Spectroscopic Analysis of Organophosphorus Pesticides Using Colorimetric Reactions
2016-07-12AmitKumarSharma
Amit Kumar Sharma
Department of Physics, College of Engineering, Teerthanker Mahaveer University NH-24, Delhi Road, Moradabad-244001, UP, India
Spectroscopic Analysis of Organophosphorus Pesticides Using Colorimetric Reactions
Amit Kumar Sharma
Department of Physics, College of Engineering, Teerthanker Mahaveer University NH-24, Delhi Road, Moradabad-244001, UP, India
OP pesticides;Optical properties;UV-Vis spectrophotometer;FTIR spectroscopy
Resume:My research is focused on environment and health safety with quality aspects.We have made notable contribution in biosensors, Synthesis and characterization of optical properties of nanomaterials, drug delivery systems, heavy metals and pesticides detection, and Computational analysis of molecule-protein interaction.Our work resulted in 15 peer reviewed research papers and 24 papers contributed in national and international conferences.
Introduction
Pesticides enter into natural water by direct application and by leaching from soil and through vegetation.Many of these pesticides present in aqueous media can undergo photochemical transformation with sunlight via direct or indirect photoreaction.The study of toxic pesticides have been systematically studied in different environments[1-3]in order to analyze their structure in view of the wide applications of these pesticides in the field of agricultural, horticultural as a pest.However, the physical data of these compounds were not calculated and the structure correlations have not been established or cleared[1, 3].Though, the solution of these problems can help to improve the sensor technology in terms of physical parameters for their detection as well as their characterization by spectroscopic method using colorimetric reactions.The general formula of OP pesticides consist mainly of phosphate or thiophosphate esters as
Where R1 and R2 are most often methyl or ethyl groups whereas R3 is a large organic group.In these compoundsXis oxygen or sulfur andYis oxygen, sulfur or nitrogen.
The used method is stable for increasing their thermal stability (at room temperature) because the number of basic characteristic vibration bands and their relative intensities vary with concentrations of the OP pesticides[4-7].


This work focused an interpretation of absorption bands of OP pesticides like methyl parathion, malathion and parathion using appropriate steps of colorimetric reactions in detail and provides the useful information for their characterization.The produced data can be used for the detection of pesticides in various environments like soil, water, vegetables or in human blood samples.It is based on a consistent methodology for the analysis of absorbance, frequencies, energies, wavelength, structure analysis, and absolute intensities of the normal vibrations of pesticides.
1 Materials and Techniques
Malathion (EC; purity 80%) and Methyl parathion (EC; purity 80 %) and Parathion (EC; purity 80%) were purchased from Singhal Pesticides, Agra, India.Hydroxylammonium chloride (99%, GR grade) was purchased from Merck, Germany.A sodium hydroxide pellet (97%) was obtained from qualigens fine chemicals, India.A Hitachi U-2800 double beam spectrophotometer (Tokyo, Japan) with UV solutions 2.1 software was used for the recording of UV-Vis absorption spectra and quantitative analysis.The IR spectrum of toxic pesticides were recorded on a Perkin-Elmer autosystem model RS-1 spectroscopy (USIC, Delhi University, Delhi, India).
A stock solution of each toxic pesticides of 100 ppm was prepared in 99% absolute methanol by using serial dilution method and further by using micro-pipettes (eppendorf-AG, Hamburg Germany) 1.5 to 0.1 ppm samples were prepared.Aqueous solution of buffer solutions (at pH 9.2), sodium hydroxide crystal (0.1 mol·L-1) and hydroxyl ammonium chloride crystal (0.05 mol·L-1) were prepared with milli Q-water.
Sodium hydroxide (0.1 mol·L-1), hydroxyl ammonium hydrochloride (0.05 mol·L-1) is used as a colorimetric agent to produce the light yellowish to dark yellowish color in various concentrations of pesticide samples (Fig.3).The used quantity of colorimetric agents of sodium hydroxide (0.1 mol·L-1), hydroxyl ammonium hydrochloride (0.05 mol·L-1) was taken 100 and 50 μL, respectively and this quantity of agents is linearity adjusted by UV-Vis spectrophotometer.
The IR spectrum of these compounds were analyzed experimentally that combined a calculation of the frequencies and shapes of the normal vibrations by colorimetric reactions with an approximated stretching bands and intensities is to be studied.
2 Experimental Results and Discussion
The UV-Vis and IR absorption spectra of malathion, methyl parathion and parathion contain bands at 319, 395, 352 nm and IR region of these pesticides show effective range from 2 900~900 cm-1, respectively.
2.1 UV-Vis analysis
In the UV-Vis study of pesticides is observed using colorimetric process[1].The application of this method uses a fixed wavelength excitation and records the absorption spectrum of pesticides.The UV-Vis absorption spectra of OP pesticides (like malathion, methyl parathion and parathion) are shown in Figure 1(a—d), and their optical parameters were calculated (Table 1).


Table 1 Optical properties of pesticides using colorimetric reactions
The shifting of UV-Vis absorption peak towards the wavelength of blue color has been observed due to these functional groups[14-16].It was also found that the concentration of pesticide increases as the absorbance of analyte is increased within the certain limit of detection, Figure 1(a—d).
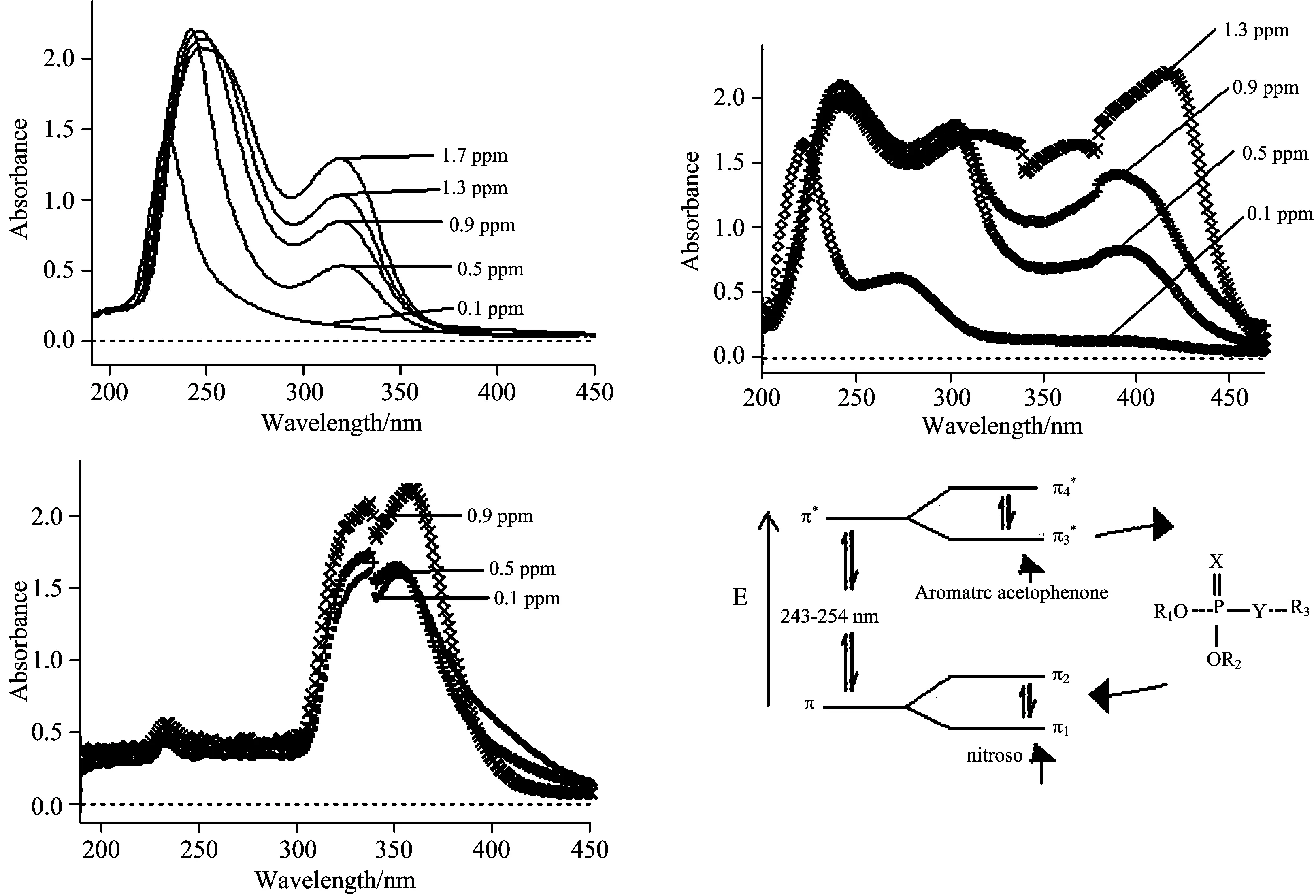
Fig.1 UV-Vis absorption spectra for quantitative analysis of (a) malathion, (b) methyl parathion, (c) parathion with various concentrations using hydrolysis method and (d) Study of possible transition states of OP pesticides
After certain limit of the colorimetric agents, the absorbance results show a non-linear phenomenon tiat will present a error in the results.Beyond this limit of detection, all results may be changed, if the change in sampling time, quantity of added color reagent, room temperature and sensitivity of using instrument.
The solvent in which the absorbing species is dissolved also has an effect on the colorimetric reactions.It can be concluded that the phenomena is due to a blue shifting and shows then→π*transitions that are shifted to shorter wavelengths (blue shift) with increasing solvent polarity.This arises from increased solvation of the lone pair, which lowers the energy of the n orbital.
2.2 FTIR analysis
In the assignments of IR bands to specific groups, we have tried to solve the maximum information towards the optical behavior in the functional groups using the colorimetric reactions.In the pure pesticides, we observed that the absorption band centered at 1 016 cm-1in malathion, 1 018 cm-1in methyl parathion and 1 020 cm-1in parathion is due to P—OH group.But in the colorimetric sample of pesticides, there is no good measurable shifting due to this group.The bands at 1 173 cm-1in malathion, 1 168 cm-1in methyl parathion and 1 160 cm-1in parathion is due to ionized P—O— group.There is some support for this in tie studies of pesticides[16]that show the acid salts of orthophosphate have their strongest band near 1 100 cm-1and analysed in Table 2.In the colorimetric IR spectra, the band at 3436 cm-1in malathion, 3 430 cm-1in methyl parathion and 3 435 cm-1in parathion is reduced and shows the —OH strongest absorption.Therefore, it is probably due to colorimetric reaction.This is strongest band in all IR spectra of pesticides.Although, its absorption increases in the colorimetric reaction and increasing in absorbance in P—OH or —OH may be conversion from these structures as-

Table 2 IR properties of pesticides for spectral band characterization in colorimetric samples
awith twelve determinations; b ND: not detected


Fig.2 IR absorption spectra for characterization of products of (a) malathion, (b) methyl parathion, and (c) parathion, at concentration level of 1 ppm

The weak bands at 3 789, 3 696, 3 662 cm-1in malathion, at 3 789, 3 696, 3 662 cm-1in methyl parathion and at 3 789, 3 696, 3 662 cm-1in parathion is due to intermolecular bonded in alcohol and phenols.But, another characteristic group 1, 4 disubstituted benzene may be here, since we analysis the molecular structure of toxic pesticides and found that methyl parathion and parathion has 1, 4 disubstituted benzene.So, it may be possible, but compare the observe bands with malathion and observe that the bands are approximately same position.Therefore, it is due to intermolecular bonded in alcohols and phenols not 1, 4 disubstituted benzene.The two closer medium bands at 795, 655 cm-1in malathion; 797, 674 cm-1in methyl parathion is due to P—O—CH3group and the three medium bands at 925, 794, 721 cm-1in parathion is due to P—O—CH2CH3group respectively, in the pure IR spectra.In the hydrolyzed spectra, there is no change in this region[16].The present method has simple procedural steps and the products are thermally stable in the room temperature.An image of colorimetric samples of pesticides is shown in Figure 3.

Fig.3 An image of color variation of pesticides with concentration (1 ppm) using colorimetric reactions for quantitatively analysis
3 Conclusion
The present studies of OP pesticides followed by colorimetric procedure and their characterization have been discussed.The detection of selected pesticides has exposed a good response with the colorimetric reaction in UV-Vis and IR characteristics.These results reveal that the amounts of malathion, methyl parathion and parathion can be determined with spectroscopic data.In the colorimetric reactions of pesticides, a new broad band is formed and it observed at 2 078 cm-1in malathion; 2 082 cm-1in methyl parathion and 2 076 cm-1in parathion, is due to thiocyanates (—RNCS) that shows the compound is toxic, because the group —CN is added with the breaking of OP pesticides compound.The optimum values and statistical parameters established a suitable correlation with the results of spectroscopic data and also be characterizes the functional groups in the molecular structure of pesticide.All data are reproducible under room environment conditions and may be applied for optimization of others pesticides.
Acknowledgements
Dr.Amit K.Sharma expresses their gratitude to Department of Science & Technology, New Delhi, Government of India for utilizing the materials and also thankful to Director, College of Engineering, Teerthanker Mahaveer University, Moradabad, Dr.M.S.Gaur, Professor, Associate Professor, Hindustan College of Science & Technology, Mathura, and Dr.Sunil Sharma, Scientist, USIC, Delhi University, Delhi for his kind supports.
[1] Gaur M S, Sharma A K, Sharma P, et al.Canadian Journal of Pure & Applied Sciences, 2008, 2(3):581.
[2] Qian S,Lin H.Anal.Chem., 2015, 87(10): 5395.
[3] Pérez-López B, Merkoçi A.Trends in Food Science & Technology, 2011, 22(11): 625.
[4] Sharma A K, Tiwari R K, Gaur M S.Journal of Biomedical Research, 2012, 26(3): 170.
[5] Sharma A K, Gaur K, Tiwari R K, et al.Journal of Biomedical Research, 2011, 25(5):335.
[6] Meng X, Schultz C W, Cui C, et al.Sensors and Actuators B: Chemical, 2015, 215: 577.
[7] Wu Jiguo, Lan Chongyu, Gilbert Yuk Sing Chan.Chemosphere, 2009, 76(9): 1308.
[8] Aragay G, Pino F, Merko?i A.Chem.Rev., 2012, 112(10):5317.
[9] Liu G, Wang J, Barry R, et al.Chemistry-A European Journal, 2008, 14(32):9951.
[10] Tankiewicz M, Fenik J, Biziuk M.TrAC Trends in Analytical Chemistry, 2010, 29(9): 1050.
[11] Sharma A K, Tiwari R K, Gaur M S, et al.Journal of Biomedical Research, 2015, 29(6):00.
[12] Khanmohammadi M, Karimi M A, Ghasemi K, et al.Talanta, 2007, 72:620.
[13] Shama S A, Amin A E, Omara H.J.Quant.Spectroscopy & Radiative Transfer, 2006, 102: 261.
[14] Sharma A K, Gaur M S, Sharma P, et al.Sensor Review, 2009, 29(1):70.
[15] Algiwale T A, Shinde C P, Purnanand.Indian J.Chemistry A, 2007, 46A: 273.
[16] Mikhalovsky I S, Samoylov M V, Wileishikova N P.J.Appl.Spectrosc., 2009, 76(1):127.
O434
A
10.3964/j.issn.1000-0593(2016)09-3033-06
Received:2015-05-28; accepted:2015-10-10
Funding Agency:We had been worked in funding projects from DST, New Delhi for extensive research in the area of nano-bio molecular spectroscopy and sensor technology development
Biography:Amit Kumar Sharma, Ph.D., CSIR RAship Assistant Professor, Department of Physics College of Engineering, Teerthanker Mahaveer University e-mail: amit_vashishtha1980@rediffmail.com, amit.db1980@gmail.com
