Analytic Study on the Characteristics of Infrared Spectrum of Oil Pool Flame
2016-07-12LIUHongtaoCHENZhiliHUTangaoLIANGJianjunLIUQiangWEIXiaolin
LIU Hong-tao,CHEN Zhi-li,HU Tan-gao,LIANG Jian-jun,LIU Qiang,WEI Xiao-lin
1. Department of Military Oil Application and Management Engineering, Logistical Engineering University,Chongqing 401311, China
2. Department of National Defense Architecture Planning and Environmental Engineering,Logistical Engineering University,Chongqing 401311, China
3. College of Science, Hangzhou Normal University, Hangzhou 311121, China
4. Department of Military Oil Supply Engineering, Logistical Engineering University,Chongqing 401311, China
Analytic Study on the Characteristics of Infrared Spectrum of Oil Pool Flame
LIU Hong-tao1,CHEN Zhi-li2*,HU Tan-gao3,LIANG Jian-jun4,LIU Qiang1,WEI Xiao-lin1
1. Department of Military Oil Application and Management Engineering, Logistical Engineering University,Chongqing 401311, China
2. Department of National Defense Architecture Planning and Environmental Engineering,Logistical Engineering University,Chongqing 401311, China
3. College of Science, Hangzhou Normal University, Hangzhou 311121, China
4. Department of Military Oil Supply Engineering, Logistical Engineering University,Chongqing 401311, China
In virtue of the severity and scale of the pollution caused by oil pool flame, space remote sensing can provide us a new way of monitoring in real time the oil pool flame pollution. Space remote sensing monitoring is based on the analysis of target spectrum characteristics. Due to lack of adequate researches on the characteristics of infrared spectrum of oil pool flame, this paper carries out the analytical study on flame spectrums of several types of oil, mixed oil and other combustible objects in outdoor space by establishing all-flame infrared testing system with the spectrum range of 1~14 μm. The results show that the spectrum curves of oil pool flame of 92#gasoline, 95#gasoline, 0#diesel, aviation kerosene and lube have similar features, that there exist characteristics emission peaks at the area of certain wave lengths—H2O characteristics emission peak for 1.1, 2.4, 2.8 and 6.3 μm, CO2characteristics emission peak for 4.2 and 4.5 μm, C—H stretching vibration emission peak for 3.4 μm, and no obvious characteristics peak for spectrum curves of 6.3 μm and above; that there is no obvious difference in the spectrum of oil pool flame among the mixtures of 92#gasoline and 0#diesel at different proportions, that the comparison of the flame spectrum of 92#gasoline with that of wood and paper shows that there appears a characteristics emission peak at 3.4 μm; that though the flame spectrum of alcohol has similar radiated emission near 3.4 μm, the proportion of its radiation intensity to that of CO2at 4.5 μm is far less than that for the flame spectrum of 92#gasoline; that the flame spectrum of honeycomb briquette is similar to that of gray body radiation. The differences in flame spectrum among all kinds of combustible materials are closely linked to their chemical compositions and burning reaction mechanisms. Comparative analysis on the spectrum characteristics at continuous area, intermission area and flue gas area shows that C—H stretching vibration peak only exists in continuous area, which proves that the emission peak is caused by the combustible reaction of oil and gas. This result is in line with the mechanism of oil pool combustion reaction. The experimental conclusion is of great significance in the remote-sensing recognition of oil pool flame based on the analysis of spectrum characteristics.
Oil pool flame;Emission spectrum;Remote-sensing recognition
Introduction
There have been a considerable number of oil depots in China and both the number and the scale are on the rise, which increases the possibility of fire and explosion and imposes potential threats on public safety. The combustion after the explosion of oil tank and the removal of its top belongs to the typical oil-pool fire non-premixed combustion with a large burning area and height, causing heat radiation pollution to the surrounding environment[1]. Currently, ground on-the-spot monitoring is the main way of monitoring oil depot fire pollution, which is hard to meet the new requirements due to its relatively backward technology. Space remote sensing technology makes possible the rapid, real-time, dynamic and big-scale monitoring of oil depot fires in a time and energy-saving way. Since remote sensing monitoring takes the spectrum characteristics of target as one of the main identification criteria, the study on the spectrum characteristics of monitoring targets is of great significance.
Currently, there are few research and reports on the spectrum characteristics of oil pool fire in open space in China and abroad. Nie Wansheng[2]studied the infrared radiation characteristics of tail flame of liquid rocket engine,mainly focusing on the characteristics emission spectrum of H2O and CO2, but the type of fuel cannot be identified only according to the characteristics emission wave band of CO2and H2O since the burning of many hydrocarbon fuels may produce CO2and H2O and there exist emission peaks of flame spectrum at the area of two wave bands. Cai Xiaoshu[3]studied the flame spectrum of diesel and other fuels within the wave band scope of visible light and near infrared (500 to 1 100 nm) by using CCD fiber spectrometer. The relevant results showed that there exists similarities among the flame spectrum of diesel, butane and candle—H2O characteristics absorption peak appearing at the area of 940 nm and that the appearance of H2O characteristics emission peak or absorption peak at 940 nm is linked to the temperature of flame. Researchers from Xi’an Electronic Science and Technology University[4]carried out spectrometer-based system integration research on flame detection and tested gasoline flame spectrum within the scope of ultraviolet, visible light and near infrared, but there are few reports on testing other fuels based on this system. Jill M. Suo-Anttila[5]used ethanol, the mixture of ethanol and toluene, and normal heptanes as the fuel and studied the flame radiation characteristics of an oil pool with a diameter of 2 m and different heights. The results showed that the products of burning selected fuels were mainly H2O and CO2, that the radiation of flue gas generated during the burning of ethanol and normal heptanes is much stronger, and that the flame near the liquid surface may produce high-level radiation, which may veil part of emission spectrum of H2O and CO2.
Infrared spectrum analysis is characterized with high efficiency, low cost and timeliness, which is widely applied to petroleum chemical industry and food processing[6-8]. In this paper, by establishing optical system and using Fourier infrared spectrometer, a testing study and comparative analysis are made on the small-scale oil pool flame of 92#gasoline, 95#gasoline, 0#diesel, aviation kerosene and lube as well as the flame of wood, coal, alcohol and paper. Besides, an analytical research is made on the spectrum characteristics of oil pool flame and the data on relevant characteristics are figured out, which lays a foundation for remote sensing-based monitoring and identification of oil depot fire pollution.
1 Experiment
Nicolet 6700 Fourier infrared spectrometer by US Thermo Fisher Scientific is adopted to test the infrared spectrum of different types of fuel. The followings are the main technical parameters of the spectrometer: the signal-noise ratio of 50 000∶1, a spectrum testing range of 1~14 μm and a spectrum resolution of 5 nm. The igniting equipment is JAJALIN electronic igniter. A oil pan with a diameter of 10 cm and a height of 3 cm is used as the vessel containing liquid fuel in the experiment. All oil material, alcohol (an ethanol content of 99%) and paper used in the experiment are purchased in the market.
The experiment was carried out in outdoor open space and an optical system was set above the flame of fuel to simulate the view finding position of space remote-sensing fire monitoring satellite sensor. The reflecting mirror is 1.5 m far from the ground surface and 1.3 m from the spectrometer. The experimental platform is shown in Fig.1. Every time before the experiment, environmental surrounding noise was measured so as to remove the influence of noise on testing results. The spectrum curve of each fuel tested was figured out based on the average value of 32 samplings. The spectrum data were normalized and the comparative analysis was made.
Fig.1 The layout of experimental platform
2 Results and Analysis
2.1 Oil pool flame spectrum analysis of different types of oil material and mixture
The atoms and molecules of different substances have different energy levels, which may emit or absorb a certain amount of photons when excited by external energy. Thus, the corresponding spectrum is the characteristics spectrum of the substance. Flame spectrum consists of continuous strip-shaped spectrum and incontinuous line-shaped spectrum. Line-shaped spectrum is a kind of spectrum line generated by the spontaneous transition radiation of an excited atom, and the characteristics of spectrum line is linked to the atomic structure, so line-shaped spectrum can be seen as characteristics spectrum. Moleculic spectrum is much more complex since the vibrational transition, rotational transition and electronic transition of the atom may produce spectrum, so moleculic spectrum is the integration of vibrational, rotational and electronic spectrum, thus becoming a strip-shaped one. Oil pool flame consists of such combustion products as CO2, H2O and carbon black particles, and also consists of substances participating in combustion, including O2. Those molecules with a symmetrical structure like O2and N2do not produce characteristics emission or absorption when excited by external energy[3], having a limited impact on the spectrum characteristics of oil pool flame.
Fig.2 shows the oil pool flame spectrums of all types of oil. Within the wave band of 1~14 μm, 0#diesel, 92#gasoline, 95#gasoline, aviation kerosene and lube have similar features in flame spectrum curve—a weak H2O emission peak at 6.3 μm, no obvious characteristics for all spectrum curves after 6.3 μm, and the range of the characteristics wave band of all oil spectrum curves at 1~5 μm. There exist H2O characteristics emission peaks in the vicinity of 1.1,2.4 and 2.8 μm and CO2emission peaks at 4.2 and 4.5 μm. For all oil pool flame spectrums, there exists a C—H stretching vibration emission peak at 3.4 μm[9]. The combustion of oil may produce not only such complete combustion products as H2O and CO2, but also incomplete combustion products—carbon black particles, due to low equivalent ratio of oil gas and oxygen. The flame light is generated by heated carbon black particles. The emission spectrums of 1.5~1.75 μm are mainly produced by lighting carbon particles. The smoke quantity of diesel is relatively higher, so its flame spectrum emission intensity is higher than those of other types of oil in the vicinity of 1.75 μm.
Since the intensity of oil pool flame heat radiation is high and the heat convection between flame and oil surface is strong, liquid oil inside the oil tank may become boiling and spill out due to the heat transfer of flame heat radiation and tank walls, causing the burning of the mixture of different types of oil. Therefore, testing analysis was made on the flame spectrum of oil mixture. At the ratio of 1∶5, 1∶10, 5∶1 and 10∶1 respectively, 92#gasoline and 0#diesel is mixed. The characteristics of the mixture’s flame spectrum were measured. As shown in Fig.3, the mixture of 92#gasoline and 0#diesel at different proportions is similar to each type of oil in flame spectrum characteristics, showing no ocvious unique characteristics. Its main characteristics radiation is from H2O emission peaks in the vicinity of 1.1, 2.4 and 2.8 μm, CO2emission peaks at 4.2 and 4.5 μm, and C—H stretching vibration emission peak at 3.4 μm.

Fig.2 Flame spectrum of several types of oil pool flame
The experimental results show that there is no obvious difference between the flame spectrum of each oil and that of the mixed oil. The ignition point of gasoline is relatively low and the octane value of 95#gasoline is higher than tha of 92#gasoline. The ignition point of diesel is higher than that of gasoline, and the ignition point of aviation kerosene is somewhere between that of gasoline and that of diesel since it is made of kerosene and other additives. Lube is made of mineral oil and various types of additives. The main component of 92#gasoline, 0#diesel, 95#gasoline and aviation kerosene is made from crude oil, consisting of hydrocarbons, so there is no obvious difference in oil pool flame spectrum characteristics.

Fig.3 Oil pool flame spectrum of the mixtures of 92#gasoline and 0#diesel at different proportions
2.2 Analysis of oil pool flame spectrum characteristics at different combustion positions
As is shown in Fig.4, oil pool flame can be divided into continuous position, intermission position and smoke plume position. Combustion happens first at continuous position where there is adequate fuel. The upper middle part of flame is the intermission position containing all kinds of complete combustion products and incomplete combustion products.The top part of flame is the smoke position mainly containing complete combustion products and carbon black particles generated by imcomplete combustion.

Fig.4 Layout of oil pool characteristics at different burning positions
One characteristics wave band of oil pool flame spectrum measured in the experiment, which is the emission peak at 3.4 μm, is generated by the stretching vibration of carbon-hydrogen bond. The characteristics wave bands of CO2and H2O exist in each of the remaining fuel spectrum lines. It can be deduced that C—H emission peak at 3.4 μm is the characteristics of reactants in the early stage of oil pool flame combustion. In order to verify the deduction, only emitted light of flame at a certain height is allowed to pass through the baffle by regulating the height of oil pan and setting a baffle between 92#gasoline pool flame and infrared spectrometer, so as to measure the spectrums at different positions of 92#gasoline pool flame. The results are shown in Fig.5.

Fig.5 92# gasoline pool flame spectrum characteristics at different burning positions
Fig.5 shows that there is no emission peak at 3.4 μm in the intermission position and smoke plume position, but in the continuous position of 92#oil pool flame. Gasoline is a kind of complex mixture of hundreds of hydrocarbons, including alkane, cyclane, alkene and arene. The split products in the initial components of oil gas or the intermediate products in the process of combustion may emit characteristics radiation in high temperature environment, so that there may appear a characteristics wave band in flame spectrum. The continuous position of oil pool flame is of adequate fuel and complex chemical composition and reaction. In the process of gasoline combustion, the first is the chemical reaction between air and the oil gas evaporated from the liquid surface, which is the beginning of chain reaction. The concentration of free radical gradually increases, which participates in chemical reaction, generating several types of middle products. The experimental results show that the characteristics emission peak of oil pool flame at 3.4 μm is from the initial-state substance participating in oil gas combustion chemical reaction, which is the characteristics radiation produced by hydrocarbon gas, due to the evaporation caused by flame heating, in the high-temperature continuous position of flame.
2.3 Comparative analysis of oil pool flame spectrum and other combustible material flame spectrum
Fig.6 indicates the flame spectrums of other types of fuel with the spectrum characteristics at the wave band range of 1~5 μm, among which the most obvious one is CO2strong emission peak at 4.5 μm. The flame spectrum curve of honeycomb briquette is a continuous spectrum with a distinguished spectrum line—a strong characteristics of a trough and two peaks within the range of 4~5 μm and the almost equivalent intensity of two peaks. Honeycomb briquette is made from raw coal, carbide sawdust and other components. The burning of coal can produce combustible gas in high-temperature environment, which reacts with oxygen in the air-the process of flame burning-producing CO2and H2O. After that, when the coal solid does not produce combustible gas, the coal solid becomes solid waste consisting of coal particles, charcoal particle and carbon black, which may emit continuous spectrum, playing a leading role in the flame spectrum of honeycomb briquette. The coal flame spectrum figured out through the testing system is a mixture of spectrums of burning coal flame and high-tempearture solid waste. The radiation of solid waste leads to the grey body radiation charactersitics of honeycomb briquette flame spectrum. There appear CO2characteristics peaks at 2.7 and 4.5 μm and H2Ocharacteristics peaks at 2.8 and 6.3μm.
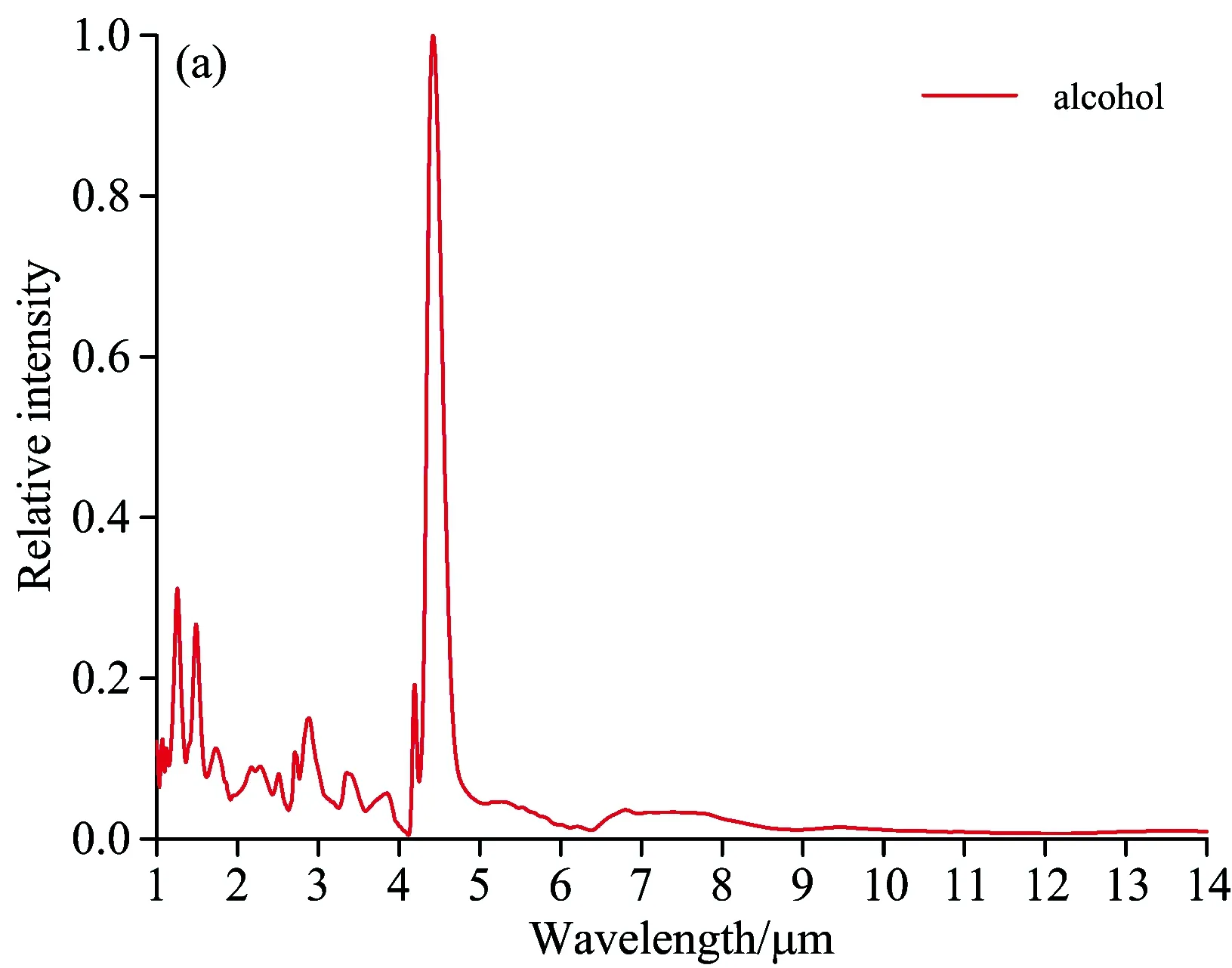
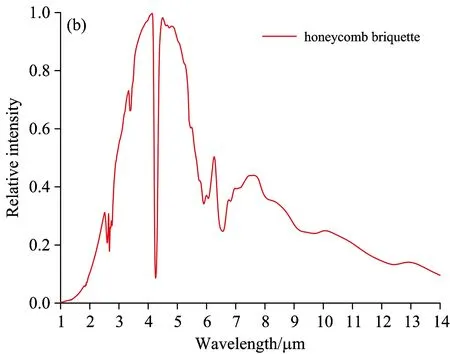
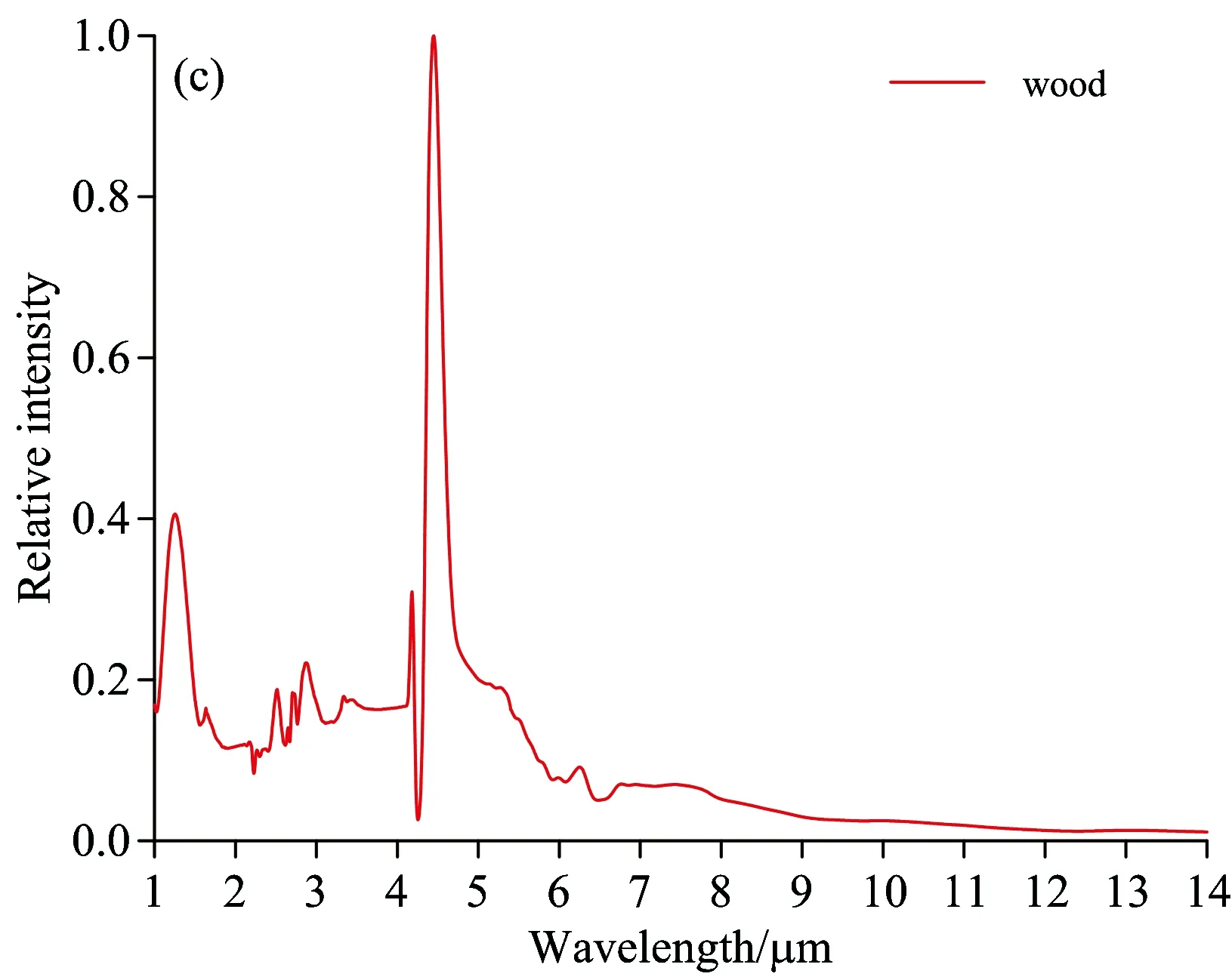
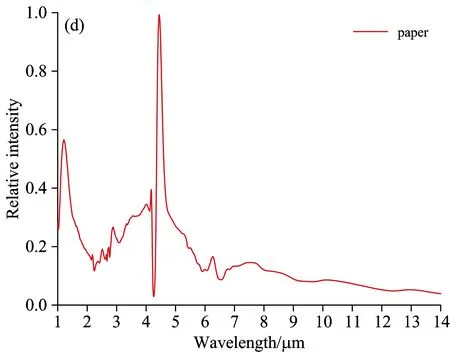
Fig.6 Flame spectrum of alcohol, honeycomb briquette, wood and paper
Fig.6 shows that there are some differences between the flame spectrum of 92#gasoline pool and that of other fuels:
Within the wave band of 1~1.5 μm, 92#gasoline pool flame spectrum has an H2O emission peak at 1.1 μm, while the alcohol flame spectrum has two H2O emission peaks at 1.3 and 1.5 μm respectively. For 92#gasoline pool flame spectrum, there are four emission peaks at the wave-band range of 2.5~3 μm while three emission peaks for alcohol flame spectrum. At 3.4 μm, 92#gasoline pool flame spectrum has a strong emission peak caused by C—H stretching vibration, and the ratio of its radiation intensity and that of CO2at 4.5 μm is 30∶100. The strength of C—H vibration is linked to chemical radicals. Alcohol flame spectrum has a weak C—H vibration in the vicinity of 3.4μm, and the ratio of its radiation intensity and that of CO2at 4.5 μm is 8∶100. Alcohol flame spectrum has a weak emssion peak at 3.8 μm. Alcohol consists of ethanol, whose combustion reaction is complicated, including 383 elementary reactions[10]with many intermission products. Due to the extremely short-term existence or low radiation intensity of these intermission products, there is no characteristics emission spectrum line in flame spectrum. The composition of gasoline is much more complex than that of alcohol, so there is no elaborate and uniform mechanism of combustion chemical reaction available. The differences between the burning flame spectrum of gasoline and that of alcohol lie in the chemical compostion and combustion reaction mechanism. The differences between the flame spectrum of paper and that of 92#gasoline lie in the wave-band range of 2.5~3 μm. The paper flame spectrum has a CO2emission peak at 2.7 μm and no C—H stretching vibration peak at 3.4 μm. For wood flame spectrum, the difference is that there is no C—H stretching vibration peak at 3.4 μm. Both wood and paper are solid fuel, whose combustion reaction is similar to that of coal. There are differences among the flame spectrums of gasoline, wood and paper due to the differences in chemical composition and chemical reaction mechanism. The complete combustion products of wood and paper are CO2and H2O, so the main characteristics of their flame spectrums appear at the emission wave band of these two combustion products.
There is a high possibility of large-scale fire pollution happening to oil depot, forest and coalfield. Currently there is few space remote-sensing data on oil depot fire, and the recognition of forest fire and coalfield fire is based on high-temperature target algorithm. The flame spectrum characteristics are seldom taken as the judgment criterion for fire disaster. The understanding of the differences among the flame spectrums of oil, wood and coal is of great significance in remote-sening recognition of these three types of large-scale fire pollution. The spectrum characteristics analysis can be made on high-temperature targets based on the remote-sensing data. Since the complex compostion of air may cause the attenuation of radiation intensity of oil pool flame, there need more further researches.
3 Conclusion
By establish a Frouier infradred spetrometer-based optical testing system, this paper tests and analyzes the spectrum characteristics of several types of oil pool flame, mixed oil flame and other combustible material flame within the wavelength of 1~14 μm as well as the spectrum characteristics of different positions of oil pool flame. The results show that:
(1) the spectrum characteristics of oil pool flame of 92#gasoline, 95#gasoline, 0#diesel, aviation kerosene and lube have similar features—there exist characteristics emission peaks at the area of certain wave lengths—H2O characteristics emission peak for 1.1, 2.4 and 2.8 μm, CO2characteristics emission peak for 4.2 and 4.5 μm, C—H stretching vibration emission peak for 3.4 μm, and no obvious characteristics peak for spectrum curves of 6.3 μm and above.
(2) 92#gasoline and 0#diesel are mixed at the ratio of 1∶5, 1∶10, 5∶1 and 10∶1 respectively to measure the flame spectrum characteristics of the mixtures. The results show no obvious difference in flame spectrum characteristics between the mixtures and each type of oil.
(3) For 92#gasoline pool flame, its C—H stretching vibration peak at 3.4 μm is located in the continuous position of the flame and is not found neither in the intermission position or plume position, which indicates that C—H stretching vibration peak is caused by the initial-state substance of oil gas combustion reaction—hyrdrocarbon steam.
(4) Compared with those of paper and wood flame spectrums, the main characteristics of oil pool flame spectrum is the emission peak at the wavelength of 3.4 μm. Though the flame spectrum of alcohol has similar radiation in the vicinity of 3.4 μm, the ratio of its radiation intensity to that of CO2at 4.5 μm is far less than that for 92#gasoline flame spectrum. The flame spectrum of honeycomb briquette is different from that of other fuels, having the characteristics of a trough and two peaks within the range of 4~5 μm and having the grey body radiation characteristics within the scope of tested spectrums. The differences among the flame spectrums of fuels lie in the chemical composition and combustion reaction mechanism.
The study in this paper has laid a foundation for remote-sensing detection and recognition of oil pool fire, which is of great significance for further research.
[1] CHEN Zhen, WEI Xiaolin. Procedia Engineering,2014,84: 514.
[2] NIE Wan-sheng, YANG Jun-hui, HE Hao-bo. Journal of Natioal Uniersity of Defense Technology, 2005, 27(5): 92.
[3] CAI Xiao-shu, JI Kun, ZHAO Zhi-jun. Journal of Engineering Thermophysics,2004, 25(1): 173.
[4] Han Ming,Lin Xiao-chun,An Yu-ying. Journal of Applied Optics,2007,28(3): 342.
[5] Jill M Suo-Anttila, Thomas K Blanchat, Allen J Ricks, et al. Proceedings of the Combustion Institute, 2009,32(2): 2571.
[6] LIU Ya-fei,YUAN Hong-fu,SONG Chun-feng. Spectroscopy and Spectral Analysis,2014,34(11): 2929.
[7] ZHU Shi-ping,WANG Gang,YANG Fei. J. Infrared and Millimeter Waves,2008,27(2): 129.
[8] CHEN Quan-sheng,ZHAO Wen-jie,ZHANG Hai-dong. Acta Optica Sinica,2006,26(6): 933.
[9] Phani K Raj. Journal of Hazardous Materials, 2007, 142(3): 724.
[10] Nick M Marinov. International Journal of Chemical Kinetic, 1999, 31(3): 183.
*通讯联系人
O657.3
A
油料池火焰红外光谱特性分析研究
刘洪涛1,陈志莉2*,胡潭高3,梁建军4,刘 强1,魏小林1
1. 后勤工程学院军事油料应用与管理工程系,重庆 401311
2. 后勤工程学院国防建筑规划与环境工程系,重庆 401311
3. 杭州师范大学理学院,浙江 杭州 311121
4. 后勤工程学院军事供油工程系,重庆 401311
油罐池火燃烧污染强度大、范围广,航天遥感可成为实时动态监测油罐池火灾污染的新途径。航天遥感监测以目标光谱特性分析为基础,针对油料池火焰光谱特性研究不足的现状,通过构建全火焰红外测试系统,在室外开放空间条件下对多种油料及混合油料池火焰光谱,其他可燃物火焰的发射光谱进行了测试分析研究,光谱范围1~14 μm。结果表明:92#汽油、95#汽油、0#柴油、航空煤油、润滑油池火焰的光谱曲线特征相似,在特定的波长处存在特征发射峰,在1.1,2.4,2.8及6.3 μm附近存在H2O特征发射峰,在4.2及4.5 μm附近存在CO2发射峰,在3.4 μm处存在C—H伸缩振动发射峰,6.3 μm后各光谱曲线无明显特征峰。92#汽油与0#柴油以不同比例混合的池火焰光谱与各油料池火焰光谱相比也无明显差别。92#汽油池火焰光谱与木柴及纸张火焰光谱相比,在3.4 μm处存在特征发射峰;酒精火焰光谱虽然在3.4 μm附近也有类似辐射发射,但辐射强度与4.5 μm处CO2的辐射强度之比远低于92#汽油池火焰光谱在此两波段处辐射强度之比;蜂窝煤火焰光谱近似灰体辐射光谱。各燃料火焰光谱的差异主要由燃料的化学组成及燃烧反应机理的差异决定的。对92#汽油池火焰连续区、间歇区及烟气区的光谱特性进行了比较分析,结果表明3.4 μm处的C—H伸缩振动峰只存在于连续区,证明了该发射峰是参与燃烧化学反应的油气产生的,该结果与油料池火燃烧反应机理吻合。实验结论对基于光谱特性分析的油料池火焰遥感识别具有重要借鉴意义。
油料池火焰;发射光谱;遥感识别
2015-06-24,
2015-11-05)
Foundation item:Natural Science Foundation of China(21377166)
10.3964/j.issn.1000-0593(2016)10-3442-07
Received:2015-06-24; accepted:2015-11-05
Biography:LIU Hong-tao,(1988-),a doctor at Department of Military Oil Application and Management Engineering, Logistical Engineering University e-mail: 382106994@qq.com *Corresponding author e-mail: 1012262034@qq.com
猜你喜欢
杂志排行
光谱学与光谱分析的其它文章
- Gd靶激光等离子体光源离带辐射及其等离子体演化的研究
- Probing the Binding of Torasemide to Pepsin and Trypsin by Spectroscopic and Molecular Docking Methods
- Mn(Ⅱ)-5-Br-PADAP共沉淀-火焰原子吸收光谱法测定虾、贝样中的镉
- Near Infrared Spectroscopy Study on Nitrogen in Shortcut Nitrification and Denitrification Using Principal Component Analysis Combined with BP Neural Networks
- 内蒙古草原植被最大光能利用率取值优化研究
- 健康和糖尿病大鼠红细胞荧光光谱非线性程度差异
