GnRH主动免疫对公猪体内粪臭素代谢的影响
2016-07-14随芬芬韩兴发曹晓涵杜小刚孟风艳储明星曾宪垠
随芬芬,韩兴发,曹晓涵,杜小刚,孟风艳,储明星,曾宪垠*
(1.四川农业大学生命科学学院原子能农业应用研究室,雅安 625014;2.中国农业科学院北京畜牧兽医研究所,农业部畜禽遗传资源与种质创新重点实验室,北京 100193)
GnRH主动免疫对公猪体内粪臭素代谢的影响
随芬芬1,韩兴发1,曹晓涵1,杜小刚1,孟风艳1,储明星2,曾宪垠1*
(1.四川农业大学生命科学学院原子能农业应用研究室,雅安 625014;2.中国农业科学院北京畜牧兽医研究所,农业部畜禽遗传资源与种质创新重点实验室,北京 100193)
旨在探讨促性腺激素释放激素(Gonadotropin releasing hormone,GnRH)主动免疫对公猪体内粪臭素代谢的影响。36只公猪随机分为免疫组(10周龄时初免,18周龄加免)、手术去势组(1周龄外科阉割)及完整对照组(不作任何处理)。应用ELISA检测血清中睾酮、雌二醇及雄烯酮含量,采用高效液相色谱检测脂肪中粪臭素、雄烯酮和血液中雄烯酮含量,荧光定量PCR检测肝中粪臭素代谢相关基因mRNA表达量,ELISA检测肝中粪臭素代谢相关蛋白表达。结果显示,GnRH主动免疫后,血清睾酮浓度显著下降(P<0.05),睾丸严重萎缩。免疫组和手术组公猪血清中睾酮、雌二醇、雄烯酮浓度和脂肪中粪臭素、雄烯酮含量相似(P>0.05),均显著低于对照组(P<0.05)。免疫组公猪肝中CAR、COUP-TF1、CYP2E1、CYB5A、CYP2C49、GSTO2 mRNA表达变化与手术组相似(P>0.05),均显著高于对照组(P<0.05);CYP2A19 mRNA表达量手术组显著高于免疫组(P<0.05),免疫组显著高于对照组(P<0.05);而PXR、SULT1A1 mRNA表达量在3组间无明显差异(P>0.05)。免疫组公猪肝中CYP2A19、CYP2C49和GSTO2蛋白表达变化与手术组相似(P>0.05),均显著高于对照组(P<0.05); CYP2E1和CYB5A蛋白表达量在手术组最高,对照组最低,免疫组介于两组之间,与两组均有显著性差异(P<0.05);而SULT1A1蛋白表达量在3组间无明显差异(P>0.05)。综上表明,GnRH主动免疫降低公猪血清中睾酮、雌二醇和雄烯酮含量,上调肝CYP450s和GSTO2基因和蛋白表达,加速粪臭素在肝中的代谢,从而降低公猪膻味。
GnRH主动免疫;肝;粪臭素代谢;公猪膻味
粪臭素是引起公猪膻味(Boar taint)的2种物质之一,主要由色氨酸在大肠中经微生物降解产生,机体吸收后经肝代谢,代谢产物主要由尿液排出,其中未被代谢的部分经外周血液循环,沉积在脂肪组织中,形成“膻味”[1-2]。粪臭素在动物肝中的代谢主要分为I相代谢和II相代谢,I相代谢主要是给粪臭素加上亲水性的羟基;II相代谢是通过I相添加的羟基连接磺基等水溶性基团,以增强粪臭素的水溶性,便于经尿液排出体外[3-6]。研究表明细胞色素P450家族中CYP2E1、CYP2A19、CYB5A和CYP2C49主要参与粪臭素I相代谢,而磺基转移酶(SULT1A1)和谷胱甘肽-S-转移酶2(GSTO2)主要参与粪臭素II相代谢反应[7]。孤儿核受体((Constitutive androstane receptor,CAR)和(Pregnane X receptor,PXR))通过调节机体内外源物质新陈代谢进而参与粪臭素代谢[8-9]。
有研究表明,GnRH主动免疫可有效抑制睾丸发育,降低动物生殖激素浓度及公猪肉中“膻味”物质含量,从而去除公猪肉中的“膻味”,已成为最有望替代传统外科阉割去势方法[10-15]。GnRH主动免疫能有效降低公猪肉中粪臭素含量[16-17],其主要原因是对粪臭素在肝中的代谢产生影响[18]。GnRH主动免疫对公猪粪臭素代谢影响的分子机制至今鲜见报道。为此,本试验拟研究GnRH主动免疫后公猪体内相关激素含量变化和肝中粪臭素代谢相关基因(CYP2E1、CYP2A19、CYB5A、CYP2C49、GSTO2 和SULT1A1 )mRNA和蛋白表达变化,以阐明GnRH主动免疫对公猪体内粪臭素代谢影响的分子机制。
1 材料与方法
1.1疫苗制备
G6k-GnRH-tandem的合成采用R.H.Meloen等[19]报道的方法,以赖氨酸、色氨酸等10种氨基酸为原料,并用D型赖氨酸(D-lysine)取代第6位的甘氨酸(Glysine),合成品用高压液相色谱纯化。G6k-GnRH-tandem -dimer (TDK-GnRH)合成采用DMSO法将合成的G6k-GnRH-tandem纯化物进行二聚化,得到GnRH并列体二聚物(TDK-GnRH)。EDCI法将TDK-GnRH与鸡卵清蛋白(OVA)连接,形成免疫复合抗原,并与specol佐剂充分乳化。
1.2试验动物选取及处理
1.2.1试验动物的选择、分组及饲养管理选取10周龄、体重一致、健康状况良好的大白×长白×杜洛克公猪36头,按体重随机分为3组,每组12头,组Ⅰ为正常完整对照组、组Ⅱ为GnRH免疫组、组Ⅲ为手术去势组。每组分3圈饲养,每圈4头猪。试验期间动物统一饲喂商品日粮和自由饮水。
1.2.2动物免疫程序免疫组公猪10周龄初免,18周龄加免,采用颈部肌肉注射,注射剂量为2 mL(62.5 μg G6k-GnRH-tandem),对照组公猪不作任何处理,手术组公猪于1周龄进行外科阉割去势。
1.2.3样品采集初免当天颈静脉采血,以后每4周采血1次,直至屠宰。每次所采血样在4 ℃放置过夜,3 000 r·min-1离心20 min,制备血清,-20 ℃保存。所有试验猪于育肥期末(26周龄)屠宰。屠宰后立即采集睾丸、肝和背部脂肪组织,用电子天平称睾丸重量,用游标卡尺测量每侧睾丸长度和宽度,睾丸体积釆用公式:V=(4*π(宽度/2)2(长度/2))/4进行计算,记录为每对睾丸的平均体积。用生理盐水清洗采取组织后迅速放入液氮中备用,再转入-80 ℃超低温冰箱保存备用。
1.3激素含量测定
2.1.2 手术禁忌证 TURBT并无绝对禁忌证,但在遇到以下情况时,应在患者一般情况调整好或病情基本稳定后手术:①系统疾病。如严重的高血压、急性心肌梗死、未能控制的心力衰竭、严重的心律失常、近期发生脑血管意外者;严重的支气管哮喘、肺气肿合并肺部感染、肺功能显著减退者;严重的肝、肾功能异常;全身出血性疾病;严重糖尿病,血糖未能有效控制者;精神障碍、不能配合手术者。②局部或专科疾病。如急性泌尿生殖系统感染;严重的尿道狭窄或尿道闭锁,经尿道扩张或尿道内切开术仍不能置入电切镜鞘者;髋关节强直,不能采取截石位者。
采用酶联免疫试剂盒测血清中睾酮和雌二醇浓度[20]。睾酮灵敏度是0.05 ng·mL-1,最低检测值是0.1 ng·mL-1;雌二醇灵敏度是25 pg·mL-1,最低检测限值是20 pg·mL-1。采用高效液相色谱检测血液雄烯酮的浓度及脂肪中粪臭素和雄烯酮含量[21]。
1.4基因表达测定
使用苯酚-氯仿法提取肝总RNA,利用反转录试剂盒将其反转录成cDNA,再以cDNA为模板在特定引物下进行定量检测。95 ℃10 s;95 ℃变性5 s,58~61 ℃退火+延伸25 s,40个循环,最后进行熔解曲线分析以检测PCR扩增产物的特异性,每个样品3次重复。目的mRNA相对表达水平采用标准曲线法进行计算。引物序列见表1。
表1引物序列
Table 1Primer sequences of body tissue genes

基因Gene收录号GenBankaccessionNo.引物序列(5'-3')Primersequence长度/bpLength退火温度/℃AnnealingtemperatureCOUP-TF1XM_003123784.1F:CACTACGGCCAATTCACCTGCR:GCCCACTTTGAGGCACTTCTTG16288.6CARNM001037996F:CCGCCATATGGGCACTATGTR:GCGAAATGCATGAGCAGAGA15488.7PXRNM001038005F:CACCAGCAGGTGCATTAATGTCR:ATGCCCAGAAGGTAGGAAGGA16487.6CYB5ANM_001001770F:CCGAACAGTCCGACAAAGCCR:CACCTCCAGCTTGTTCCCTTAA16887.4CYP2E1NM_214421F:ACCCTGAGATACGGGCTCCTAAR:ACGGCATCCAGGTAGGGCAT14086.0GSTO2NC_010456.4F:CAGCATGAGATTCTGTCCTTTCGR:TGGCACCAGACCTGAGGGATT13986.8SULT1A1NM_213765F:GACCACAGCATCTCAGCCTTCAR:GGTTACAGCCTGCCATCTTCTCA12187.1CYP2A19NM_214417F:GGAGAAGAAGAATCCTGACACCGR:GCCTCCACATCCGGTTTCTT14488.6CYP2C49NM_214420F:TCCCCAACCCAGAGGTGTTR:CCTTCTCCCACACAAATTCGTT15288.2β-actinDQ845171.1F:GGCCGCACCACTGGCATTGTCATR:AGGTCCAGACGCAGGATGGCG10460
应用Real-time PCR测定肝中CAR、PXR、COUP-TF1(转录因子)、CYP2E1、CYP2A19、CYB5A、CYP2C49、SULT1A1、GSTO2 mRNA相对表达量。
1.5蛋白表达测定
取一部分肝组织,称重,液氮研磨,加入500 μL PBS缓冲液(pH=7.4)并充分震荡,3 000 r·min离心20 min,仔细收集上清。应用酶联免疫法进行酶含量测定,严格按照ELISA试剂盒(Antibodies-online.com)说明书进行操作。
1.6数据处理
利用SAS 9.0统计学软件中GLM对所测数据进行单因子方差分析,采用Duncan法进行数据间多重比较,结果以“平均值±标准差”表示。P<0.05表示差异显著,P<0.01表示差异极显著。
2 结 果
数据处理过程剔除4头犯病公猪,其中对照组2头,手术组与免疫组各1头,最后统计各组动物数分别为对照组10头,免疫组11头,手术组11头。当样品激素含量(睾酮、雌二醇)低于最低检测值时,以最低检测限值统计。
2.1血清抗GnRH抗体滴度
如图1所示,公猪初免后产生较好的免疫反应,免疫后血清抗GnRH抗体滴度显著升高,初免后4周上升到32%,屠宰时(初免后16周)抗体滴度上升到63%。
2.2睾丸重量和体积
如表2所示,GnRH主动免疫后,免疫去势公猪睾丸严重萎缩,免疫去势公猪睾丸重量和体积均下降到对照组33%(P<0.01)。
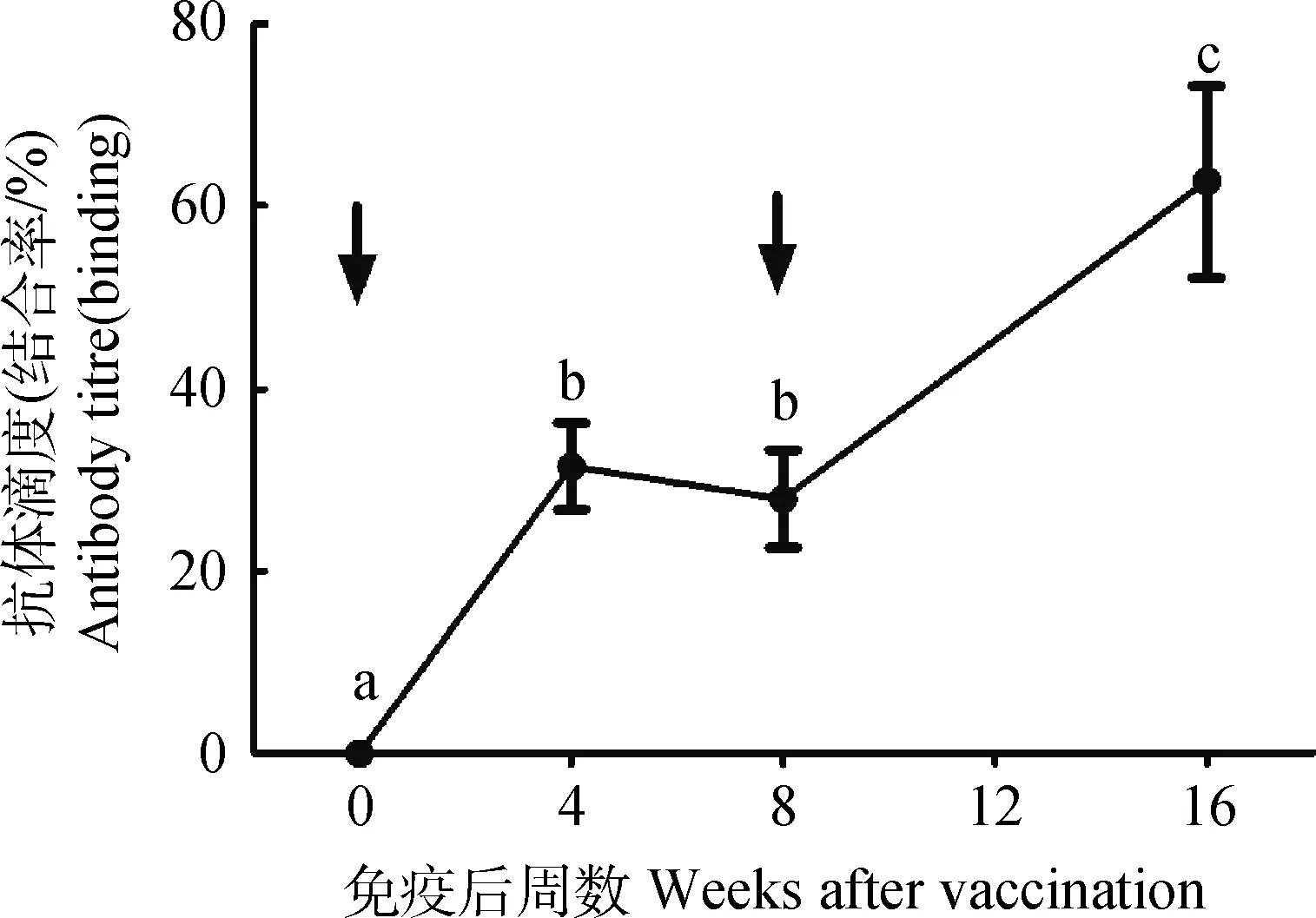
不同字母表示差异显著(P<0.05);箭头表示免疫时间点Different letters among treatments donate a significant difference (P<0.05);arrows indicate time-points of vaccination图1 血清抗GnRH抗体滴度(n=11)Fig.1 Serum anti-GnRH antibody titers (n=11)
2.3血清中睾酮、雌二醇和雄烯酮激素浓度
GnRH主动免疫显著降低公猪血清睾酮,雌二醇和雄烯酮的浓度(表 2)。屠宰时,免疫组公猪血清中睾酮、雌二醇和雄烯酮浓度与手术组相似(P>0.05),且极显著低于完整对照组(P<0.01)。
2.4背部脂肪中粪臭素和雄烯酮含量
屠宰时背部脂肪中粪臭素和雄烯酮含量见表2。与完整对照组相比,GnRH主动免疫显著降低脂肪中粪臭素和雄烯酮含量(P<0.05);手术组公猪脂肪中粪臭素和雄烯酮含量与免疫组相似(P>0.05),均显著高于完整对照组(P<0.05)。
表2屠宰时睾丸重量、体积,脂肪中粪臭素和雄烯酮及血液中激素的含量
Table 2Testicular weight,volume,skotole and androstenone in fat and hormones in plasma of entire male,immuno- castrates,surgical castrates at slaughter

项目Items对照组(n=10)Entiremale免疫组(n=11)Immuno-castrates手术组(n=11)Surgicalcastrates睾丸重量/gTesticularweight229.07±56.61a73.59±35.66b—睾丸体积/cm3Testicularvolume185.31±48.45a61.40±25.65b脂肪中膻味物质Boartaintcompoundsinfat粪臭素/(ng·g-1)Skatole45.33±9.84a16.48±4.23b14.62±1.54b雄烯酮/(μg·g-1)Androstenone1.06±0.08a0.17±0.04b0.16±0.04b血液中激素Hormonesinplasma睾酮/(ng·mL-1)Testosterone5.80±0.46a0.15±0.03b0.11±0.01b雌二醇/(pg·mL-1)E271.96±7.57a32.61±3.84b27.53±2.34b
同行不同上标小写字母表示差异显著(P<0.05)
Different lower-case superscripts letter within row donates a significant difference (P<0.05)
2.5肝组织中CAR、PXR和COUP-TF1 mRNA表达变化
以β-actin为参考基因,肝组织中CAR、PXR和COUP-TFmRNA相对表达量如图2所示。免疫组和手术组公猪肝中CAR、COUP-TF1 mRNA表达量相似(P>0.05),均显著高于对照组(P<0.05);PXRmRNA表达量在3组间无明显差异 (P>0.05)。
2.6肝组织中CYP2E1、CYP2A19、CYP2C49、CYB5A、GSTO2和SULT1A1 mRNA相对表达量
以β-actin为参考基因,肝组织中CYP2E1、CYP2A19、CYP2C49、CYB5A、GSTO2、SULT1A1的mRNA相对表达量见图3。免疫组公猪肝中CYP2E1、CYP5A、CYP2C49、GSTO2 mRNA表达量与手术组相似(P>0.05),均显著高于对照组(P<0.05);CYP2A19 mRNA表达量手术组显著高于免疫组(P<0.05),免疫组显著高于对照组(P<0.05);而SULT1A1 mRNA表达量在3组间无明显差异(P>0.05)。

处理间不同字母表示差异显著(P<0.05)。下同Different letters among treatments donate a significant difference (P<0.05).The same as below图2 完整对照组、免疫组和手术组肝组织中CAR、PXR和COUP-TF1 mRNA表达量Fig.2 mRNA expression of CAR,PXR,COUP-TF1 in the liver from intact males,immuno-castrates and surgical castrates
2.7肝组织中CYP2E1、CYP2A19、CYP2C49、CYB5A、GSTO2和SULT1A1蛋白表达量
CYP2E1、CYP2A19、CYP2C49、CYB5A、GSTO2和SULT1A1蛋白表达量如图4。免疫组公猪肝中CYP2A19、CYP2C49和GSTO2蛋白表达量与手术组相似(P>0.05),均显著高于对照组(P<0.05);公猪肝中CYP2E1和CYB5A蛋白表达量在手术组最高,对照组中最低,免疫组在两者之间,与两组均有显著性差异(P<0.05),SULT1A1蛋白表达量在3组间无明显差异(P>0.05)。

1.CYP2E1;2.CYP2A19;3.CYP2C49;4.CYB5A;5.GSTO2;6.SULT1A1图3 完整对照组、免疫组和手术组肝组织中CYP2E1、CYP2A19、CYP2C49、CYB5A、GSTO2、SULT1A1 mRNA表达Fig.3 mRNA expression of CYP2E1,CYP2A19,CYP2C49,CYP5A,GSTO2,SULT1A1 in the liver from intact males,immuno-castrates and surgical castrated pigs
3 讨 论
GnRH主动免疫后,血液中睾酮含量显著降低,睾丸严重萎缩,与前人研究结果一致[10-12,14]。本研究中GnRH主动免疫公猪脂肪粪臭素和雄烯酮含量下降到外科阉割公猪水平,表明GnRH主动免疫可有效降低公猪肉中由粪臭素和雄烯酮沉积引起的膻味,与文献[14,16]研究结果一致。
免疫组公猪脂肪粪臭素含量降低,主要是由于肝中参与粪臭素代谢的酶活性增加,加速肝粪臭素代谢。而肝中粪臭素代谢是由细胞色素P450家族的一系列酶催化完成,可分为Ⅰ相代谢和Ⅱ相代谢两个阶段[8]。 I相代谢中,发现细胞色素P450家族中的CYP2E1、CYP2A、CYB5A和CYP2C49是催化粪臭素氧化产生具羟基、氨基和巯基,使粪臭素进入II相代谢反应的重要酶[18,22]。粪臭素II相代谢主要由磺基转移酶(SULT1A1)和谷胱甘肽-S-转移酶2(GSTO2)参与完成[8-9]。其中,SULT1A1主要使I相代谢产物加上磺基团增加其水溶性,便于排出体外[23];而GSTO不仅可以转运粪臭素的代谢物排出体外,还可以与粪臭素结合提高粪臭素在组织中转运速度[7]。本研究GnRH主动免疫显著上调公猪肝粪臭素代谢I相代谢CYP2E1、CYP2A19、CYP2C49、CYB5A,及II相代谢GSTOmRNA和蛋白表达水平,表明GnRH主动免疫能有效增强公猪肝对粪臭素的亲水代谢反应和转运能力,继而提高公猪体内粪臭素代谢的能力。

1.CYP2E1;2.CYP2A19;3.CYP2C49;4.GSTO2;5.CYB5A;6.SULT1A1图4 完整对照组、免疫组和手术组肝组织中CYP2E1、CYP2A19、CYP2C49、GSTO2、CYB5A和SULT1A1的蛋白表达量Fig.4 Protein expression of CYP2E1,CYP2A19,CYP2C49,GSTO2,CYB5A,SULT1A1 in the liver from intact males,immuno-castrates and surgical castrated pigs
GnRH主动免疫后,引起CYP450 mRNA和蛋白表达水平增高,主要原因是免疫后公猪血清中性激素显著下调,而性激素可以直接或间接作用于CYP450,影响其活性。性激素是调节粪臭素代谢CYP450酶活性一个重要因子,睾酮可以抑制CYP2E1、CYP2A19的活性[23-26],而雌二醇和雄烯酮主要对CYP2E1表达有抑制作用[16,27]。与上述研究结果相同,本研究中手术去势与免疫组相似,睾酮、雌二醇及雄烯酮含量显著降低,而公猪肝粪臭素代谢CYP2E1、CYP2A、CYB5A、CYP2C49和GSTO2基因表达和蛋白水平显著上调,也表明性激素对肝粪臭素代谢重要酶具有抑制作用。由此推测,GnRH主动免疫可能也是主要通过降低睾丸性激素合成,继而增强肝粪臭素相关代谢酶的活性,从而降低公猪脂肪粪臭素含量。
性激素除直接抑制CYP450s表达和活性外,还可以与核受体结合从而启动一些粪臭素代谢相关基因的转录[8,25,26]。参与粪臭素代谢的主要核受体包括CAR和PXR。其中CAR主要在肝上表达,由性激素和雄烯酮前体物抑制其活性,通过改变核上配体结合的部位来启动CYB5A、CYP2A和CYP2B基因的转录[28]。本研究中与对照组相比,免疫组和手术组肝CARmRNA表达显著上调而对PXR无显著作用。CAR表达增加可启动肝上CYP2A19、CYB5A转录来加速粪臭素I相代谢反应,从而降低脂肪中粪臭素含量。因此,GnRH主动免疫可通过上调公猪肝脏核受体CAR表达,上调CYP450s基因表达和蛋白活性,加速粪臭素I相氧化反应来有效减少脂肪粪臭素含量。
4 结 论
综上表明,GnRH主动免疫公猪通过下丘脑-垂体-性腺轴降低睾丸产生的类固醇激素睾酮、雌二醇、雄烯酮的合成,从而直接或间接通过核受体CAR上调肝中CYP450s和GSTO2基因表达和蛋白活性,加快肝粪臭素代谢,从而降低公猪背部脂肪中粪臭素含量。
[1]JENSEN M T,COX R P,JENSEN B B.3-methylindole (skatole) and indole production by mixed populations of pig fecal bacteria [J].ApplEnvironMicrobiol,1995,61(8):3180-3184.
[2]CLAUS R,RAAB S,RÖCKLE S.Skatole concentration in blood plasma of pigs as influenced by the effects of dietary factors on gut mucosa proliferation[J].JAnimPhysiolAnimNutr,1996,76(1-5):170-179.[3]BABOL J,SQUIRES E J,LUNDSTRÖM K.Hepatic metabolism of skatole in pigs by cytochrome P4502E1 [J].JAnimSci,1998,76(3):822-828.
[4]BABOL J,SQUIRES E J,LUNDSTRÖM K.Relationship between oxidation and conjugation metabolism of skatole in pig liver and concentrations of skatole in fat[J].JAnimSci,1998,76(3):829-838.
[5]DIAZ G J,SQUIRES E J.Role of aldehyde oxidase in the hepaticinvitrometabolism of 3-methylindole in pigs[J].JAgricFoodChem,2000,48(3):833-837.
[6]DIAZ G J,SQUIRES E J.Metabolism of 3-methylindole by porcine liver microsomes:responsible cytochrome P450 enzymes[J].ToxicolSci,2000,55(2):284-292.
[7]GUNAWAN A,SAHADEVAN S,CINAR M U,et al.Identification of the novel candidate genes and variants in boar liver tissues with divergent skatole levels using RNA deep sequencing[J].PLoSOne,2013,8(8):e72298.
[8]RASMUSSEN M K,ZAMARATSKAIA G.Regulation of porcine hepatic cytochrome P450 — implication for boar taint[J].ComputStructBiotechnolJ,2014,11(19):106-112.
[9]GRAY M A,SQUIRES E J.Effects of nuclear receptor transactivation on steroid hormone synthesis and gene expression in porcine Leydig cells[J].JSteroidBiochemMolBiol,2013,133:93-100.
[10]ZENG X Y,TURKSTRA J A,VAN DE WIEL D F,et al.Active immunization against gonadotrophin-releasing hormone in Chinese male pigs[J].ReprodDomestAnim,2001,36(2):101-105.
[11]ZENG X Y,TURKSTRA J A,MELOEN R H,et al.Active immunization against gonadotrophin-releasing hormone in Chinese male pigs:effects of dose on antibody titer,hormone levels and sexual development[J].AnimReprodSci, 2002,70(3-4):223-233.
[12]ZAMARATASKAIA G,RYDHMER L,ANDERSSON H K,et al.Long-term effect of vaccination against gonadotropin-releasing hormone,using improvac,on hormonal profile and behaviour of male pigs[J].AnimReprodSci,2008,108(1-2):37-48.
[13]EINARSSON S,ANDERSSON K,WALLGREN M,et al.Short- and long-term effects of immunization against gonadotropin-releasing hormone,using Improvac,on sexual maturity,reproductive organs and sperm morphology in male pigs[J].Theriogenology,2009,71(2):302-310.
[15]TURKSTRA J A,VAN DER STAAY F J,STOCKHOFE-ZURWIEDEN N,et al.Pharmacological and toxicological assessment of a potential GnRH vaccine in young-adult male pigs [J].Vaccine,2011,29 (21):3791-3801.
[16]BRUNIUS C,ZAMARATSKAIA G,ANDERSSON K,et al.Early immunocastration of male pigs with Improvac®- effect on boar taint,hormones and reproductive organs [J].Vaccine, 2011,29(51):9514-9520.[17]ZAMARATASKAIA G, RYDHMER L, ANDERSSON H K,et al. Long-term effect of vaccination against gonadotropin-releasing hormone, using Improvac TM,on hormonal profile and behavior of male pigs[J].AnimReprodSci,2008,108(1-2):37-48.
[18]ZAMARATSKAIA G,SQUIRE E J.Biochemical,nutritional and genetic effects on boar taint in entire male pigs[J].Animal,2009,3(11):1508-1521.
[19]MELOEN R H,TURKSTRA J A,LANKHOF H,et al.Efficient immunocastration of male piglets by immunoneutralization of GnRH using a new GnRH-like peptide[J].Vaccine,1994,12(8):741-746.
[20]NERINGA S,RONALDAS B,JÜRATÉ S,et al.Effect of active immunization against GnRH on “boar taint”,testes and accessory sex gland in matured boars[J].VetZootech-lith,2014,65(87):1392-2130.
[21]VERHEYDEN K,NOPPE H,ALUWÉ M,et al.Development and validation of a method for simultaneous analysis of the boar taint compounds indole,skatole and androstenone in pig fat using liquid chromatography-multiple mass spectrometry[J].JChromatogrA,2007,1174 (1-2):132-137.
[22]WIERCINSKA P,LOU Y,SQUIRES E J.The roles of different porcine cytochrome P450 enzymes and cytochrome b5A in skatole metabolism [J].Animal,2012,6(5):834-845.
[23]KOJIMA M,DEGAWA M.Sex differences in the constitutive gene expression of sulfotransferases and UDP-glucuronosyltransferases in the pig liver:androgen-mediated regulation[J].DrugMetabPharmacokinet,2014,29(2):192-197.
[24]CHEN G,CUE R A,LUNDSTROM K,et al.Regulation of CYP2A6 protein expression by skatole,indole and testicular steroids in primary cultured pig hepatocytes[J].DrugMetabDispos,2008,36 (1):56-60.
[25]KOJIMA M,DEGAWA M.Serum androgen level is determined by autosomal dominant inheritance and regulates sex-relatedCYPgenes in pigs[J].BiochemBiophysResCommun, 2013,430(2):833-838.
[26]ZAMARATSKAIA G,GILMORE W J,LUNDSTRÖM K,et al.Effect of testicular steroids on catalytic activities of cytochrome P450 enzymes in porcine liver microsomes[J].FoodChemToxicol,2007,45(4):676-681.[27]DORAN E,WHITTINGTON F W,WOOD J D,et al.Cytochrome P4502E1 (CYP2E1) is induced by skatole and this induction is blocked by androstenone in isolated pig hepatocytes[J].ChemBiolInteract,2002,140(1):81-92.
[28]GRAY M A,POLLOCK C B,SCHOOK L B,et al.Characterization of porcine pregnane X receptor,farnesoid X receptor and their splice variants[J].ExpBiolMed(Maywood),2010,235(6):718-736.
(编辑程金华)
Effects of Active Immunization against GnRH on Skatole Metabolism in Male Pigs
SUI Fen-fen1,HAN Xing-fa1,CAO Xiao-han1,DU Xiao-gang1,MENG Feng-yan1,CHU Ming-xing2,ZENG Xian-yin1*
(1.IsotopeResearchLaboratory,CollegeofLifeScience,SichuanAgriculturalUniversity,Ya’an625014,China;2.KeyLaboratoryofFarmAnimalGeneticResourcesandGermplasmInnovationofMinistryofAgriculture,InstituteofAnimalScience,ChineseAcademyofAgriculturalSciences,Beijing100193,China)
The objective of the present study was to investigate the effects of active immunization against GnRH on skatole metabolism in boars.Thirty-six boars at the age of 10 weeks were randomly allocated to 3 groups.Included intact males(not administrated),Immuno-castrates(immunized against GnRH at 10 and 18 weeks of age),and Surgical castrates (surgically castrated at the age of one week).The hormone levels of testosterone and estradiol in plasma were determined by ELISA,the androstenone levels in plasma and androstenone,skatole levels in fat were determined by HPLC.The mRNA expression of skatole-metabolism genes in the liver were analyzed by real-time fluorescence quantitative PCR technique,and the protein expression of them were determined by ELISA.Active immunization against GnRH in boars resulted in a atrophy of testes and reduction of plasma testosterone levels (P<0.05).Plasma testosterone,androstenone,estradiol levels and fat androstenone and skatole levels in immunized boars were similar to that of surgical castrated boars (P>0.05),in which were significantly lower than that of intact controls (P<0.05).In the liver,CARCOUP-TF1,CYP2E1,CYB5A,CYP2C49,GSTO2 mRNA expression in immune-castrated boars were similar to that of surgical castrated boars (P>0.05),which were significantly higher than that of intact controls (P<0.05);theCYP2A19 mRNA expression in surgical castrated pigs was higher than in intact controls (P<0.05),whileCYP2A19 mRNA expression in immunized boars was in between,which was significantly different from either surgical castrates or intact controls (P<0.05),PXRandSULT1A1 mRNA expression were not significantly different among 3 groups (P>0.05).In the liver,the CYP2E1,CYB5A protein expression were highest for surgical castrated pigs and lowest for intact controls,with vaccinated pigs at an intermediate level;CYP2A19,CYP2C49,GSTO2 protein expression in surgical castrated boars were similar to that of surgical castrated boars (P>0.05),in which both were significantly higher than intact controls(P<0.05);but SULT1A1 protein expression had no significant difference among 3 groups (P>0.05).Active immunization against GnRH in boars decreased plasma testosterone,estradiol and androstenone levels,increasedCYP450s andGSTO2 mRNA and protein expression and to reduce boar-taint by accelerating skatole metabolism in the liver.
active immunization against GnRH;liver;skatole metabolism;boar taint
10.11843/j.issn.0366-6964.2016.06.008
2015-12-01
四川农业大学双支计划(00770107);四川省教育厅重点项目(15ZA0005)
随芬芬(1989-),女,山东济宁人,硕士生,主要从事动物生殖免疫调控研究,E-mail:suifen90@163.com
曾宪垠,博士生导师,主要从事动物生理与生殖免疫调控研究,E-mail:yzeng1966@163.com
S828.3
A
0366-6964(2016)06-1140-07
