生物质炭对土壤无机污染物迁移行为影响研究进展
2016-08-15张栋刘兴元赵红挺
张栋, 刘兴元, 赵红挺*
(1.杭州电子科技大学材料与环境工程学院环境材料与应用技术研究所,杭州 310018;2.广东大众农业科技股份有限公司,广东 东莞 523169)

生物质炭对土壤无机污染物迁移行为影响研究进展
张栋1, 刘兴元2, 赵红挺1*
(1.杭州电子科技大学材料与环境工程学院环境材料与应用技术研究所,杭州 310018;2.广东大众农业科技股份有限公司,广东 东莞 523169)
生物质炭材料来源广泛,制备工艺相对简单,且具备丰富的含氧官能团、发达的孔隙结构和表面电荷,对有机污染物和各类无机污染物(重金属、氮磷、放射性元素)均具有良好的潜在吸附能力,被认为是一种低成本、高效的新型环境功能吸附材料。本文针对重金属、氮磷等土壤无机物,在介绍生物质炭基本性质的基础上,综述了生物质炭吸附无机污染物的机制,探讨了应用于无机污染土壤缓解和修复的可行性,并指出了相应的发展趋势。生物质炭的基本特性受来源材料性质、裂解温度等主要因子的影响,其碳含量和结构、H/C比值、孔隙结构、pH等性质有较大差异,这也导致生物质炭对重金属、氮磷等无机污染物的吸附机制包含了表面物理吸附、络合作用、静电引力、阳离子交换、共沉淀、碘-碳特殊作用等多种机制。然而,受土壤复杂理化性质和生物活性、生物质炭迁移性和稳定性等因素影响,生物质炭在无机污染土壤缓解和修复中的应用有很大潜力,但尚存在不确定性、调控性差等问题,甚至反而会活化土壤中的污染物。因此,在应用生物质炭缓解和修复重金属污染土壤时,应充分考虑土壤性质、污染程度和类型与生物质炭性质的匹配度。生物质炭更适合pH和有机质含量较低的镉、铅、铜、锌等重金属污染土壤;与低温生物质炭相比,高温生物质炭的适用范围更广。
生物质炭; 吸附; 重金属; 土壤; 修复
Summary Biochar is a carbon-rich product obtained from thermal treatment and pyrolysis of various plant- and animal-based biomass. The biomass for preparation of biochar had extensive sources, and the treatment is usually easy-operation, mainly thermochemical decomposition under a poor-oxygen condition. Biochar has been considered as a low-cost and high-efficiency sorbent for both organic and inorganic contaminants including heavy metals, radioactive elements, nitrogen and phosphate, due to its abundant O-containing functional groups and surface charges, advanced micro- and macro-pore structures, and rich carbon content.
In this paper, recent research progress on biochar with regards to its mechanisms and potential applications in remediation of inorganic contaminated soils was reviewed. The key parameters controlling biochar’s properties include pyrolysis temperatures and feedstock types, resulting in biochar with great difference in surface areas, pore size distribution, pH, H/C ratio, ion-exchange capacity, and carbon content. Therefore, the sorption mechanisms of inorganic pollutants varied with different properties of biochar. The sorption mechanisms of inorganic pollutants such as heavy metal, radioactive elements, nitrogen and phosphate were summarized as well as their potential applications in real soil condition. Several different possible mechanisms were proposed: 1) electrostatic outer-sphere complexation due to surface cationic exchange; 2) surface complexation with active O-containing functional groups such as carboxyl and hydroxyl groups; 3) electrostatic attraction of anionic inorganic pollutants such as phosphate and arsenic to protonated groups under alkaline pH; 4) co-precipitation of heavy metal and phosphate with organic matter and mineral oxides on surface of the biochar or pre-sorbed metal ions; 5) specific binding of iodide with aromatic carbon in biochar; 6) to donate electrons for mitigating/reducing heavy metal such as chromium; 7) physical adsorption of heavy metals onto biochar’s surface; 8) changing the pH of point of zero charge (pHpzc) to immobilize or mobilize heavy metals.
Generally and undoubtedly, the use of biochar as an environmental sorbent can have strong implications. It can effectively sorb various organic and inorganic contaminants in aqueous solutions. However, due to soil complexity, whether biochar is suitable for the remediation of inorganic contaminated soil is still unclear. These confused results could attribute to: 1) high dissolved organic carbon contents of soil at the increased pH induced by biochar addition may mobilize heavy metal leaching and/or form high available species; 2) electrostatic repulsion between anionic heavy metal ions and negatively charged biochar surface may enhance the desorption of heavy metal from soil-biochar matrix; 3) changing soil pH may result in mobilization or immobilization of heavy metals; 4) the transportation of biochar in soil system may influence the mitigation of sorbed heavy metals; 5) the availability of heavy metal sorbed by biochar to soil microorganism or plants; 6) the stability and biodegradation of biochar is also an uncertain factor for the application of biochar in the remediation of inorganic contaminated soil.
Based on the limited information, we proposed that biochars, especially those pyrolyzed at high temperature were suitable for the remediation of the low-pH and/or low dissolved organic carbon soil contaminated with cadmium, lead, copper, zinc and other heavy metals. Furthermore, further researches on interactions among soil-biochar-pollutants and field applications for remediation of contaminated soil are urgently needed.
生物质炭是生物质在缺氧或无氧条件下裂解形成的多孔、低密度的富碳材料[1-4]。近年来,生物质炭因具有温室气体控制、改良土壤性质、缓解土壤污染的作用[5-9],已成为国内外环境研究领域新的关注热点(图1A)。生物质炭制备材料来源广泛(图1B),具备丰富的含氧官能团、发达的孔隙结构和表面电荷,与有机污染物、重金属、氮磷、放射性元素等其他无机污染物有较强的亲和作用,被认为是一种低成本、高效的新型环境功能吸附材料[10-13]。已有文献综述介绍了生物质炭调控有机污染物的环境过程及其机制[8,14],本文针对重金属、放射性元素、氮磷等无机污染物,从生物质炭的性质、吸附无机污染物的作用机制和影响因子等方面深入论述了生物质炭对土壤无机污染物迁移行为的影响。
1 生物质炭的基本特性
生物质炭的主要特性标志是含碳丰富,且富含烷基和芳香结构[9,15-16],通常呈碱性或弱碱性[3]。生物质炭主要成分为纤维素、羰碳、羧酸及其衍生物、呋喃、吡喃、脱水糖、苯酚、烷烃及烯烃的衍生物等复杂有机碳化物[17],官能团较丰富,但极性官能团较少,以羧基、羟基等为主[18];从微观上看,生物质炭多由紧密堆积、高度扭曲的芳香环片层结构组成[9],多孔特性显著,具有较大的比表面积[19-20]。
生物质炭的富碳、多孔、结构和官能团特性及其广泛多样性,使其能在复杂环境污染物修复中具有较强的应用前景[2,8]。目前,学界普遍认可材料来源和裂解温度是影响生物质炭的特性和环境应用的主要因子。生物质炭的元素组成和存在形态在一定程度上决定了生物质炭的官能团、结构和比表面积,进而决定其环境功能。生物质炭的组成元素为碳、氢、氧、氮、磷等,主要受材料来源和裂解温度等因素的影响。AHMAD等[8]在综述中介绍了生物质炭来源对元素组成及其关系的影响。对同一材料来源的生物质炭,各元素比例主要受裂解温度的影响。随裂解温度的升高,生物质炭的碳元素、矿物灰分、磷等含量增大[21-24];氧、氢、硫等元素含量降低[21-23,25];氮元素含量变化规律不明确,可略有富集或下降[21,26],甚至可能先富集后降低[21-22]。生物质炭中碳元素含量随制备温度的升高会显著增大,其存在形态也会发生变化。如KEILUWEIT等[21]发现,在不同温度(100~700 ℃)条件下制备的木材生物质炭和禾草生物质炭中碳元素含量均随温度升高而增大,分别从50.6%和48.6%增大到92.3%和94.2%。此外,H/C比值也随裂解温度的升高而降低,通常在100~500 ℃阶段降低迅速,在500 ℃以上降低缓慢[25]。H/C比值的降低意味着碳元素的存在趋于芳环结构,特别是在较高温度(500 ℃以上),主要为硬碳芳环结构[25],而羧基、醚键等极性基团降低,傅里叶变换红外光谱分析也证明了这种推断[21-22,25]。这不利于生物质炭与重金属离子的络合,但有利于生物质炭对碘离子等的捕获。

图1 2003—2015年生物质炭相关论文增长情况(A)及生物质炭来源解析(B)Fig.1 Growth in peer-reviewed publications on biochar from 2003—2015 based on Web of ScienceTM (A) and feedstock analysis of biochar (B)
灰分也是生物质炭的重要组成部分,因其含有Na+、K+、Mg2+、Ca2+等碱基阳离子的氧化物或碳酸盐,使生物质炭溶于水后通常呈碱性。YUAN等[27]通过X射线衍射图谱和碳酸盐定量分析、傅里叶变换红外光谱和zeta电位分析表明,羧酸盐(COO—)是几种作物秸秆生物质炭碱性的主要贡献者。生物质炭pH与灰分含量呈正相关,且均与裂解温度和来源材料有关[3,23,27-28]。裂解温度越高,灰分含量高,则生物质炭pH越高。UCHIMIYA等[23]使用棉籽壳在不同温度(200~650 ℃)烧制生物质炭,其灰分和pH均随温度升高而增大。来源材料灰分含量高,则制成生物质炭的pH也较高。如在550 ℃下用干材烧制的生物质炭pH为9.49(灰分为3.5%),而灰分含量更高的禽畜粪肥生物质炭pH为10.26(灰分为44.4%)[28]。
2 生物质炭吸附无机污染物的机制
无机污染物(主要指重金属,也包括放射性元素和氮磷等),主要通过采矿、冶炼、农药化肥施用、金属加工、火电厂和核电厂、废水和污泥等人类活动进入到环境中[29-33]。与有机污染物不同,重金属和放射性元素通常难以通过生物降解途径去除[29,34];碳基材料常被应用至污染水体或土壤中,可降低重金属生物有效性、生态毒性和风险[35]。目前,生物质炭对环境污染物的吸附研究主要集中于有机污染物,对重金属、氮磷等无机污染物环境行为的影响近年关注度增加,但研究工作仍相对较少[2,8,14,16],特别对碘、铯等常见放射性元素环境行为的作用研究更是几近空白。

此外,生物质炭还有一些特殊作用:在生物质炭上的含氧基团可以将Cr(Ⅵ)还原为Cr(Ⅲ),在吸附和吸持污染物的同时还可以将其转化为毒性较小的形态[49-50];生物质炭可降低等电点时的pH,从而改变重金属的存在形态[23];生物质炭比表面积较大,为表面物理吸附作用提供了良好的平台[8,19-20]。
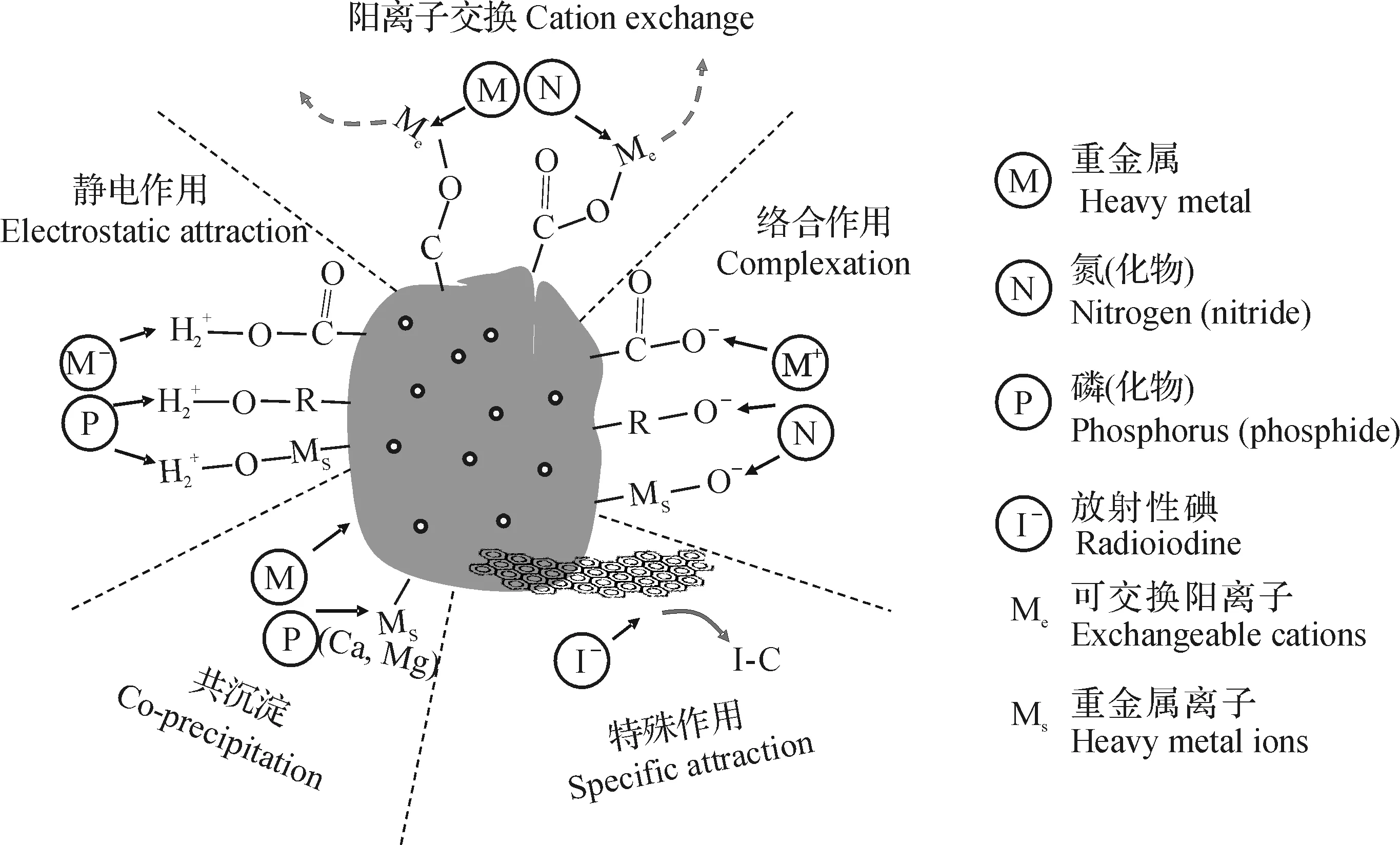
图2 生物质炭与无机污染物的相互作用机制[8,36]Fig.2 Interaction and underlying mechanisms of biochar and inorganic pollutants[8,36]
3 生物质炭在无机污染土壤修复中的应用潜力
我国土壤无机污染主要集中在重金属污染,且部分地区污染程度较重。据《全国土壤污染状况调查公报》,我国土壤Cd、Hg、As、Cu、Pb、Cr、Zn、Ni等8种无机污染物点位超标率分别为7.0%、1.6%、2.7%、2.1%、1.5%、1.1%、0.9%和4.8%[51]。特别是我国大多数城市近郊农田均受到了不同程度的重金属污染,严重影响到粮食产品的安全,需要进行修复或控制[52-56]。如NIU等[56]评估了我国土壤11种重金属的生态风险,发现82%的样品中Cd处于高风险状态,过半数样品中Cu、Pb、Zn等污染物处于中高度风险状态。ZHU等[57]研究发现,粤北稻米Cd含量中位值达到0.33 mg/kg,远超GB 2762—2012规定的最高值(0.20 mg/kg);由此导致人体月摄入Cd量达到55.01 μg/kg,比对照地区摄入量高出3.4倍。目前,重金属污染土壤修复技术主要有热脱附、电动修复、淋洗技术、稳定和固化技术、植物修复、微生物修复及联合修复技术等[53,58-62]。对有生产任务的轻污染农田土壤,稳定和固化技术可能在缓解土壤重金属污染风险、生产安全农产品或粮食中具有较强优势。
生物质炭对环境中的重金属、放射性元素等无机污染物表现出了良好的吸附和亲和性能[8]。然而,土壤环境体系复杂,pH、土壤可溶性有机物(碳)含量、污染物种类和浓度都可能影响生物质炭在缓解和修复重金属污染土壤中的作用;因此,需对其在土壤修复中的应用进行可行性评价。近年来,生物质炭在无机污染土壤修复中的应用研究详见表1。
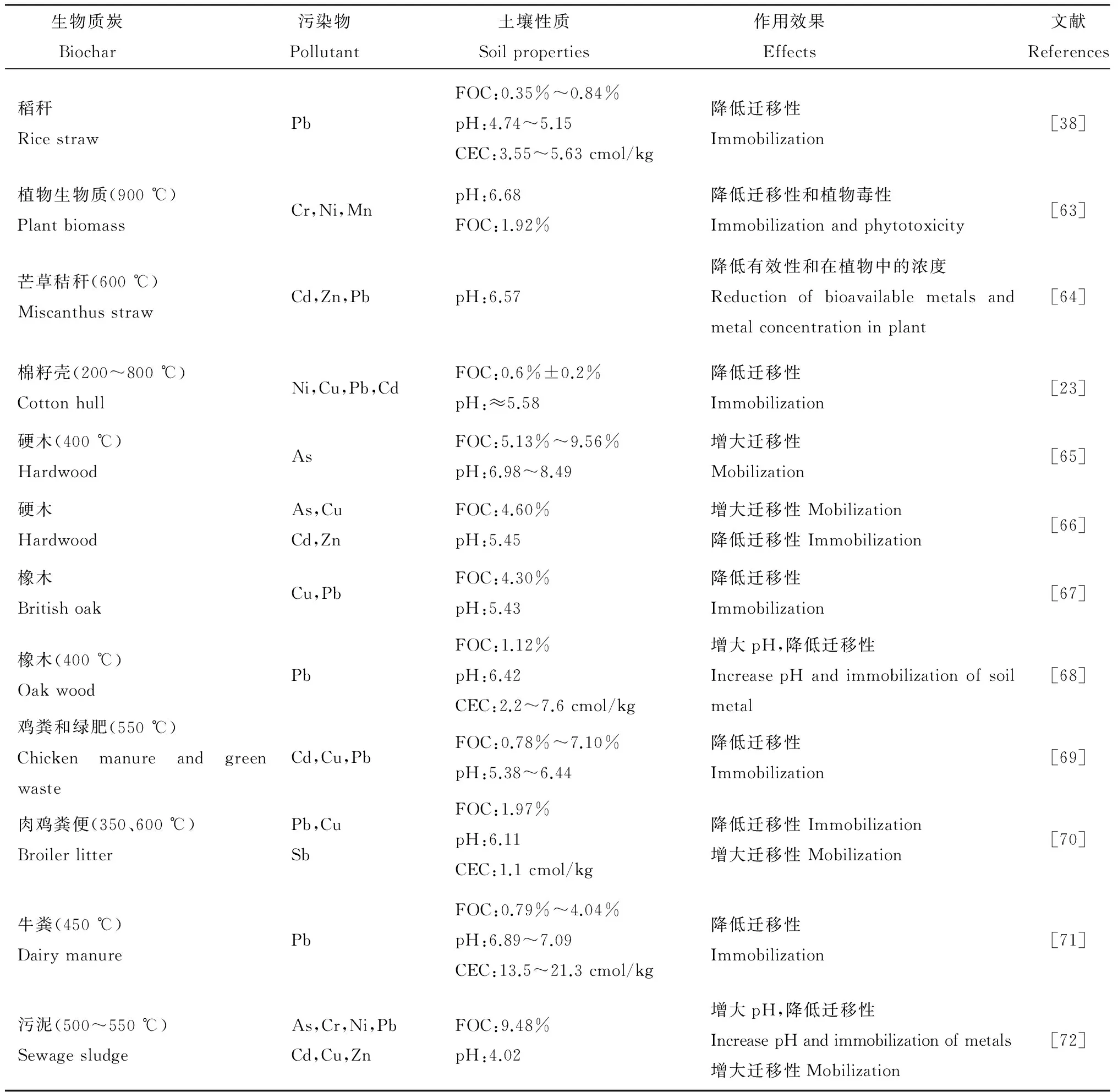
表1 生物质炭对土壤中重金属迁移行为的影响
FOC:有机碳质量分数;CEC:阳离子交换量。
FOC: Fraction of organic carbon; CEC: Cation exchange capacity.
生物质炭是吸附、固定和钝化土壤重金属的良好材料,很多研究也证明了添加生物质炭可以降低土壤重金属(Cu、Pb、Zn、Cd、Ni等)的迁移性、生物有效性和生物毒性[23,38,63-64,66-72]。然而,由于土壤环境的复杂性,即使添加同样的生物质炭对不同污染物也会产生不同的效果[51],常见的影响因素有:1)生物质炭增大土壤pH,使土壤高有机碳含量对某些重金属产生活化作用,如高含量有机碳更易于产生溶解性铜[66,72];2)静电位阻效应阻止重金属吸附到土壤固相,如阴离子型锑[70];3)生物质炭对土壤pH的影响,导致As、Cu等迁移活性的增大[65-66];4)生物质炭的稳定性和迁移性也可能导致吸附态重金属重新进入土壤溶液或随生物质炭在土壤中迁移,但其作用机制和调控方法尚不明确。
虽然在现有研究中生物质炭在无机污染土壤修复中的应用表现出不同甚至相反的结果,但总体上生物质炭还是具有较大的应用潜力。如:1)在pH低的酸性土壤中,我国普遍存在的Cd、Cu、Pb、Zn等重金属迁移活性和生态风险大[73-75],添加生物质炭可提高土壤微环境pH,有利于降低重金属迁移性。2)土壤有机质含量也是影响重金属形态的重要因子,对有机质含量较低的土壤,生物质炭可提供丰富的官能团,易于与重金属(离子)进行阳离子交换、络合等作用,降低其迁移性;而对有机质含量相对较高的土壤,添加低温生物质炭有可能释放出可溶性有机质,活化重金属[65-66],此时可选择具有更多稳定芳环结构的高温生物质炭,提供难利用的有机相[23,64,69]。
4 小结
生物质炭在有机及无机污染物控制方面具有独特的优势,是一种良好的吸附剂,在环境修复方面具有较大的应用前景。生物质炭制备来源广泛,制备条件特别是裂解温度范围较宽,这是其优势,但也导致生物质炭基本特性、组成、吸附性能和机制、环境行为等都存在较大差异。
虽然已经初步探明了生物质炭与重金属、氮磷等无机污染物的相互作用及其机制,但鉴于土壤性质的复杂性,将生物质炭用于无机污染土壤的控制和修复还存在较大的随机性和不确定性。毋庸置疑,生物质炭在污染土壤缓解和修复领域必将发挥重要作用。现阶段我们应加大力度,完善理论体系和应用技术,一方面构建来源材料、制备条件与生物质炭性能的关系,完善多种多类污染物共存与生物质炭的相互作用;另一方面加快生物质炭用于土壤修复的进程,针对土壤性质和实际污染状况,选择性使用生物质炭,克服生物质炭在土壤中的稳定性和迁移性等瓶颈,提高生物质炭的利用效率;此外,还需拓展生物质炭的应用领域,拓宽处理的污染物种类。
[1]LEHMANN J, RILLIG M, THIES J,etal. Biochar effects on soil biota: A review.SoilBiology&Biochemistry, 2011,43:1812-1836.
[2]BEESLEY L, MORENO-JIMÉNEZ E, GOMEZ-EYLES J,etal. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils.EnvironmentalPollution, 2011,159:3269-3282.
[3]LEHMANN J. Bio-energy in the black.FrontiersinEcologyandtheEnvironment, 2007,5:381-387.
[4]SOHI S P, KRULL E, LOPEZ-CAPEL E,etal. A review of biochar and its use and function in soil.AdvancesinAgronomy, 2010,105:47-82.
[5]LEHMANN J. A handful of carbon.Nature, 2007,447:143-144.
[6]LEHMANN J, GAUNT J, RONDON M. Bio-char sequestration in terrestrial ecosystem: A review.MitigationandAdaptationStrategiesforGlobalChange, 2006,11:403-427.
[7]NOVAK J M, BUSSCHER W, LAIRD D L,etal. Impact of biochar amendment on fertility of a southeastern coastal plain soil.SoilScience, 2009,174(2):105-112.
[8]AHMAD M, RAJAPAKSHA A U, LIM J E,etal. Biochar as a sorbent for contaminant management in soil and water: A review.Chemosphere, 2014,99:19-33.
[9]LEHMANN J, JOSEPH S.BiocharforEnvironmentalManagement:ScienceandTechnology. UK: Earthscan Ltd., 2009:1-12.
[10]CAO X D, MA L, GAO B,etal. Dairy-manure derived biochar effectively sorbs lead and atrazine.EnvironmentalScience&Technology, 2009,43:3285-3291.
[11]YAO Y, GAO B, CHEN J J,etal. Engineered biochar reclaiming phosphate from aqueous solutions: Mechanisms and potential application as a slow-release fertilizer.EnvironmentalScience&Technology, 2013,47:8700-8708.
[12]徐秋桐,邱志腾,章明奎.生物炭对不同pH土壤中碳氮磷的转化与形态的影响.浙江大学学报(农业与生命科学版),2014,40(3):303-313.
XU Q T, QIU Z T, ZHANG M K. Effects of biochar application on transformation and chemical forms of C, N and P in soils with different pH.JournalofZhejiangUniversity(AgricultureandLifeSciences), 2014,40(3):303-313. (in Chinese with English abstract)
[13]杜敬霆,孙朋飞,赵宇华,等.壳聚糖-生物炭微球对甲基红的吸附及微球菌剂的吸附增益效应.浙江大学学报(农业与生命科学版),2015,41(2):228-236.
DU J T, SUN P F, ZHAO Y H,etal. Biosorption of chitosan-biochar microsphere for methyl red and biosorption enhancing effect of microsphere-microbe complex.JournalofZhejiangUniversity(AgricultureandLifeSciences), 2015,41(2):228-236. (in Chinese with English abstract)
[14]季雪琴,孔雪莹,钟作浩,等.秸秆生物炭对疏水性有机污染物的吸附研究综述.浙江农业科学,2015,56(7):1114-1118.
JI X Q, KONG X Y, ZHONG Z H,etal. A review of sorption of hydrophobic organic compounds using straw-derived biochar.JournalofZhejiangAgriculturalSciences, 2015,56(7):1114-1118. (in Chinese)
[15]GLASER B, HAUMAIER L, GUGGENBERGER G,etal. Black carbon in soils: The use of benzenecarboxylic acids as specific markers.OrganicGeochemistry, 1998,29(4):811-819.
[16]李力,刘娅,陆宇超,等.生物炭的环境效应及其应用的研究进展.环境化学,2011,30(8):1411-1421.
LI L, LIU Y, LU Y C,etal. Review on environmental effects and applications of biochar.EnvironmentalChemistry, 2011,30(8):1411-1421. (in Chinese with English abstract)
[17]张阿凤,潘根兴,李恋卿.生物黑炭及其增汇减排与改良土壤意义.农业环境科学学报,2009,28(12):2459-2463.
ZHANG A F, PAN G X, LI L Q. Biochar and the effect on C stock enhancement, emission reduction of greenhouse gases and soil reclaimation.JournalofAgro-EnvironmentScience, 2009,28(12):2459-2463.
[18]CORNELISSEN G, GUSTAFSSON O, BUCHELI T D,etal. Extensive sorption of organic compounds to black carbon, coal, and kerogen in sediments and soils: Mechanisms and consequences for distribution, bioaccumulation, and biodegradation.EnvironmentalScience&Technology, 2005,39(18):6881-6895.
[19]CAO X D, HARRIS W. Properties of diary-manure-derived biochar pertinent to its potential use in remediation.BioresourceTechnology, 2010,101(14):5222-5228.
[20]ÖZÇIMEN D, ERSOY-MERIÇBOYU A. Characterization of biochar and bio-oil samples obtained from carbonization of various biomass materials.RenewableEnergy, 2010,35:1319-1324.
[21]KEILUWEIT M, NICO P S, JOHNSON M G,etal. Dynamic molecular structure of plant biomass-derived black carbon (biochar).EnvironmentalScience&Technology, 2010,44:1247-1253.
[22]CHEN Z M, CHEN B L, CHIOU C T. Fast and slow rates of naphthalene sorption to biochars produced at different temperatures.EnvironmentalScience&Technology, 2012,46:11104-11111.
[23]UCHIMIYA M, WARTELLE L H, KLASSON K T,etal. Influence of pyrolysis temperature on biochar property and function as a heavy metal sorbent in soil.JournalofAgriculture&FoodChemistry, 2011,59:2501-2510.
[24]SILBER A, LEVKOVITCH I, GRABER E R. pH-dependent mineral release and surface properties of cornstraw biochar: Agronomic implications.EnvironmentalScience&Technology, 2010,44:9318-9323.
[25]CHEN B L, CHEN Z M. Sorption of naphthalene and 1-naphthol by biochars of orange peels with different pyrolytic temperatures.Chemosphere, 2009,76:127-133.
[26]CHEN B L, CHEN Z M, LÜ S F. A novel magnetic biochar efficiently sorbs organic pollutants and phosphate.BioresourceTechnology, 2011,102:716-723.
[27]YUAN J H, XU R K, ZHANG H. The forms of alkalis in the biochar produced from crop residues at different temperatures.BioresourceTechnology, 2011,102:3488-3497.
[28]SINGH B P, HATTON B J, SINGH B,etal. Influence of biochars on N2O emission and nitrogen leaching from two contrasting soils.JournalofEnvironmentalQuality, 2010,39(4):1224-1235.
[29]ADRIANO D C.TraceElementsinTerrestrialEnvironments. New York, USA: Springer, 2001:133-165.
[30]USMAN A R A, LEE S S, AWAD Y M,etal. Soil pollution assessment and identification of hyperaccumulating plants in chromate copper arsenate (CCA) contaminated sites, Korea.Chemosphere, 2012,87:872-878.
[31]OTERO N, VITORIA A, SOLER A,etal. Fertiliser characterisation: Major, trace and rare earth elements.AppliedGeochemistry, 2005,20:1473-1488.
[32]XU S, FREEMAN S P H T, HOU X,etal. Iodine isotopes in precipitation: Temporal responses to129I emissions from the Fukushima nuclear accident.EnvironmentalScience&Technology, 2013,47:10851-10859.
[33]HOU X L, FOGH C L, KUCERA J,etal. Iodine-129 and Caesium-137 in Chernobyl contaminated soil and their chemical fractionation.ScienceofTotalEnvironment, 2013,308:97-109.
[34]ZHANG X K, WANG H L, HE L Z,etal. Using biochar for remediation of soils contaminated with heavy metals and organic pollutants.EnvironmentalScienceandPollutionResearch, 2013,20:8472-8473.
[35]IPPOLITO J A, STRAWN D G, SCHECKEL K G,etal. Macroscopic and molecular investigations of copper sorption by a steam-activated biochar.JournalofEnvironmentalQuality, 2012,41:1150-1156.
[36]CHOUNG S, UM W, KIM M,etal. Uptake mechanism for iodine species to black carbon.EnvironmentalScience&Technology, 2013,47:10349-10355.
[37]CUI X Q, HAO H L, ZHANG C K,etal. Capacity and mechanisms of ammonium and cadmium sorption on different wetland-plant derived biochars.ScienceoftheTotalEnvironment, 2016,539:566-575.
[38]JIANG T Y, JIANG J, XU R K,etal. Adsorption of Pb(Ⅱ) on variable charge soils amended with rice-straw derived biochar.Chemosphere, 2012,89:249-256.
[39]DING Y, LIU Y X, WU W X,etal. Evaluation of biochar effects on nitrogen retention and leaching in multi-layered soil columns.WaterAir&SoilPollution, 2010,213(1/2/3/4):47-55.
[40]WANG M C, SHENG G D, QIU Y P. A novel manganese-oxide/biochar composite for efficient removal of lead(Ⅱ) from aqueous solutions.InternationalJournalofEnvironmentalScienceandTechnology, 2015,12:1719-1726.
[41]EBERHARDT T L, MIN S H, HAN J S. Phosphate removal by refined aspen wood fiber treated with carboxymethyl cellulose and ferrous chloride.BioresourceTechnology, 2006,97(18):2371-2376.
[42]YAO Y, GAO B, INYANG M,etal. Biochar derived from anaerobically digested sugar beet tailings: Characterization and phosphate removal potential.BioresourceTechnology, 2011,102(10):6273-6278.
[43]ZENG Z, ZHANG S D, LI T Q,etal. Sorption of ammonium and phosphate from aqueous solution by biochar derived from phytoremediation plants.JournalofZhejiangUniversity-ScienceB(Biomedicine&Biotechnology), 2013,14(12):1152-1161.
[44]WAND S S, GAO B, ZIMMERMAN A R,etal. Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite.BioresourceTechnology, 2015,175:391-395.
[45]GERRITSE R G. Prediction of travel times of phosphate in soils at a disposal site for wastewater.WaterResearch, 1993,27(2):263-267.
[46]INYANG M, GAO B, YAO Y,etal. Removal of heavy metals from aqueous solution by biochar derived from anaerobically digested biomass.BioresourceTechnology, 2012,110:50-56.
[47]LU H L, ZHANG W H, YANG Y X,etal. Relative distribution of Pb2+sorption mechanisms by sludge-derived biochar.WaterResearch, 2012,46:854-862.
[48]KONG H L, HE J, GAO Y Z,etal. Cosorption of phenanthrene and mercury(Ⅱ) from aqueous solution by soybean stalk-based biochar.JournalofAgricultureandFoodChemistry, 2011,59:12116-12123.
[49]DONG X, MA L Q, LI Y. Characteristics and mechanisms of hexavalent chromium removal by biochar from sugar beet tailing.JournalofHazardousMaterials, 2011,190:909-915.
[50]CHOPPALA G K, BOLAN N S, MALLAVARAPU M,etal. The influence of biochar and black carbon on reduction and bioavailability of chromate in soils.JournalofEnvironmentalQuality, 2012,41:1175-1184.
[51]环境保护部和国土资源部.全国土壤污染状况调查公报(2014).中国环保产业,2014(5):10-11.
The Ministry of Environmental Protection and the Ministry of Land and Resources. Bulletin on national survey of soil contamination (2014).ChinaEnvironmentalProtectionIndustry, 2014(5):10-11. (in Chinese)
[52]张小敏,张秀英,钟太洋,等.中国农田土壤重金属富集状况及其空间分布研究.环境科学,2014,35(2):692-703.
ZHANG X M, ZHANG X Y, ZHONG T Y,etal. Spatial distribution and accumulation of heavy metal in arable land soil of China.EnvironmentalScience, 2014,35(2):692-703. (in Chinese with English abstract)
[53]樊霆,叶文玲,陈海燕,等.农田土壤重金属污染状况及修复技术研究.生态环境学报,2013,22(10):1727-1736.
FAN T, YE W L, CHEN H Y,etal. Review on contamination and remediation technology of heavy metal in agricultural soil.EcologyandEnvironmentalSciences, 2013,22(10):1727-1736. (in Chinese with English abstract)
[54]李海光,施加春,吴建军.污染场地周边农田土壤重金属含量的空间变异特征及其污染源识别.浙江大学学报(农业与生命科学版),2013,39(3):325-334.
LI H G, SHI J C, WU J J. Spatial variability characteristics of soil heavy metals in the cropland and its pollution source identification around the contaminated sites.JournalofZhejiangUniversity(AgricultureandLifeSciences), 2013,39(3):325-334. (in Chinese with English abstract)
[55]HUANG S S, LIAO Q L, HUA M,etal. Survey of heavy metal pollution and assessment of agricultural soil in Yangzhong District, Jiangsu Province, China.Chemosphere, 2007,67:2148-2155.
[56]NIU L L, YANG F X, XU C,etal. Status of metal accumulation in farmland soils across China: From distribution to risk assessment.EnvironmentalPollution, 2013,176:55-62.
[57]ZHU P, LIANG X X, WAND P,etal. Assessment of dietary cadmium exposure: A cross-sectional study in rural areas of south China.FoodControl, 2016,62:284-290.
[58]PAGE M M, PAGE C L. Electroremediation of contaminated soils.JournalofEnvironmentalEngineering, 2002,128(3):208-219.
[59]DERMONT G, BERGERON M, MERCIER G,etal. Soil washing for metal removal: A review of physical/chemical technologies and field applications.JournalofHazardousMaterials, 2008,152:1-31.
[60]BOLAN N, KUNHIKRISHNAN A, THANGARAJAN R,etal. Remediation of heavy metal(loid)s contaminated soils: To mobilize or to immobilize?JournalofHazardousMaterials, 2014,266:141-166.
[61]MEAGHER R B. Phytoremediation of toxic elemental and organic pollutants.CurrentOpinioninPlantBiology, 2000,3:153-162.
[62]KHAM M S, ZAIDI A, WANI P A,etal. Role of plant growth promoting rhizobacteria in the remediation of metal contaminated soils.EnvironmentalChemistryLetters, 2009,7:1-9.
[63]HERATH I, KUMARATHILAKA P, NAVARATNE A,etal. Immobilization and phytotoxicity reduction of heavy metals in serpentine soil using biochar.JournalofSoilsandSediments, 2015,15:126-138.
[64]HOUBEN D, EVRARD L, SONNET P. Mobility, bioavailability and pH-dependent leaching of cadmium, zinc and lead in a contaminated soil amended with biochar.Chemosphere, 2013,92:1450-1457.
[65]HARTLEY W, DICKINSON N M, RIBY P,etal. Arsenic mobility in brownfield soils amended with green waste compost or biochar and planted withMiscanthus.EnvironmentalPollution, 2009,157:2654-2662.
[66]BEESLEY L, MORENO-JIMÉNEZ E, GOMEZ-EYLES J L. Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil.EnvironmentalPollution, 2010,158:2282-2287.
[67]KARAMI M, CLEMENTE R, MORENO-JIMÉNEZ E,etal. Efficiency of green waste compost and biochar soil amendments for reducing lead and copper mobility and uptake to ryegrass.JournalofHazardousMaterials, 2011,191:41-48.
[68]AHMAD M, LEE S S, YAND J E,etal. Effects of soil dilution and amendments (mussel shell, cow bone, and biochar) on Pb availability and phytotoxicity in military shooting range soil.EcotoxicologyandEnvironmentalSafety, 2012,79:225-231.
[69]PARK J H, CHOPPALA G K, BOLAN N S,etal. Biochar reduces the bioavailability and phytotoxicity of heavy metals.Plant&Soil, 2011,348:439-451.
[70]UCHIMIYA M, BANNON D I, WARTELLE L H,etal. Lead retention by broiler litter biochars in small arms range soil: Impact of pyrolysis temperature.JournalofAgriculture&FoodChemistry, 2012,60:5035-5044.
[71]CAO X D, MA L, LIANG Y,etal. Simultaneous immobilization of lead and atrazine in contaminated soils using dairy-manure biochar.EnvironmentalScience&Technology, 2011,45:4884-4889.
[72]KHAN S, CHAO C, WAQAS M,etal. Sewage sludge biochar influence upon rice (OryzasativaL.) yield, metal bioaccumulation and greenhouse gas emissions from acidic paddy soil.EnvironmentalScience&Technology, 2013,47:8624-8632.
[73]廖敏,黄昌勇,谢正苗.pH对镉在土水系统中的迁移和形态的影响.环境科学学报,1999,19(1):81-86.
LIAO M, HUANG C Y, XIE Z M. Effect of pH on transport and transformation of cadmium in soil water system.ActaScientiaeCircumstantiae, 1999,19(1):81-86. (in Chinese with English abstract)
[74]BRADL H B. Adsorption of heavy metal ions on soils and soils constituents.JournalofColloidandInterfaceScience, 2004,277:1-18.
[75]BASTA N T, RYAN J A, CHANEY R L. Trace element chemistry in residual-treated soil: Key concepts and metal bioavailability.JournalofEnvironmentalQuality, 2005,34:49-63.
Research progress in effects of biochar on transport of inorganic pollutants in soil.JournalofZhejiangUniversity(Agric. &LifeSci.), 2016,42(4):451-459
ZHANG Dong1, LIU Xingyuan2, ZHAO Hongting1*
(1.InstituteofEnvironmentalMaterials&Applications,CollegeofMaterials&EnvironmentalEngineering,HangzhouDianziUniversity,Hangzhou310018,China; 2.GuangdongDazhongAgricultureScienceCo.,Ltd.,Dongguan523169,Guangdong,China)
biochar; sorption; heavy metal; soil; remediation
国家自然科学基金(41271249,21407037);浙江省自然科学基金(LQ14B070006);广东省东莞市引进创新科研团队项目(2014607101003).
Corresponding author):赵红挺(http://orcid.org/0000-0002-4562-4576),Tel:+86-571-87713572,E-mail:hzhao@hdu.edu.cn
联系方式:张栋(http://orcid.org/0000-0002-7329-6370),Tel:+86-571-86919158,E-mail:zhangdong@hdu.edu.cn
2016-01-31;接受日期(Accepted):2016-06-23;网络出版日期(Published online):2016-07-19
X 131; X 53
A
URL:http://www.cnki.net/kcms/detail/33.1247.S.20160719.1832.002.html
