断肢再生对中华绒螯蟹蜕壳、生长及相关基因表达的影响
2016-08-15岳武成陈娇慈元吉黄姝王军王成辉
岳武成, 陈娇, 慈元吉, 黄姝, 王军, 王成辉
(上海海洋大学农业部淡水水产种质资源重点实验室,上海 201306)

断肢再生对中华绒螯蟹蜕壳、生长及相关基因表达的影响
岳武成, 陈娇, 慈元吉, 黄姝, 王军, 王成辉*
(上海海洋大学农业部淡水水产种质资源重点实验室,上海 201306)
以中华绒螯蟹(Eriocheirsinensis)幼蟹为材料,研究人工压力法切断其4个步足(左侧第1、3步足和右侧第2、4步足)后的蜕壳、生长与相关基因的表达规律。结果表明:断肢蟹当期蜕壳的步足再生率为7.6%,第2次蜕壳后的再生率为91.6%;断肢蟹与正常蟹的成活率相当;断肢蟹在断肢当期的蜕壳周期显著长于正常蟹(P<0.01),但蜕壳后增质量率显著高于正常蟹(P<0.05),而第2次的蜕壳周期显著短于正常蟹(P<0.01),但蜕壳后增质量率与正常蟹差异无统计学意义(P>0.05);经2次蜕壳后,断肢蟹与正常蟹的整体蜕壳周期差异无统计学意义(P>0.05)。相关基因表达差异分析表明:断肢蟹与正常蟹在胰岛素样生长因子2基因IGF2、维甲类X受体基因RXR、蜕皮抑制激素基因MIH的表达上差异无统计学意义(P>0.05);但断肢蟹的蜕皮激素受体基因EcR的表达量显著高于正常蟹(P<0.05),而肌肉生长抑制素基因MSTN的表达量却显著低于正常蟹(P<0.05)。综上表明:中华绒螯蟹幼蟹发生断肢后能在2个蜕壳周期内完成再生,且与正常蟹在成活率、蜕壳周期和蜕壳后增质量率上差异无统计学意义;EcR和MSTN基因对促进中华绒螯蟹再生肢体的生长发育有重要作用;断肢蟹可在生产上继续使用。
中华绒螯蟹; 断肢再生; 增质量率; 蜕壳周期; 基因表达
Summary Limb regeneration is a regrowth phenomenon when limbs of animals are truncated by external forces or self-cut in response to predation, which is a self-protection mechanism formed during the long-term evolution process. Currently, amphibians with tails, and crustaceans belonging to arthropods were intensively studied. The results indicated that the feeding ability, growth and development were greatly affected by regeneration. Molting could be accelerated and the ovary development could be promoted by limb regeneration, inducing expression of the related genes. Chinese mitten crab (Eriocheirsinensis) has the ability of limb regeneration like other crustaceans in its life cycle. However, the molecular mechanism of regeneration forE.sinensiswas still unclear. Therefore, study on limb regeneration ofE.sinensiscan supplement basic biological knowledge for Chinese mitten crab, and provide practical guidance for development of aquaculture industries.
A total of 80 juvenile Chinese mitten crabs (40 males and 40 females) were randomly sampled to autotomy treatments with four walking legs (the first and third legs on the left, the second and fourth legs on the right), and the same number of male and female individuals were employed as the control group without any treatment (intact crab). They were separately reared in forty tanks with each of 60 L for two molt cycle, and two crabs with cut limbs (one male, one female) and two intact crabs (one male, one female) were kept in each tank. Body mass, carapace length and carapace width were measured and recorded at the initial stocking stage, and 48 h after the first and second molting, respectively. Meanwhile, the eyes and muscle tissues (regeneration limbs and intact limbs) were collected quickly after the second molt and were stored at -80 ℃ for quantitative real-time polymerase chain reaction (qRT-PCR) analysis of myostatin geneMSTN, insulin-like growth factor-2 geneIGF2, molting hormone receptor geneEcR, retinoid X receptor geneRXR, and molt-inhibiting hormone geneMIH.
The results showed that limb regeneration rate was only 7.6% after the first molt, but reached 91.6% after the second molt; the survival rates of the amputated crabs and intact crabs were roughly equal. Longer inter-molting days (P<0.05) were observed for the amputated crabs than intact crabs, and the body mass-gaining rate was higher than intact crabs (P<0.05) after the first molting. Shorter inter-molting days (P<0.05) were observed for amputated crabs than intact crabs, and no significant difference (P>0.05) was observed for the body mass-gaining rate of the amputated and intact crabs after the second molting. Overall, there was no significant difference of average inter-molting days between the amputated and intact crabs after the two molting. The related gene expression analysis indicated that there were no significant difference (P>0.05) between the amputated and intact crabs inIGF2,RXRandMIHgenes. However, there were significant higher expression (P<0.05) ofEcRgene and lower expression ofMSTNgene for amputated crabs (P<0.05) than the intact crabs.
In conclusion, Chinese mitten crab can regenerate new legs in two molt cycles; there are no significant differences on the survival rate, mass-gaining rate and molt cycles between the amputated and intact crabs. TheEcRandMSTNgenes play important roles in promoting the leg regeneration, and the amputated crab can be kept for further aquaculture.
断肢再生是动物肢体在受到外力截断或在逃避敌害时发生自切后重新长出的现象,是动物在长期进化过程中形成的自我保护机制[1]。断肢再生一直是再生科学研究的重要课题。目前,断肢再生研究较多的物种为有尾两栖类、节肢动物门以及其下属的甲壳类。基础生物学研究表明,断肢再生过程对动物的摄食能力、生长、发育等有较大影响[2-4]。动物发生断肢后机体处于高应激状态,其社会行为会发生巨大改变,机体的生长发育会受到较大影响。对节肢动物门蜘蛛类[5]和蟹类[6]的断肢再生研究表明,机体在发生断肢后,生长减缓,发育时间(蜕皮或蜕皮周期)延长,用以机体修复。断肢再生还会加速甲壳类蜕壳,促进卵巢发育,诱导相关基因表达(如蜕皮激素受体基因EcR的高表达)[6-7]。一些研究还发现,动物肢体或肌肉再生时,肌肉生长抑制基因MSTN会表达下调,进而使再生肢芽呈现出爆发式的生长现象[8-10]。由此可见,断肢再生过程和动物的生长发育是相互作用的。
目前,国际上对锯缘青蟹(Scyllaserrata)、陆地蟹(Gecarcinuslateralis)、拟穴青蟹(Scyllaparamamosain)等相关蟹类的断肢再生开展了研究,发现断肢再生受到激素、温度、光周期以及动物发育程度等多种因素的调控[6,11],而相关分子机制研究也揭示了断肢再生、蜕壳与生长相关基因(MIH、EcR、RXR)的关系[6-7,12]。中华绒螯蟹(Eriocheirsinensis)与其他蟹类一样都具有较强的断肢再生能力,而目前尚无中华绒螯蟹断肢再生研究的相关报道。在生产中,许多断肢蟹特别是在蟹种放养选择时常被抛弃,从而造成种质浪费和经济损失。开展中华绒螯蟹断肢与再生研究,不仅对丰富中华绒螯蟹基础生物学研究具有重要意义,而且对其产业发展也具有重要的实践指导作用。本文以中华绒螯蟹幼蟹为试验材料,通过人工压力迫使幼蟹断肢来研究其肢体再生现象,探讨中华绒螯蟹断肢后对其生长和相关基因表达的影响,了解新肢体再生所需的时间或蜕壳周期。
1 材料与方法
1.1试验材料与饲养管理
取本实验室中华绒螯蟹配套选育系A的同一家系Ⅴ期仔蟹160只,随机分为2组:断肢组(试验组)和正常组(对照组),每组雌雄各40只。断肢前测量试验蟹体质量、壳长、壳宽等性状指标,然后对断肢组采用压力法迫使其自切掉左侧第1、3步足和右侧第2、4步足,共4个步足。将断肢组与对照组分别放养在40个容积为60 L的水族箱中,每个水族箱放养4只中华绒螯蟹(断肢组和正常组雌雄各1只)。在水族箱内种植伊乐藻(Elodeanuttallii),每天早晚各投喂1次饲料,每3天换水1次,试验期间保持各个水族箱的养殖环境基本一致。
1.2生长测定与组织采集
试验期间,每天观察中华绒螯蟹的蜕壳情况,记录蜕壳时间。蜕壳2 d(48 h)后测量中华绒螯蟹的体质量、壳长、壳宽3个生长性状。试验时间为2个蜕壳周期,即供试中华绒螯蟹在水族箱内完成2次蜕壳后结束。
当全部供试中华绒螯蟹个体完成第2次蜕壳并测定其生长性状后,立即采集每只中华绒螯蟹个体的眼柄和肌肉组织,经液氮速冻后保存于-80 ℃冰箱中,备用。
1.3相关基因表达分析
随机挑选断肢组和正常组各5只雄蟹,用RNA提取试剂盒(美国Axygen公司)提取其眼柄、正常蟹步足肌肉、断肢蟹再生步足肌肉的总RNA。使用大连宝生物公司的反转录试剂盒(PrimeScriptTMRT-PCR Kit)进行反转录。根据本实验室已有的肌肉生长抑制素基因MSTN、蜕皮激素受体基因EcR、维甲类X受体基因RXR、蜕皮抑制激素基因MIH、胰岛素样生长因子2基因IGF2的定量实时聚合酶链式反应(quantitative real-time polymerase chain reaction, qRT-PCR)引物(表1)进行PCR扩增。
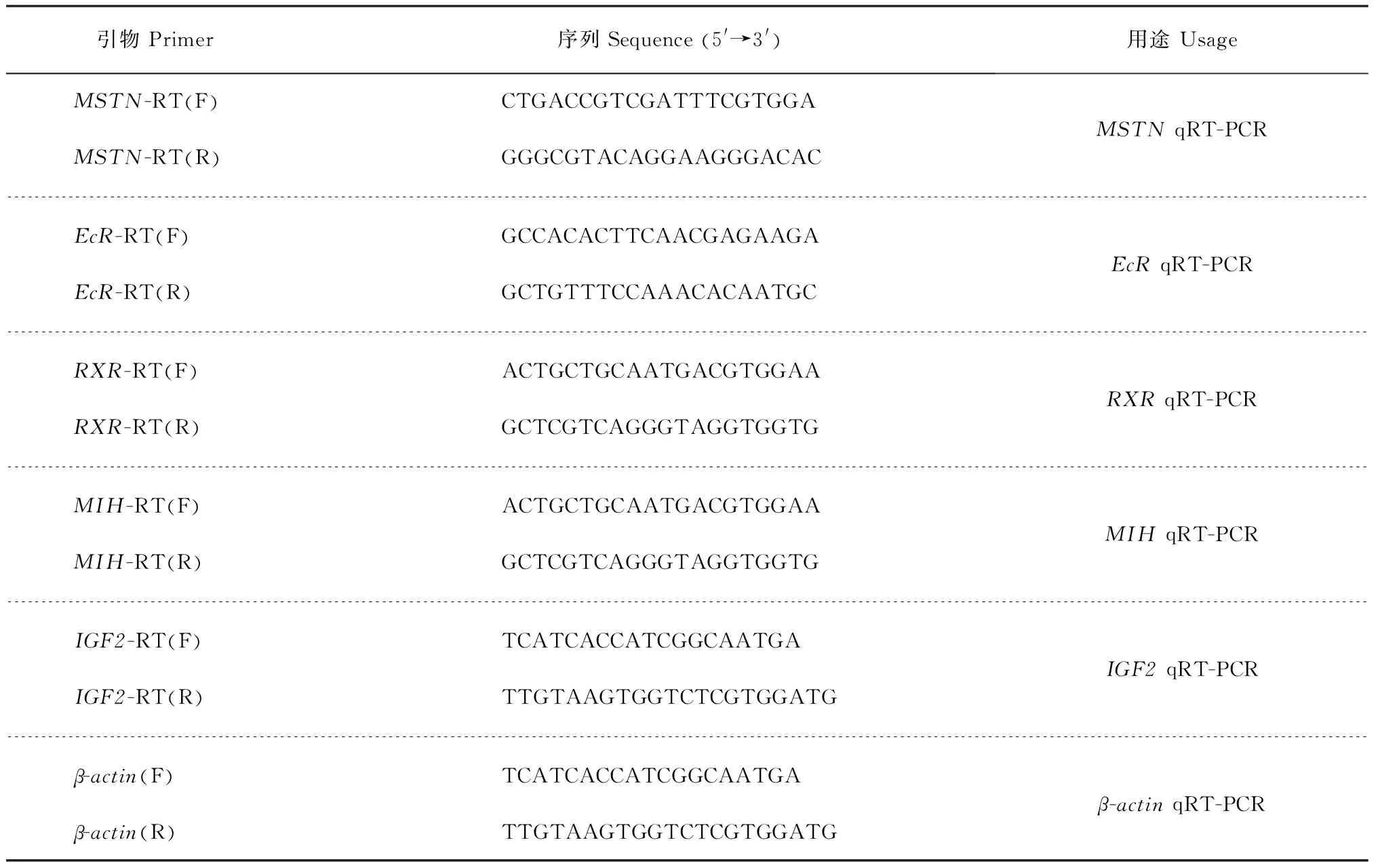
表1 定量实时聚合酶链式反应引物信息
1.4数据处理
利用SPSS 20.0、Excel 2013等软件对生长性状进行单因素方差分析和显著性检验。采用2-ΔΔCT方法[13]对定量实时PCR数据进行基因表达差异检验。
2 结果
2.1中华绒螯蟹增质量率差异
断肢中华绒螯蟹在每次蜕壳后的步足再生与体质量增长情况如表2所示。第1次蜕壳后的断肢再生率平均仅为7.6%,第2次蜕壳后断肢再生率达91.6%。表明中华绒螯蟹须经历2次蜕壳后才能完成断肢再生过程,而有极个别个体经2次蜕壳后也未完成断肢再生。断肢组由于切除了4个步足,其起始体质量与正常组存在显著差异,因而每次蜕壳后的体质量也与正常组存在统计学上的显著差异(P<0.05)。但从增质量率看,断肢组在第1次蜕壳后的增质量率显著高于正常组,特别是断肢雄蟹的增质量率(58.6%)显著高于正常雄蟹(47.7%)(P<0.05),而断肢雌蟹与正常雌蟹的增质量率不存在统计学上的显著差异(P>0.05)。第2次蜕壳后,断肢组再生后的步足小于正常蟹的步足,其平均体质量也显著小于正常蟹(P<0.05),但蜕壳后增质量率不存在统计学上的显著差异(P>0.05)。经2次蜕壳后,断肢蟹与正常蟹的成活率较为接近,均在60%以上。

表2 断肢蟹和正常蟹的断肢再生率与蜕壳后增质量率差异


2.2中华绒螯蟹蜕壳周期差异
通过对平均蜕壳周期(中华绒螯蟹所有个体完成一次蜕壳所经历的平均天数)分析发现,断肢组在去步足后到完成第1次蜕壳所需的平均天数(10.38 d)显著高于正常组(6.73 d)(P<0.01,图1A),而断肢组第2次平均蜕壳周期(18.25 d)显著短于正常组(23.47 d)(P<0.01,图1B),但前后2次蜕壳整体上不存在统计学上的显著差异(P>0.05,图1C)。以上结果表明,中华绒螯蟹在受到断肢刺激后会延长当期的蜕壳时间,随着断肢再生和步足的重新生长,则会显著缩短蜕壳周期,即附肢再生有刺激蜕壳的作用。

A:第1次蜕壳周期差异;B:第2次蜕壳周期差异;C:2次蜕壳周期平均差异。♀:雌性;:雄性。短栅上的不同大写或小写字母分别表示在P<0.01或P<0.05水平差异有统计学意义。A: Difference in the first inter-molting period; B: Difference in the second inter-molting period; C: Mean difference in the two molting periods. ♀: Female; : Male. Different capital or lowercase letters above bars show statistically significant differences at the 0.01 or 0.05 probability level, respectively.图1 断肢蟹和正常蟹的蜕壳周期差异Fig.1 Molt cycle difference between leg-amputated crabs and intact crabs
2.3中华绒螯蟹基因表达差异

短栅上的不同小写字母表示在P<0.05水平差异有统计学意义。Different lowercase letters above bars show statistically significant differences at the 0.05 probability level.图2 断肢蟹和正常蟹的相关基因表达差异Fig.2 Gene expression difference between leg-amputated crabs and intact crabs
从图2可以发现:在断肢蟹与正常蟹的步足肌肉中,胰岛素样生长因子2基因IGF2、维甲类X受体基因RXR和眼柄中的蜕皮抑制激素基因MIH的表达在统计学上无显著差异(P>0.05);而断肢蟹步足肌肉的蜕皮激素受体基因EcR的表达显著高于正常蟹(P<0.05),肌肉生长抑制素基因MSTN的表达显著低于正常蟹(P<0.05)。该结果初步表明,断肢具有促进中华绒螯蟹蜕壳和再生肢体肌肉生长的作用。
3 讨论
生长与蜕壳是中华绒螯蟹的重要生物学特性,而断肢再生以及再生肢体的生长发育都离不开蜕壳。本研究表明,中华绒螯蟹幼蟹在断肢后经历2次蜕壳可完成再生,再生肢体的外部形态接近正常肢体水平,但能观察到显著的大小差异,也存在个别中华绒螯蟹在第1次蜕壳后肢体发生再生或第2次蜕壳后肢体也无法完成再生的情况。YASUDA等[14]研究发现,寄居蟹(Pagurusmiddendorffii)的大螯能在一次蜕壳后完成再生;HOPKINS[15]对不同蜕壳时期的大西洋砂招潮蟹(Ucapugilator)进行了肢体再生研究,发现肢芽生长是在蜕壳间期而不是蜕壳前期和后期进行的。说明甲壳类动物完成肢体再生需要的蜕壳周期存在差异。肢体再生所经历的蜕壳周期,一方面可能由断掉肢体的生理功能决定,另一方面还和蟹本身所处的蜕壳时期有关。本研究选取的是蜕壳前期的中华绒螯蟹,断肢再生所需蜕壳周期符合一般规律,当然也不排除因为采样误差导致的个别中华绒螯蟹出现提前再生和延迟再生的情况。
从本试验中华绒螯蟹的蜕壳周期与增质量率可以看出,断肢再生过程对2次蜕壳周期及2次蜕壳后增质量率的影响存在差异。断肢处理对中华绒螯蟹当期蜕壳具有抑制作用,此阶段蜕壳周期延长,蜕壳后增质量率较正常组高,且肢体未再生。然而,中华绒螯蟹在第2个蜕壳周期时,断肢蟹蜕壳周期显著缩短,蜕壳后正常蟹和断肢蟹增质量率无显著差异,且出现肢体再生。因而,从试验起始到完成2次蜕壳的总经历时间无显著差异,即在第2次蜕壳内,断肢蟹和正常蟹的蜕壳是基本同步的。这一现象与JOHNSON等[16]对幽灵蛛(Holocnemuspluchei)的研究结果相似。此外,WRINN等[5]对狼蛛(Schizocosaocreata)的研究表明,断肢刺激会改变动物机体的能量分配,造成机体生长延缓和肢芽爆发式生长以维护动物的生存机制。综合上述研究结果说明,中华绒螯蟹在断肢再生过程中机体具有调整能量收支的能力,延长蜕壳周期有利于动物机体的修复,同时再次储存能量以备下一次蜕壳及肢体再生;而第2次蜕壳周期缩短,是机体自我调整以适应群体蜕壳活动从而提高生存概率的表现。
本研究表明,眼柄中的蜕皮抑制激素基因MIH以及肌肉中的胰岛素样生长因子基因IGF2、维甲类X受体基因RXR在断肢蟹和正常蟹中表达无显著差异,而蜕皮激素受体基因EcR和肌肉生长抑制素基因MSTN的表达存在显著差异,即EcR基因在再生肢体中高表达,而MSTN基因在再生肢体中低表达。EcR基因是甲壳类蜕壳活动调控的重要因子,随着研究的逐渐深入,越来越多的证据显示EcR还与甲壳类的肢体再生相关[12,17-19]。DAS等[18]通过RNA干扰技术证明EcR/RXR在肢芽形成早期对芽基细胞增殖具有重要作用;而CHUNG等[20]发现EcR基因在大西洋砂招潮蟹(U.pugilator)蜕壳周期不同阶段的非再生腹肢中稳定表达,而在再生腹肢的肢芽中高表达。这说明EcR不仅参与了蜕壳调控,而且还参与了芽基的形成和肢芽的发育。本研究发现EcR在再生肢体中仍然高表达,说明EcR基因还对再生肢体的生长发育具有重要作用。MSTN基因是动物肌肉形态发生的负调控因子,与肌肉再生也存在重要联系[8,10]。甲壳动物在蜕壳过程中肌肉形态和结实程度会发生巨大变化。相关研究发现,MSTN在蜕壳前期(D4)肌肉中显著下调,蜕壳完成后逐渐恢复到正常水平[21]。本研究中华绒螯蟹第2次蜕壳后再生肢体还未能完全达到正常肢体的形态和功能,再生肢体需要继续生长发育,MSTN在再生肢体中特异的低表达符合动物再生肢体在后续生长蜕壳中逐渐完善的生物学特性。
4 结论
中华绒螯蟹幼蟹发生断肢后能在2个蜕壳周期内完成再生;断肢对中华绒螯蟹的成活率无显著影响;与正常蟹相比,断肢蟹第1次蜕壳后增质量率升高而第2次蜕壳后其值间差异无统计学意义;断肢蟹第1次蜕壳周期延长而第2次蜕壳周期缩短,正常蟹与断肢蟹的总蜕壳周期间差异无统计学意义;EcR和MSTN基因对促进中华绒螯蟹再生肢体的生长发育有重要作用。综上表明,断肢蟹在生产上可以用于后续养殖生产。
[1]MATTONI C I, GARCíA-HERNáNDEZ S, BOTERO-TRUJILLO R,etal. Scorpion sheds ‘tail’ to escape: Consequences and implications of autotomy in scorpions (Buthidae:Ananteris).PLoSONE, 2015,10(1):755-757.
[2]DIAZ-GUISADO D, GAYMER C F, BROKORDT K B,etal. Autotomy reduces feeding, energy storage and growth of the sea starStichasterstriatus.JournalofExperimentalMarineBiologyandEcology, 2006,338:73-80.
[3]BARRíA E M, GONZáLEZ M I. Effect of autotomy and regeneration of the chelipeds on growth and development inPetrolistheslaevigatus(Guérin, 1835) (Decapoda, Anomura, Porcellanidae).Crustaceana, 2008,81(6):641-652.
[4]IRAETA P, SALVADOR A, DíAZ J A. Effects of caudal autotomy on postnatal growth rates of hatchlingPsammodromusalgirus.JournalofHerpetology, 2012,46(3):342-345.
[5]WRINN K, UETZ G. Impacts of leg loss and regeneration on body condition, growth, and development time in the wolf spiderSchizocosaocreata.CanadianJournalofZoology, 2007,85(7):823-831.
[6]MYKLES D L. Interactions between limb regeneration and molting in decapod crustaceans.AmericanZoologist, 2001,41(3):399-406.
[7]KURUP K N, ADIYODI R G. Multiple limb autotomy can trigger either ovarian growth or somatic growth in the freshwater crab,Paratelphusahydrodromous(Herbst).GeneralandComparativeEndocrinology, 1984,56(3):433-443.
[8]MCCROSKERY S, THOMS M, PIATT L,etal. Improved muscle healing through enhanced regeneration and reduced fibrosis in myostatin-null mice.JournalofCellScience, 2005,118(15):3531-3541.
[9]MACLEA K S, ABUHAGR A M, PITTS N L,etal. Rheb, an activator of target of rapamycin in the blackback land crab,Gecarcinuslateralis: Cloning and effects of molting and unweighting on expression in skeletal muscle.JournalofExperimentalBiology, 2012,215(4):590-604.
[10]WAGNER K R, LIU X, CHANG X,etal. Muscle regeneration in the prolonged absence of myostatin.ProceedingsoftheNationalAcademyofSciencesoftheUSA, 2005,102(7):2519-2524.
[11]GONG J, YU K, SHU L,etal. Evaluating the effects of temperature, salinity, starvation and autotomy on molting success, molting interval and expression of ecdysone receptor in early juvenile mud crabs,Scyllaparamamosain.JournalofExperimentalMarineBiologyandEcology, 2015,464:11-17.
[12]DÉMEUSY N, MARGUERITE C, MORINIRE M,etal. Changes in ecdysteroid levels during regeneration of a limb inPilumnushirtellus.ComparativeBiochemistryandPhysiologyPartA:Physiology, 1994,108(2/3):239-243.
[13]LIVAK K J, SCHMITTGEN T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCTmethod.Methods, 2001,25(4):402-408.
[14]YASUDA C I, MATSUO K, WADA S. Rapid regeneration of the major cheliped in relation to its function in male-male contests in the hermit crabPagurusmiddendorffii.PlanktonandBenthosResearch, 2014,9(2):122-131.
[15]HOPKINS P M. Regeneration of walking legs in the fiddler crabUcapugilator.IntegrativeandComparativeBiology, 1993,33(3):348-356.
[16]JOHNSON S A, JAKOB E M. Leg autotomy in a spider has minimal costs in competitive ability and development.AnimalBehaviour, 1999,57(4):957-965.
[17]DURICA D S, WU X, ANILKUMAR G,etal. Characterization of crab EcR and RXR homologs and expression during limb regeneration and oocyte maturation.MolecularandCellularEndocrinology, 2002,189(1/2):59-76.
[18]DAS S, DURICA D S. Ecdysteroid receptor signaling disruption obstructs blastemal cell proliferation during limb regeneration in the fiddler crab,Ucapugilator.MolecularandCellularEndocrinology, 2012,365(2):249-259.
[19]GONG J, YE H H, XIE Y J,etal. Ecdysone receptor in the mud crabScyllaparamamosain: A possible role in promoting ovarian development.JournalofEndocrinology, 2015,224(3):273-287.
[20]CHUNG A C, DURICA D S, HOPKINS P M. Tissue-specific patterns and steady-state concentrations of ecdysteroid receptor and retinoid-X-receptor mRNA during the molt cycle of the fiddler crab,Ucapugilator.GeneralandComparativeEndocrinology, 1998,109(3):375-389.
[21]COVI J A, BADER B D, CHANG E S,etal. Molt cycle regulation of protein synthesis in skeletal muscle of the blackback land crab,Gecarcinuslateralis, and the differential expression of a myostatin-like factor during atrophy induced by molting or unweighting.TheJournalofExperimentalBiology, 2010,213(1):172-183.
Effects of limb regeneration on molt, growth and related gene expression in Chinese mitten crab (Eriocheir sinensis).JournalofZhejiangUniversity(Agric. &LifeSci.), 2016,42(4):502-508
YUE Wucheng, CHEN Jiao, CI Yuanji, HUANG Shu, WANG Jun, WANG Chenghui*
(KeyLaboratoryofFreshwaterFisheriesGermplasmResources,MinistryofAgriculture,ShanghaiOceanUniversity,Shanghai201306,China)
Chinese mitten crab (Eriocheirsinensis); limb regeneration; mass-gaining rate; molt cycle; gene expression
上海市中华绒螯蟹产业技术体系项目[沪农科(产)字2010-4号];上海市科委崇明科技专项(13391912102);上海市工程技术中心建设项目(03DZ2251800)。
Corresponding author):王成辉(http://orcid.org/0000-0002-6523-7610),Tel:+86-21-61900439, E-mail:wangch@shou.edu.cn
联系方式:岳武成(http://orcid.org/0000-0001-6965-3788),E-mail:547203691@qq.com
2015-09-10;接受日期(Accepted):2015-12-16;网络出版日期(Published online):2016-07-18
S 917.4
A
URL:http://www.cnki.net/kcms/detail/33.1247.S.20160718.2054.020.html
