Role of Interfacial Viscosity and pH in L-Phenylalanine,L-Tryptophan Molecular Rotors
2016-07-12MeenakshisundaramKamatchiSankaranarayanan
N.Meenakshisundaram, Kamatchi Sankaranarayanan
1.Centre for Nonlinear Science and Engineering, School of Electrical and Electronics Engineering,SASTRA University, Thanjavur 613401, India 2.DST-INSPIRE Faculty, Department of Energy and Environment,National Institute of Technology, Trichy 620015, India
Role of Interfacial Viscosity and pH in L-Phenylalanine,L-Tryptophan Molecular Rotors
N.Meenakshisundaram1, Kamatchi Sankaranarayanan2*
1.Centre for Nonlinear Science and Engineering, School of Electrical and Electronics Engineering,SASTRA University, Thanjavur 613401, India 2.DST-INSPIRE Faculty, Department of Energy and Environment,National Institute of Technology, Trichy 620015, India
Protein folding involves the aminoacid sequence to come forth and form an energy minimized structure.Recently molecular crowding leading to increase in viscosity is said to be one of the major concerns affecting protein folding.Many external fluorescent probes are used to detect such increases in viscosity.Since most of the protein sequences contain L-Phe and L-Trp, in this study we have used these aminoacids as probes to detect changes in viscosity.This study will help to advance the knowledge on molecular crowding effects in protein folding.
Protein folding; Molecular crowders; Interfacial viscosity; Fluorescent probes
Received:2015-07-20; accepted:2015-11-24
Introduction
Amino acids play central roles both as building blocks of proteins and as intermediates in metabolism.The 20 amino acids that are found within proteins convey a vast array of chemical versatility.The precise amino acid content, and the sequence of those amino acids, of a specific protein, is determined by the sequence of the bases in the gene that encodes that protein.The chemical properties of the amino acids of proteins determine the biological activity of the protein.Proteins not only catalyze all (or most) of the reactions in living cells, they control virtually all cellular process.In addition, proteins contain within their amino acid sequences the necessary information to determine how that protein will fold into a three dimensional structure, and the stability of the resulting structure.The field of protein folding and stability has been a critically important area of research for years, and remains today one of the great unsolved mysteries.Especially, the effect of molecular crowding in cells on proteins are of much importance.In this regard, L-Phenylalanine (L-Phe) and L-tryptophan (L-Trp) being present in most of the proteins and highly sensible to spectroscopy can be used as probes to study the role of molecular crowding in proteins.
L-Phe is an essential amino acid, is a derivative of alanine with a phenyl substituent on the β carbon.Phenylalanine is quite hydrophobic and thus always found buried within a protein.The π electrons of the phenyl ring can stack with other aromatic systems and often do within folded proteins, adding to the stability of the structure.L-Trp, is also an essential amino acid, is the largest of the amino acids.It is also a derivative of alanine, having an indole substituent on the β carbon.The indole functional group absorbs strongly in the near ultraviolet part of the spectrum.The indole nitrogen can hydrogen bond donate, and as a result, tryptophan, or at least the nitrogen, is often in contact with solvent in folded proteins.
In this work, role of viscogens like sucrose, trehalose, polyethyleneglycol (PEG) and glycerol on the L-Phe and L-Trp have been studied.L-Phe and L-Trp can act as molecular rotors in presence of these viscogens.Molecular rotors are fluorophores whose fluorescence emission changes as a function of the viscosity of their microenvironment[1].Molecular rotors have been highlighted as promising candidates for measurements of local viscosity using the change of fluorescence quantum yield[2].Several such molecular rotors suitable for imaging of viscosity of internal cellular organelles have been reported[3-9], including specific probes for lysosomes[10]and mitochondria[11]as well as genetically targeted probes[12].
Recent study has shown that aminoacids have different shear viscosities near to different lipids[13].Thus in this study L-Phe and L-Trp have been used as molecular rotors, at two different pH and for sensing the viscous environment.
1 Experimental Methods
1.1 Materials
L-Phe and L-Trp were purchased from Sigma, USA with >99% purity.The viscogens Sucrose, Trehalose, Glycerol, Polyethyleneglycol 400 were purchased from Merck.All aqueous solutions were prepared with distilled water further purified with Milli-Q water system (Millipore, resistivity greater than 18.2 MΩ).For the experiments, 10 mmol·L-1phosphate buffer (pH 7.5) and 10 mmol·L-1acetate buffer (pH 4.5) was used.The viscogens were taken at 1%W/Vconcentrations in this study.
1.2 UV-Vis spectroscopy
The samples were analyzed using UV-1800-Shimadzu spectrophotometer with quartz cells of 1 cm path length.The corresponding samples (PEG, Glycerol, Trehalose, Sucrose) with matching concentrations were used as reference for the measurements.
1.3 Steady state fluorescence spectroscopy
The steady state fluorescence spectroscopy was performed using Cary Eclipse Spectrometer with theλex=280 nm.
1.4 Steady state anisotropy measurements
Fluorescence anisotropy is measured by first exciting a molecule with vertically polarized light, then observing the fluorescence intensity of emitted light passing through a polarizer held alternately parallel to and perpendicular to the direction of polarization of the incident light.The anisotropy is related to these intensities by equation 1 as follows
(1)
whereI‖is the intensity of emitted light measured in the direction parallel to excitation andI⊥is the intensity of emitted light measured in the direction perpendicular to excitation.The solution was excited at 280 nm.
1.5 QCM Measurements
Plasma cleaned (exposed to UV/ozone for 10 min.) gold-coated quartz substrates were used for all QCM measurements.Measurements were performed with 10 μL of temperature-stabilized and degassed sample liquid which was delivered to the chamber containing the sensor crystal to ensure a complete exchange of the liquid.This ensures that processes of adsorption and surface adlayer changes can be followed in situ while subsequently exposing different solutions to the surface.All measurements were performed at a temperature of 24~25 ℃.QCM sensors quartz crystal microbalance, crystal holders, and polished gold AT cut 5 MHz gold crystals of 25 mm diameter crystals from Maxtek were used for the study.The oscillation frequency was measured using a Maxtek RQCM with phase lock oscillator with independent crystal measurement channel.Data acquisition was performed using the Maxtek RQCM Data Logging software (v.1.6.0) on a PC connected through an RS 232 serial interface.A sampling rate of 1/60 Hz was employed for all experiments.Any baseline drift was regulated using the coarse and fine capacitance adjustments.Upon interaction of protein with the surface of a sensor crystal, changes in the resonance frequency, Δf, are related to attached mass governed by the Sauerbray’s equation
Δm=-(μρ)1/2(Δf)/(2f0)2
(2)
f0is the resonance frequency of the crystal (5 MHz at 25 ℃),μis the shear modulus of the quartz crystal (2.947×1011g·s-2·cm-1at room temperature), andρis the density of quartz (2.648 g·cm-3).
Shear viscosity is then given by the equation
η=(19.627×(Δf)2×10-7)/ρ
(3)
2 Results and Discussion
Figure1showsthestructureofL-PheandL-Trp,asindicatedbythearrowsthesetwoaminoacidscanhaverotationsabouttheaxisinhigherviscosityenvironments.ThegeneralpropertiesofthesetwoaminoacidsaregiveninTable1.
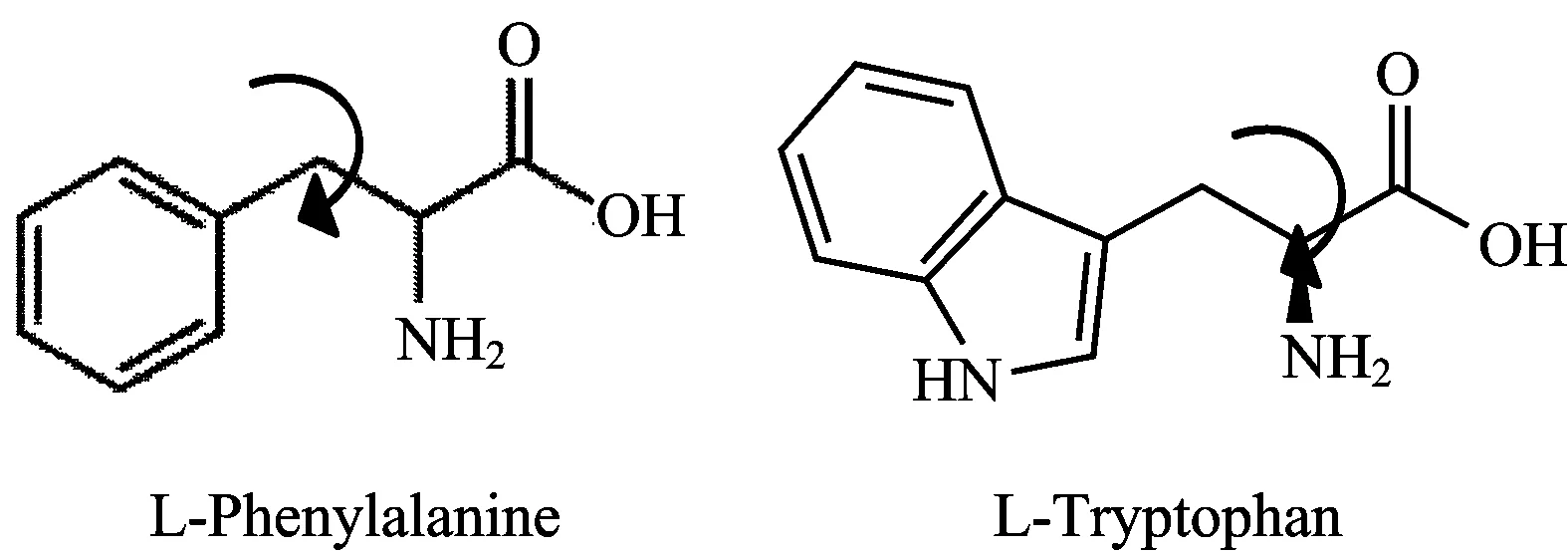
Fig.1 Structure of L-Phe and L-Trp

Table 1 Properties of L-Phe and L-Trp
In the present study we have chosen two pH 4.5 and 7.5 below and above the pI of L-Phe and L-Trp.The ground state and excited state dynamics of the aminoacids should give a basic idea of the usability of these systems as molecular rotors.Figure 2 presents the UV-Vis absorbance and steady state fluorescence emission intensity of L-Phe and L-Trp in two different pH and in presence of different viscogens.

Fig.3 Shear viscosity vs steady state fluorescence intensity for L-Phe and L-Trp in viscogens
It can be seen clearly for pH above pI, the absorbance and fluorescence emission intensity are inversely related.This may be due to the charges present in the system competing with the excited state lifetime leading to such electrostatic interactions.
Shear viscosities of these samples were measured using Quartz crystal microbalance.Figure 3 shows the shear viscosity of these aminoacids and the corresponding fluorescence emission intensities for L-Phe and L-Trp in viscogens.For sake of clarity, the shear viscosities are arranged in increasing order.
It can be seen clearly that L-Phe and L-Trp at pH above pI show the trend of a molecular rotor which shows a linear increase in steady state fluorescence emission intensity with increasing viscosity.
Steady state fluorescence usually arises from a single transition from excited state to ground state.Interaction of the exciting light with a molecule can be described as an interaction of the electric field component of the light with the relevant transition (electrical) dipole moment of the molecule.Thus, the absorption of the light quantum is proportional to the cosine of the angle between the two directions, i.e.between the excitation light polarization plane and the transition moment vector.

Fig.4 (a) Angle θ between transition dipole moment parallel and light polarization plane (b) anisotropy and (c) angle θ for control and in presence of viscogens
Consequently, excitation by linear polarized light leads to an anisotropic spatial distribution of the excited molecules: those with transition dipole moment parallel to the light polarization can be excited, whereas those in a perpendicular position cannot, known as photo selection.The resulting anisotropy can persist even up to the later moment of fluorescence emission, yielding partially polarized emitted light.Such fluorescence polarization will decay faster with higher rotational diffusion of the excited molecule, and it can be diminished further, e.g.by an excitation energy transfer.The rotational diffusion depends on the (micro)viscosity of the environment, and on the size and shape of the excited molecule.Thus in this work we have used fluorescence polarization studies.
Thus anisotropy arising due to the polarization excitation spectra can be used to identify partially overlapping electronic transitions, thus useful in identifying molecular rotors.The anisotropy stays constant if the fluorophore is fixed in space, and in presence of viscous environment there can be changes in the anisotropy, leading to changes in the transition dipole moment and the angleθ.Anisotropy is related to the angleθ(explained in Fig.4) by equation (3)
r=0.6cos2θ-0.2
(3)
Thusθvalues close to 90° indicates that transition dipole moment is perpendicular to the light polarization and values near 0° transition dipole moment is parallel.It can be seen from Figure 4 that L-Phe at both pH show low angles in the presence of viscogens, leading to the point that L-Phe are best suitable for molecular rotors.L-Trp on the other hand, show angles close to 90 indicating that they are possibly not the best choice for molecular rotors.
3 Conclusions
This study shows that L-Phe and L-Trp in the presence of viscogens like sucrose, trehalose, glycerol and PEG follows electrostatic interactions.The changes in anisotropy shows clearly that L-Phe are best suited as molecular rotors.This study will thus advance the knowledge of using L-Phe as a internal probe for molecular crowding studies in proteins.
[1] Haidekker M A, Theodorakis E A.Org.Biomol.Chem.,2007,5:1669.
[2] Kuimova M K, Yahioglu G, Levitt J A, et al.J.Am.Chem.Soc., 2008, 130: 6672.
[3] Kuimova M K, Yahioglu G, Levitt J A, et al.J.Am.Chem.Soc., 2008, 130: 6672.
[4] Levitt J A, Kuimova M K, Yahioglu G, et al.J.Phys.Chem.C, 2009,113:11634.
[5] Raut S, Kimball J, Fudala R, et al.Phys.Chem.Chem.Phys., 2014, 16: 27037.
[6] Peng X, Yang Z, Wang J, et al.J.Am.Chem.Soc.,2011,133:6626.
[7] Kuimova M K, Botchway S W, Parker A W, et al.Nat.Chem., 2009, 1: 69.
[8] Liu T, Liu X, Spring D R, et al.Sci.Rep., 2014, 4: 5418.
[9] Battisti A, Panettieri S, Abbandonato G, et al.Anal.Bioanal.Chem., 2013, 405: 6223.
[10] Wang L, Xiao Y, Tian W, et al.J.Am.Chem.Soc., 2013, 135: 2903.
[11] Yang Z, He Y, Lee J H, et al.J.Am.Chem.Soc., 2013, 135: 9181.
[12] Gatzogiannis E, Chen Z, Wei L, et al.Chem.Commun., 2012, 48: 8694.
[13] Sankaranarayanan K, Dhathathreyan A.Coll.Surf.B: Biointerfaces, 2012,91:63.
O657.3
A
10.3964/j.issn.1000-0593(2016)05-1629-05
*Corresponding author: Sankaranarayanan, PhD e-mail: kamatchi.sankaran@gmail.com; kamatchi@nitt.edu
