6]是利用血液内源性对比剂脱氧血红蛋白、无创地评价组织氧代谢,反映血流动力学和病生理学的一种功能成像。原理为通过梯度多回波不同TE扫描,计算MRI信号与TE比值斜率,从而获得弛豫率或越低代表脱氧血红蛋白含量越少、氧分压越高。1936年Pauling首先提出脱氧血红蛋白具顺磁性效应,随后1990年Ogawa研究[7]发现脱氧血红蛋白顺磁性效应能够增加血管与周围组织磁敏感差异,高场磁共振能够反映由此产生的所谓血氧水平依赖增强效应。肾血流量约占25%心输量、皮髓质灌注比例约为9∶1,髓质电解质转运需大量耗氧,同时肾皮质和髓质氧分压(PaO2)分别约为50 mmHg和10~20 mmHg,即正常生理状态髓质处于低灌注、低氧分压及高耗氧环境[8],这种解剖与生理功能的特殊使肾脏成为BOLD-fMRI应用的基础及理想器官。1996年Prasad[9]首先进行肾脏实验以来,BOLD-fMRI健康肾和异常肾病[10-12]的应用逐渐成为研究热点并开启无创检测活体肾内氧含量、客观反映肾组织代谢、病生理状态的新时代。同时BOLD-fMRI信号与肾内氧含量关系亦通过氧敏感光纤探针测量得到证实[13]。
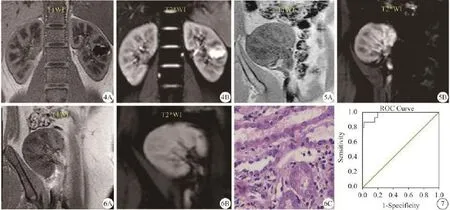
图4 原位肾T1WI(A)和T2*WI图(B):皮髓质分辨(CMD)对比清楚,左肾囊肿不影响评价 图5 移植正常肾:T1WI(A)CMD较原位肾降低,但T2*WI原始图(B)CMD仍对比清楚 图6 急性排斥肾:T1WI(A)CMD接近原位肾,但T2*WI原始图(B)CMD对比消失,组织学(HE ×40)证实T细胞免疫排斥(C)。提示T1WI不能准确反映肾功能异常,但代表评价肾氧合状态的T2*WI则可在急性排斥早期清楚反映这种变化 图7 ROC图:以髓质=24.67 Hz为最佳界值,BOLD-fMRI诊断急性排斥移植肾AUC为0.975,Se和Sp分别为86.7%和98.5%Fig. 4 T1WI(A) and T2*WI(B) from a kidney in situ: It showed clearly the corticomedullary differentiation(CMD), cyst in left renal didn't affect the measurement of. Fig. 5 A transplanted renal: It displayed clearly CMD on T2*WI(B), but it had a lower clearity on T1WI(A) compared with those of kidney in situ(Fig.4A).Fig. 6 A kidney with acute rejection: CMD on T2*WI was lost(B) and it was diagnosed as an cellular acute rejection by biopsy(C),however CMD on T1WI was close to kidney in situ (A). It followed that T1WI cound't reflect renal situation exactly, but T2*WI made it clear in early acute rejection which illustrates renal oxygenation. Fig. 7 ROC curve: With an area under the ROC curve of medullary=24.67 Hz as diagnose critical points, the sensibility was 86.7%, the specificity was 98.5%, and the accuracy was 0.975 in the prediction of kidneys with early acute rejection.
课题随机选择100名志愿者纳入3组试验,应用BOLD-fMRI肾扫描显示,术后1周至4周急性排斥肾移植15例中,髓质值(19.36±3.94) Hz远低于原位肾(29.73±2.92) Hz与移植正常肾(29.80±2.75)Hz,而原位肾与移植正常肾髓质间及3组间皮质无明显差异,说明急性排斥异常主要发生于肾髓质,与组织学对照,笔者认为原因可能是以下综合作用:(1)炎症反应、氧化应激、细胞因子释放使肾灌注下降,但因血流重新分布导致髓质含氧血红蛋白增加;(2)肾实质及微血管炎性损伤或肾小管细胞代谢率下降使氧的利用受损、含氧血红蛋白增多;(3)髓质氧耗减少比血流灌注下降程度更显著,导致髓质脱氧血红蛋白减少;(4)肾髓质PaO2约为10~20 mmHg,髓质氧含量轻微增加即可引起脱氧血红蛋白含量明显降低。而皮质差异不明显可能为皮质PaO2约50 mmHg处于解离曲线上段,氧含量轻微变化不会以髓质相似程度影响脱氧血红蛋白浓度,BOLD-fMRI尚难于显示其氧合状态小幅改变。以病理为标准,髓质= 24.67 Hz为界值,课题BOLD-fMRI诊断急性排斥肾AUC为0.975,Se与Sp分别为86.7%和98.5%,提示扫描对急性排斥诊断具有很高价值。课题结论与国内外研究报道[14-16]认为急性排斥肾髓质显著低于功能正常移植肾及原位肾,皮质之间则无明显差异基本一致,但不同机型、参数设置与场强结果不完全一致,Park等[15]应用3.0 T (20回波)研究显示移植肾急性排斥组(4例)髓质值(22.5±5.6) Hz明显低于功能正常组(8例)(31.8±3.4)Hz和原位正常肾(10例)(31.4±5.0) Hz,而皮质驰豫率(13.0±2.6、14.6±1.8、15.2±2.0)无明显统计学差异,其研究髓质变化与本试验接近,但皮质值则略小,分析原因可能与其入组病例数过少、参数设置及个体差异等有关。Sadowski[16]以1.5 T扫描功能正常与急性排斥移植肾,结果发现两组皮质平均值(分别为12.6 Hz和12.7 Hz)无显著差异,而髓质(分别为24.3 Hz和16.2 Hz)组间差异明显,这与本研究亦基本一致,定量测量值较小为场强不同所致,在3.0 T与1.5 T对比研究[17-18]中可见两者接近两倍关系,即3.0 T测量值约为1.5 T 的两倍[双肾皮、髓质值[17]:3.0 T为(18.73±1.59) Hz和(31.45±6.84) Hz,1.5 T为(11.20±0.81) Hz和(15.56±0.93) Hz]。
课题同时应用BOLD-fMRI序列于初始、1年及2年时刻水模扫描数据比较,目的在于评价磁场波动,从而反映志愿者数据可比性,结果发现标准水模3次扫描无统计学差异(P>0.05),这与龙玲莉等[18]研究基本一致,说明志愿者数据具有可比性。以标准水模监测磁体稳定性研究已有相关报道[19-20],但作为移植肾数据可靠性与科学性评估比较尚未见报道,这也是本课题创新之处,目的和意义在于确保一定时间内驰豫参数、以及不同设备或强场间的准确性和可比性。
笔者分析图像或处理原始数据发现,扫描总成功率约98.0%(148/151),A组中左肾2个、右肾1个因明显磁敏感伪影被剔除外其余148个肾脏均满足定量检测,A与B组肾脏无肿胀渗出,原始T2*WI图随TE延长髓质信号降低、产生明显BOLD效应;C组肾肿胀渗出,T2*WI图随TE延长皮髓质对比始终欠佳(图4B、5B)。A组T1WI肾皮髓质分辨(CMD)清晰(图4A),B与C组肾CMD有所下降但无明确规律,可见依赖T1WI定性并不十分可靠(图5A、6A)。受肠气影响T2*WI肾边缘可出现一定程度磁敏感伪影,可前后移动、改变匀场位置或尝试轴位采集解决。最后,随ROI位置变化及部分容积效应可发生一定测量偏差。
本研究尚存不足:(1)扫描仅一种机型完成,结论可能不完全适用不同型号或其它厂商、场强设备;(2)仅纳入术后1~4周排斥肾脏且病例数较少,结果可能有所偏倚;(3)未结合缺血、肾小管坏死等肾病探讨。
综上所述,BOLD-fMRI可基本实现移植肾急性排斥早期诊断,临床3.0 T MRI应用中,笔者推荐以髓质=24.67 Hz为阈值诊断移植肾急性排斥,可获得高准确率、特异性及较高敏感度。
致谢:感谢广西医科大学黄高明教授、我院信息中心蓝华分别提供统计学和论文图像处理指导!
[References]
[1]Womer KL, Kaplan B. Recent developments in kidney transplantation-a critical assessment. Am J Transplant, 2009,9(6): 1265-1271.
[2]Schwarz A, Gwinner W, Hiss M, et al. Safety and adequacy of renal transplant protocol biopsies. Am J Transplant, 2005, 5(8): 1992-1996.
[3]Masin-Spasovska J, Spasovski G, Dzikova S, et al. Do we have to treat subclinical rejections in early protocol renal allograft biopsies? Transplant Proc, 2007, 39(8): 2550-2553.
[4]Zhang JL, Rusinek H, Chandarana H, et al. Functional MRI of the kidneys. J Magn Reson Imaging, 2013, 37(2): 282-293.
[5]Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant, 2014,14(2): 272-283.
[6]Yang ZH, Feng F, Wang XY. A guide to technique of magnetic resonance imaging-criterion of examination, clinical strategy and application of new techniques. Beijing: People's military medical press, 2014: 303.
杨正汉, 冯逢, 王霄英. 磁共振成像技术指南-检查规范、临床策略及新技术应用. 北京: 人民军医出版社, 2014: 303.
[7]Ogawa S, Lee TM, Kay AR, et al. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA, 1990, 87(24): 9868-9872.
[8]Chou SY, Porush JG, Faubert PF. Renal medullary circulation: Hormonal control. Kidney Int, 1990, 37(1): 1-13.
[9]Prasad PV, Edelman RR, Epstein FH.Noninvasive evaluation of intrarenal oxygenation with BOLD MRI. Circulation, 1996,94(12): 3271-3275.
[10]Jiang ZX, Wang Y, Ding JL, et al. Assessment of renal injury in diabetic nephropathy using blood oxygenation level-depentent MRI. Chin J Magn Reson Imaging, 2015, 6(7): 524-528.
蒋振兴, 王毓, 丁玖乐, 等. 血氧水平依赖MRI评估糖尿病肾病肾功能损伤的研究. 磁共振成像, 2015, 6(7): 524-528.
[11]Ebrahimi B, Textor SC, Lerman LO. Renal relevant radiology: renal functional magnetic resonance imaging. Clin J Am Soc Nephrol, 2014, 9(2): 395-405.
[12]Zhang JL, Morrell GR, Lee VS. Blood oxygen level-dependent MR in renal disease: moving toward clinical utility. Radiology,2013, 268(3): 619-621.
[13]Neugarten J. Renal BOLD-MRI and assessment for renal hypoxia. Kidney Int, 2012, 81(7): 613-614.
[14]Prasad PV. Functional MRI of the kidney: tools for translational studies of pathophysiology of renal disease. Am J Physiol Renal Physiol, 2006, 290(5): 958-974.
[15]Park SY, Kim CK, Park BK, et al. Evaluation of transplanted kidneys using blood oxygenation level-dependent MRI at 3.0 T: a preliminary study. AJR, 2012, 198(5): 1108-1114.
[16]Sadowski EA, Djamali A, Wentland AL, et al. Blood oxygenlevel -dependent and perfusion magnetic resonance imaging: detecting differences in oxygen bioavailability and blood flow in transplanted kidneys. Magn Reson Imaging, 2010, 28(1): 56-64.
[17]Liu JH, Liu AL, Ning DX, et al. Blood oxygen level dependent MRI in kidney of healthy volunteers: comparison between 1.5 T and 3.0 T MRI. BME & Clin Med, 2011, 15(3): 251-253.
刘静红, 刘爱连, 宁殿秀, 等. 正常志愿者肾脏血氧水平依赖MRI-1.5 T与3.0 T MRI对比. 生物医学工程与临床, 2011,15(3): 251-253.
[18]Tumkur S, Vu A, Li L, et al. Evaluation of intrarenal oxygenation at 3.0 T using 3-dimensional multiple gradientrecalled echo sequence. Invest Radiol, 2006, 41(2): 181-184.
[19]Long LL, Peng P, Huang ZK, et al. Liver iron quantification by 3.0 T MRI: calibration on a rabbit model. Chin J Magn Reson Imaging, 2012, 3(6): 451-455.
龙莉玲, 彭鹏, 黄仲奎, 等. 铁超负荷兔模型3.0 T MRI定量肝铁沉积可行性研究. 磁共振成像, 2012, 3(6): 451-455.
[20]Huang HB, Zhou YL, Huang GX, et al. Feasibility of multipleecho GRE with parameters optimized protocol at 3.0 T MRI. Chin J Magn Reson Imaging, 2015, 6(7): 529-534.
黄海波, 周亚丽, 黄桂雄, 等.前瞻性3.0 T MRI梯度多回波序列参数优化可行性研究. 磁共振成像, 2015, 6(7): 529-534.
Value of BOLD-fMRI to transplanted kidneys with acute rejection: a preliminary study
HUANG Hai-bo1, HUANG Gui-xiong1*, LIU Xu-yang2, GUAN Jun1, QIN Ming1,LI Da-chuang1, YANG Jian-jun3
14 Nov 2015, Accepted 25 Dec 2015
Objective: To explore the value of BOLD-fMRI to early diagnose transplanted kidneys with acute rejection. Materials and Methods: Study protocol was approved by local ethics committee; informed consent was obtained. A MR special model which included fifteen vials containing 0-3.2 mM manganese chloride in hydrochloric acid solution, and a total of 100 velunteers were enrolled and divided into three groups, as follows: Group A, 51 cases with healthy kidneys in situ; group B,34 transplantation with stable renal function for at least 3 months after operating; and group C, 15 iliac renal allografts with early acute rejection from 1 week to 4 weeks after operating. T2W axial/coronal, T1W coronal and a coronal fat-saturated multiecho GRE with 12 echos (9.2-53.2 ms) were performed on a 3.0 T scanner during normal breathing or breath-holding. CMR tools was used to calculate the value ofin MR model vias, renal cortex, medulla respectively after MRI. Receiver operating characteristic (ROC) curve was used to predict the kidneys with early acute rejection andthresholdvalue were identified to discriminate between transplanted kidneys with acute rejection, those with normal function, and healthy native renals. Results: No statistical significances were found forvalues among repeated scanning on phantom(P>0.05). The value of(Hz) on renal medulla(19.36±3.94) with acute rejection was significantly lower than those of medulla both in group A(29.73±2.92) and B(29.80±2.75) (P<0.05), however no statistical significances were found between group A and B(P>0.05), and foron renal cortex among three groups(P>0.05). The value ofon medulla was higher than those on cortex both group A and B, moreover no statistical significance was found forbetween left and right kidney in situ(P>0.05). With a medullary =24.67 Hz as diagnose critical points compared to bilpsy, the sensibility was 86.7%, the specificity was 98.5%, and the accuracy was 0.975 in the prediction of kidneys with early acute rejection. Conclusion: BOLD-fMRI is of important value in the diagnosis of renals with early-stage acute rejection.
Kidney transplantation; Graft rejection; Blood oxygen level dependent; Magnetic resonance imaging, functional;Diagnostic imaging
1. 解放军第303医院医学影像科,南宁 530021
2. 解放军第303医院移植科,南宁530021
3. 解放军第303医院病理科,南宁530021
黄桂雄,E-mail: 303hgx@163.com
2015-11-14接受日期:2015-12-25
R445.2;R617
A
10.12015/issn.1674-8034.2016.06.009
