MEK/ERK signaling pathway in apoptosis of SW620 cell line and inhibition effect of resveratrol
2016-07-07HaoChenZhiLiangJinHaiXuJingzhouCentralHospitalWuhanCityHubeiProvinceChinaHuangjiahuHospitalHubeiUniversityofChineseMedicineWuhanCityHubeiProvinceChinat
Hao Chen, Zhi-Liang Jin, Hai XuJingzhou Central Hospital, Wuhan City, Hubei Province, ChinaHuangjiahu Hospital, Hubei University of Chinese Medicine, Wuhan City, Hubei Province, Chinat
MEK/ERK signaling pathway in apoptosis of SW620 cell line and inhibition effect of resveratrol
Hao Chen1*, Zhi-Liang Jin1, Hai Xu2
1Jingzhou Central Hospital, Wuhan City, Hubei Province, China
2Huangjiahu Hospital, Hubei University of Chinese Medicine, Wuhan City, Hubei Province, Chinat
ABSTRACT
Objective: To study the involvement of MAPK MEK/ERK signaling transduction pathway in the apoptosis process of SW620 tumor cell line and the inhibition effect of resveratrol.Methods: SW620 cell lines were divided into 5 groups, namely, control group, PD98059 group, low-dose resveratrol group, mid-dose resveratrol group and high-dose resveratrol group. The inhibition rate of cell proliferation was detected by MTT method. The expression of apoptotic molecules and MEK/ERK signaling pathway related proteins were assayed by realtime PCR and Western blotting. Results: Compared with control group, the proliferation of cells treated with resveratrol was significantly inhibited. In the case of apoptotic molecules, the expression of Bax, Caspase 3 and Caspase 9 was increased significantly while the expression of anti-apoptotic molecule Bcl2 was decreased significantly in resveratrol groups with a dosedependent manner. In the case of molecules in MEK/ERK signaling pathway, the expression of Ras, Raf, MEK and ERK1/2 was decreased significantly in resveratrol groups with a dose-dependent manner. Conclusions: PD98059 and resveratrol can effectively inhibit the proliferation of SW620 through inhibiting the MEK/ERK signaling pathway.
ARTICLE INFO
Article history:
Received 15 October 2015
Received in revised form 20 November 2015
Accepted 15 December 2015
Available online 20 January 2016
Keywords:
Colon cancer
Apoptosis
MEK/ERK signaling pathway
Resveratrol
Inhibition of proliferation
Tel: 18672779388
E-mail: 643025@qq.com
Foundation project: Supported by Natural Science Fund of Hubei Province (201918283).
1. Introduction
The colon cancer is one of the most clinically common malignant tumors in digestive tract, seriously affecting patients’ health and heavily burdening both patients’ family and society economically and socially[1,2]. The early stage of colon cancer is presented as abdominal distension and dyspepsia while abdominal pain before defecation, mucous stool, mucopurulent stool, anemia, feebleness, emaciation, edema and other toxic symptoms are presented in the later stage[3,4]. However, the specific pathogenesis of colon cancer still remains unveiled and there have been no effective therapeutic drugs clinically yet. MEK/ERK signaling transduction pathway is the key pathway of extracellular signal being transduced into cells and furthermore transduction of karyo gene being activated, with participation in sorts of pathological processes and close relation to occurrence, development and deterioration of tumors[5,6]. The present study analyzed the mechanism of MEK/ERK signaling pathway in the apoptosis of SW620 cells and the inhibition effect of resveratrol on cell proliferation, aiming to provide reference for clinical treatments to patients with gastric cancer and research and development relative to anti-gastric cancer new drugs.
2. Materials and methods
2.1. Reagents and equipments
The human colon cancer cell line SW620 was purchased from Institutes of Cell Biology, Chinese Academy of Sciences. The dulbecco's modified eagle medium was purchased from Gbico Company, USA. Fetal calf serum was purchased from Wuhan Procell Biological Technology Co., LTD. Dimethyl sulfoxide (DMSO) was purchased from China National Pharmaceutical Corporation. Trypsin, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) and PD98059 were purchased from Sigma Aldrich Company, USA. mRNA extraction kit was purchasedfrom BioTek Company. The reverse transcriptase of quantitative realtime PCR was purchased from Invitrogen Company. β-actin and SYBR Premix Ex TaqTMⅡ were purchased from TaKaRa Company, Japan.
Caspase 3 and Caspase 9 monoclonal antibodies were purchased from Abcam Company, USA. Ras and Raf monoclonal antibodies were purchased from Santa Cruz Company, USA. MEK and ERK1/2 antibody were purchased from Wuhan BOSTER Bioengineering LTD. Second antibody marked with horseradish peroxidase and diaminobenzidine color development kit were purchased from Beijing ZSGB-Bio LTD. Cell incubator was purchased from Shanghai Xinmiao Equipment LTD. Light Cycler 480 real-time fluorescent PCR was purchased from Roche Company. Protein electrophoresis and transfer-membrane system were purchased from ABI Company.
2.2. Grouping and medication
The SW620 cell lines were randomly divided into control group, PD98095 group, low-dose resveratrol group, mid-dose resveratrol group and high-dose resveratrol group. For PD98095 group, PD98095 (10 μmol/L) was added for incubation. A total of 1, 10 and 100 μmmol/L resveratrol were given to low-dose resveratrol group, mid-dose resveratrol group and high-dose resveratrol group, respectively. In control group, DMSO solvent was added for incubation.
2.3. Inhibition rate of proliferation by MTT assay
After the addition of relative medication and solvent for all groups, under the conditions of 37 ℃, 5% CO2, 24, 36, 48 and 72 h incubation with saturated humidity, the culture solution was abandoned and MTT was added into each well of cell incubation plate before the continuous incubation in 5% CO2for 4 h. Afterwards, DMSO was added into each well and shaken vigorously on the oscillating table until complete dissolution. The value of optical density (OD) in each well was detected by MTT assay and the inhibition rate of cell proliferation was calculated by using the formula as the follows: % inhibition rate = (OD1– OD2)/OD1××100. Where OD1was the OD value in control group and OD2was the value in the other four experimental groups.
2.4. Real-time PCR determination
The process of mRNA extraction and cDNA synthesis strictly stuck to the specification sheet. The system of real-time PCR were 20 μL: 2×qPCR Mixture 10 μL, upstream and downstream primers 20 pmol respectively, cDNA 2 μL, double distilled water 6 μL. The amplification conditions of PCR were denaturation at 95 ℃ for 10 seconds, extension at 61 ℃ for 45 s and 27 circulation in all. The quantitative analysis was conducted by using the formula as the follows: ΔCt = Ct1– Ct2, where the Ct1was Ct value of target gene and Ct2was Ct value of β-actin. The primer sequence was as the follows:
β-actin- forward: 5'-GGGAAATCGTGCGTGACAT-3', reverse: 5'-CAGGAGGAGCAATGATCTT-3';
ERK1- forward: 5'-TCCTTTGGATCTGGTCCTG-3', reverse: 5'-CCCCAGCAAGTGAGAGAAG -3';
ERK2- forward: 5'-AAGAGGTTGTTCCAAATGC-3', reverse: 5'-AGAGGCACCATTCACTGAC -3';
Caspase 3- forward: 5'-TACCACGCCACCACCGGCCCA-3', reverse: 5'-GGCATTTTGGCTGTCGTCAGGGAA-3';
Caspase 9- forward: 5'-GGCGAATTGGAGATGAACTG-3', reverse: 5'-TTCTTCCAGATGGTGAGCGA-3';
Bax- forward: 5'-CCCGAGAGGTCTTTTTCCGAG-3', reverse: 5'-CCAGCCCATGATGGTTCTGAT -3';
Bcl2- forward 1: 5'-ATGTGTGTGGAGAGCGTCAA-3', reverse: 5'-ACAGTTCCACAAAGGCATCC -3';
Ras- forward: 5'-GGATTTGATGCCTTGGGAGTCAGAC-3', reverse: 5'-ATTTTTTTCTTTGGAGTCAGTCCAT-3';
Raf- forward: 5'-AAGATGGTACAGTGGACGGC-3', Primer2: 5'-CCGTGTTCCTGGTGAAATCT-3';
MEK- forward: 5'-GACGACCAGTGGGGAGAGTA-3'; reverse: 5'-GTCATTGAGCCGACCTAA-3'.
2.5. Protein expression determined by Western blot
After treatment of SW620 cell lines, sterile phosphate buffered salinebuffer solution was used for washing 3 times for 10 min a time, and a homogenate was made in 0 ℃ ice-water bath. Coomassie brilliant blue method was used for quantification of total proteins. The mixture of extractive and loading buffer at 1:4 ratio was boiled for 5 min and then equivalent protein was gone through verticalsodium dodecyl sulfate-polyacrylamide gelelectrophoresis protein electrophoresis. After the electrophoresis, semi-dry transfer membrane was conducted. After the protein was transferred to nitrocellulose membrane, fetal calf serum was used to seal it. Then, monoclonal primary antibody was added at the dilution rate of 1:250 and cultured at room temperature for 6 h. The sterile phosphate buffered saline buffer solution was used for washing 3 times, after which second antibody was added (1:200) for reaction at room temperature for 2 h. The quantitative analysis of relative proteins was conducted with β-actin as the reference.
2.6. Statistical analysis
Data were analyzed by using SPSS19.0 software. One-way ANOVA was used for measurement data which were expressed as mean ± SD. x2test was used for enumeration data. Differences with P<0.05 were considered as statistically significant.
3. Results
3.1. Inhibition effect of resveratrol on SW620 proliferation
It was found that cell proliferation was effectively inhibited after treatment of MEK inhibitor PD98059 and resveratrol and that the inhibition rate was increased along with the increasing processing time (P<0.05). Meanwhile, the inhibition effect of resveratrol showed a dose-dependent manner (P<0.01 or P<0.05) (Table 1).
3.2. mRNA expression of relative apoptotic molecules by realtime PCR
It was found that compared with control group, mRNA expressionof Bax, Caspase 3 and Caspase 9 after treatment of PD98059 and resveratrol was significantly increased while mRNA expression of anti-apoptotic molecule Bax protein was significantly decreased (P <0.05). Furthermore, the regulation effect of resveratrol on mRNA expression of relative apoptotic molecules showed a dose-dependent manner (P<0.01 or P<0.05) (Table 2).
3.3. mRNA expression of MEK/ERK molecules by real-time PCR
Compared with control group, the mRNA expression of MEK/ ERK signaling pathway molecules Ras, Raf, MEK and ERK was significantly decreased (P<0.05). In addition, the regulation effect of resveratrol on mRNA expression of proteins in MEK/ERK signaling pathway showed a dose-dependent manner (P<0.01 or P<0.05) (Table 3).
3.4. Expression of proteins Caspase 3 and Caspase 9 by Western blotting
Compared with control group, the expression of proteins Caspase 3 and Caspase 9 was significantly increased after the treatment of PD98059 and resveratrol, with statistically significant difference (P<0.05). In the meanwhile, the regulation effect of resveratrol on expression of Caspase 3 and Caspase 9 showed a dose-dependent manner (P<0.01 or P<0.05) (Figure 1).

Figure 1. Difference in expression of apoptotic protein molecules by Western blotting.

Table 1 Inhibition effect of resveratrol on SW620 proliferation.

Table 2 Change in expression of apoptotic molecules by real-time PCR (△Ct).

Table 3 Change in expression of MEK/ERK molecules by real-time PCR (△Ct).
3.5. Expression of proteins Ras and Raf by Western blotting
Compared with control group, the expression of Ras and Raf in MEK/ERK signaling pathway was significantly decreased after the treatment of PD98059 and resveratrol, with statistically significant difference (P<0.05). Also, the regulation effect of resveratrol on expression of Ras and Raf showed a dose-dependent manner (P<0.01 or P<0.05) (Figure 2).
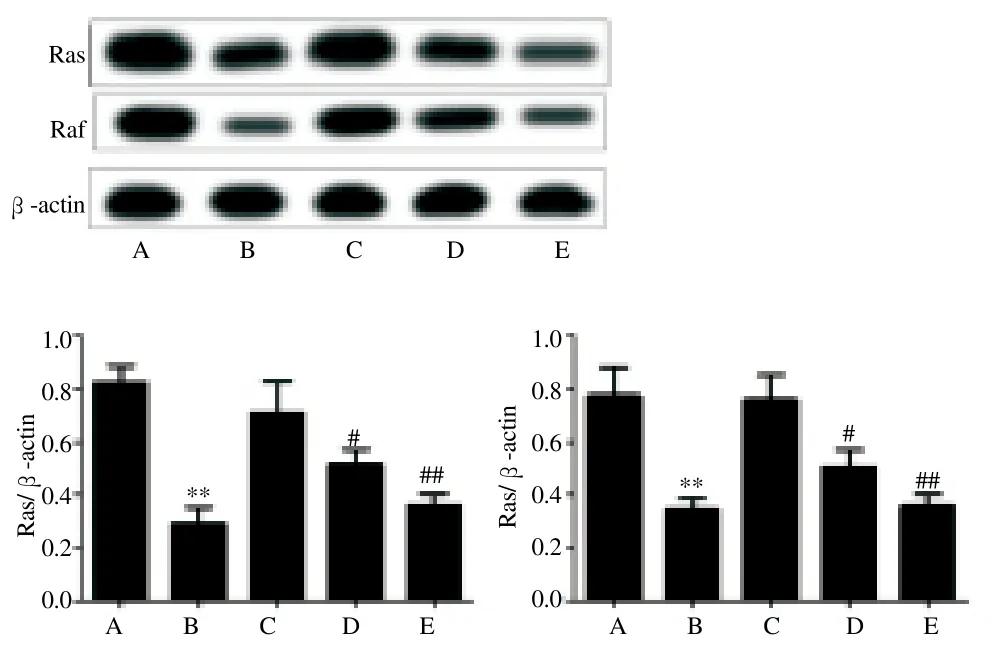
Figure 2. Difference in expression of Ras and Raf by Western blotting.
3.6. Expression of proteins MEK and ERK by Western blotting
Compared with control group, the expression of MEK and ERK1/2 in MEK/ERK signaling pathway was significantly decreased after the treatment of PD98059 and resveratrol, with statistically significant difference (P<0.05). Also, the regulation effect of resveratrol on expression of MEK and ERK1/2 showed a dosedependent manner (P<0.01 or P<0.05) (Figure 3).

Figure 3. Difference in expression of MEK and ERK1/2 by Western blotting.
4. Discussion
At the present, colon cancer is one of the most clinically common malignanttumors in department of gastroenterology, with increasing morbidity and mortality year by year[7-9]. Nevertheless, thepathogenesis of colon cancer still remains unclear for now and there are no efficient drugs clinically. The present study analyzed the mechanism of MEK/ERK signaling pathway in the apoptosis of SW620 cells and the inhibition effect of resveratrol on cell proliferation, and it is found that compared with control group, the proliferation of cell treated with reveratrol is apparently inhibited, expression of apoptotic molecules Bax, Caspase 3 and Caspase 9 is significantly increased while expression of anti-apoptotic molecule Bcl2 is significantly decreased, expression of molecules Ras, Raf, MEK, ERK in the MEK/ERK signaling pathway is significantly increased and that the effect of resveratrol shows a dose-dependent manner. Therefore, PD98059 and resveratrol can effectively inhibit the proliferation of SW620 and the inhibition process might be achieved through inhibiting MEK/ERK signaling pathway.
Cell proliferation and apoptosis is the important clue in treating malignant tumors, and also the main action mechanism of antineoplastic drugs clinically at the present[10-12]. Cell apoptosis is the ubiquitousphysiological process in cells, but turns to be weak in the cells of tumor issue[13-15]. The cell apoptosis involves some proteins, such as Bax, Caspase protein family, and so forth[16-19]. Increase in protein expression of apoptotic cells usually induces the occurrence of cell apoptosis[20-22]. In the research on induction of tumor cell apoptosis by F2/SARI, it is found that drugs promote the apoptosis of tumor cells mainly by inhibiting NFκB and the activity of transcription factor AP1[23]. In the research on antitumor mechanism of auriculoside A metabolic product, it is found that drugs possess prominent antineoplastic effect and the main actionmechanism is to promote the apoptosis process of tumor cells[24]. It is found in the present study that SW620 cells treated with MEK inhibitor PD98059 and resveratrol show proliferation inhibition of different levels, expression of apoptotic proteins Bax, Caspase 3 and Caspase 9 is significantly increased while the expression of anti-apoptotic protein Bcl2 is significantly decreased, the proliferation inhibition rate is gradually increased with increasing reaction time and that the proliferation inhibition effect of resveratrol shows a dose-dependent manner, suggesting that resveratrol can inhibit the proliferation of tumor cells by promoting the occurrence of cell apoptosis.
MEK/ERK signaling pathway is the main pathway for transduction of extracellular signals into cells, with participation in pathological and physiological processes of tumors, like proliferation, differentiation, migration, apoptosis and so on[25-27]. In the research on analysis of PI3K/AKT/ERK signaling transduction pathway in expression of gastroenteric tumor and clinicopathological correlation, it is found that though ERK protein has nothing to do with the clinical pathology of tumors, its high expression in tumor tissue participates in the occurrence and development of gastroenteric tumor at early stage[28]. In the research on effect of ERK pathway inhibitor sodium phenylacetate on salivary gland tumors in transgenic rats, it found the significantly increasing expression of proteins ERK1/2 and ph-ERK1/2 in tumor tissue, improvement in ERK pathway proteins after intervention of sodiumphenylacetate, and tumor type in the tendency of benignization, suggesting that participation of ERK in the occurrence and development of tumors and the possibility of being one of the targets in drug therapy[29]. Wu et al found that novel thiazolo-triazin compound R001 can inhibit proliferation of tumor cells by inhibiting expression of ERK signaling pathway proteins[30]. Accorded with the previous researches, it is revealed in the present study that compared with control group, expression of protein molecules Ras, Raf, MEK and ERK1/2 in MEK/ERK signaling pathway treated with MEK inhibitor PD98059 and resveratrol is significantly decreased, with statistically significant difference, and meanwhile, that the regulation effect of resveratrol on Ras and Raf shows a dose-dependent manner, suggesting that resveratrol can inhibit proliferation of colon cancer cells and promote the apoptosis of the tumor cells by inhibiting the activation of ERK signaling pathway.
In conclusion, PD98059 and resveratrol can effectively inhibit the proliferation of SW620, possibly by inhibiting MEK/ERK signaling transduction pathway.
Conflict of interest statement
We declare that we have no conflict of interest.
References
[1]Liu J, Huang H. Research on protooncogene and antioncogene in pathogenesis of colon cancer and relevant progress in pharmacotherapy. China Pharm 2014; 12(45): 4302-4304.
[2]Yang DD, Wu XL, He K, Zhang JF, Wang LK. Clinical investigation of chylous fistula after complete mesocolic excision for colon carcinoma. J Hebei Med Univ 2013; 34(12): 1517-1520.
[3]Zhao JF, Gong DP, Qing S, Yang C, Yang XJ. Research on progress in colon cancer stem cells and significance in clinical treatment. Chongqing Med 2014; 43(23): 3096-3098.
[4]An LZ. Clinical application of color Doppler ultrasound in diagnosis of colon cancer of the senile. Chin J Clin Oncol Rehabil 2013; 20(11): 1238-1239.
[5]Bianchi-Smiraglia A, Paesante S, Bakin AV. Integrinβ5 contributes to the tumorigenic potential of breast cancer cells through the Src-FAK and MEK-ERK signaling pathways. Oncogene 2013; 32(25): 3049-3058.
[6]Castro AF, Campos T, Babcock JT, Armijo ME, Martínez-Conde A, Pincheira R, et al. M-Ras induces Ral and JNK activation to regulate MEK/ERK-independent gene expression in MCF-7 breast cancer cells. J Cell Biochem 2012; 113(4): 1253-1264.
[7]Tang N, Wang YY, Shen LD. Research progress of colon cancer related genes. Med Recapitul 2012; 18(2): 207-209.
[8]Zhang X, Zhang XM, Xiao JY. Inhibitory effect of siRNA targeting CK2α gene on growth of HCT116 cells and its mechanism. J Jilin Univ (Med Ed) 2014; 40(3): 621-625.
[9]Gu SD, Mao GX. Correlation of ERCC1, BRCA1 expression and chemotherapeutic effect and prognosis of oxaliplatin regimens in advanced colon cancer tissues. Cancer Res Prev Treat 2014; 41(10): 1111-1115.
[10]Ni CW, Wang LH, Yin J. scFv-sTRAIL fusion protein targetedly induces apoptosis of tumor cells: an update. Chin J Cancer Biother 2013; 20(1): 110-114.
[11]Wang J, Zhang YY, Liu T, Guo CH, Wan YF. The mechanisms of apoptosis in Hela cells induced by Hsp90 potential inhibitor curcumin. J Mod Oncol 2014; 22(11): 2561-2565.
[12]Zhang YQ, Wu XJ, Wang YJ, Min CJ, Zhu SW, Yuan XC. Monitoring SKOV3cell apoptosis based on dynamic laser tweezers. Chin J Lasers 2014; 41(11): 1104001.
[13]Huang YN, Song C, Wei YQ, Huo HR, Zhu YY, Tan YQ. Survivin correlating with arsenic trioxide and its impacts on apoptosis of tumor cells. J Int Pharm Res 2013; 40(5): 545-549.
[14]Ouyang YY, Jiang T, Gao M, Xiao LH, Zhou Y, Dun YL, et al. Role of △ψm and Caspase 3 in the process of arsenic trioxide-induced apoptosis in adenoid cystic carcinoma ACC-2 cells. Int Eye Sci 2014; 14(2): 232-235.
[15]Wang B, Ye ZY, Li JL, Fan SP, Qin Y. Apoptosis of human cervical cancer Siha cell line induced by aspirin and its inhibition. China J Mod Med 2014; 24(4): 20-24.
[16]Ge YQ, Cheng RB, Chen Z. Research progress on molecular mechanism of tumor cell apoptosis induced by cryptotanshinone. Chin Arch Tradit Chin Med 2013; 31(12): 2631-2633.
[17]Pan WW, Cao LX, Xu Y, Shen ZF, Yi FP, Song FZ. Dendritic cells transfected with recombinant adenovirus carrying IL-24 gene induces CTLs to promote apoptosis of cervical cancer cell line CaSki. Chin J Pathophysiol 2014; 30(1): 41-47.
[18]Lan HY, Zhang JY, Chen J, Yue Y. Mechanism of resveratrol inducing apoptosis of SKOV3cells in ovarian cancer. Shandong Med J 2014; 54(1): 32-34.
[19]Cheng QL, Li HL, Huang ZQ. The study on apoptosis of human cervical cancer HeLa cell induced by Ilexgenin A from Ilex hainanensis Merr and its mechanism. Lishizhen Med Mater Med Res 2014; 25(2): 306-309.
[20]Xu WW. The preliminary study on the apoptosis of cervical cancer Hela cells induced by PJA1. Proc Clin Med 2014; 23(3): 202-204.
[21]Lu XL, Yang C, Liang XQ, Liu XM, Zhou JB. OSTP enhances paclitaxel-induced apoptosis in the ovarian cancer A2780 cell. J Med Res 2014; 43(4): 42-46.
[22]Wang B, Xing HY, Li JL, Zhu ZP, Jia LG, Qin Y. Effect of aspirin on apoptosis and proliferation of cervical cancer Hela cells. Chin J Gerontol 2014; 34(9): 2459-2462.
[23]Hu XL, Zhang CY, Yu LC, Peng XX, Gao XX. Effect of combined cancersuppressing PTEN and p53 gene transfection on apoptosis of prostate cancer PC-3m cells. Acad J Chin PLA Med Sch 2014; 35(4): 369-373.
[24]Tao F, Zhang RS. Research on anti-tumor effect and apoptosis-inducing mechanism of auriculoside A metabolic product. Chin Arch Tradit Chin Med 2013; 31(11): 2507-2509.
[25]Xiao GC, Tong SL, Zheng YB, Hao ZN, Li SB. Role of PI3K/AKT and MEK/ERK signaling pathway in tumor vascular endothelial cell migration. Chongqing Med 2015; 44(11): 1452-1456.
[26]Cai Q, Zeng FX, Dong Z, Fu JM, Yu CL. In vitro antitumor effect of MEK inhibitor CQN compounds on A549 cells. Chin J New Drugs Clin Remedies 2015; 34(4): 296-301.
[27]Guo RX, Li YM, Fan LJ, Zhang MJ, Li AJ. Exploration in effect of IGFBP-rP1 on inhibiting proliferation of endometrial cancer cell HEC-1A and its mechanism. Matern Child Health Care China 2015; 22(18): 3053-3055.
[28]Sun HT, Wang W, Zhang Y. K/AKT/ERK in gastrointestinal tumors of the expression and clinicopathological relation. Chin J Mod Drug Appl 2013; 7(6): 1-3.
[29]Shang WJ, Wang Y, Liu LM, Yang WJ, Wang ZG, Li J, et al. The effect of the ERK pathway inhibitor sodium phenylacetate on salivary gland tumors in PLAG1 transgenic mice. China J Oral Maxillofac Surg 2013; 11(5): 359-364.
[30]Wu FJ, Koirala D, Liu SJ, Jin Z, Hu C, Li DW. The antitumor activity of the novel thiazolo-triazin compound R001 in inhibiting ERK1/2 phosphorylation. Prog Mod Biomed 2012; 12(29): 5638-5642.
Contents lists available at ScienceDirect IF: 1.062
Asian Pacific Journal of Tropical Medicine
journal homepage:www.elsevier.com/locate/apjtm
doi:Document heading 10.1016/j.apjtm.2015.12.010
*Corresponding author:Hao Chen, M.D., Attending Physician, Jingzhou Central Hospital, Wuhan City, Hubei Province, China.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Evaluation of antiviral activities of Houttuynia cordata Thunb. extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection
- Non-aqueous extracts of Curcuma mangga rhizomes induced cell death in human colorectal adenocarcinoma cell line (HT29) via induction of apoptosis and cell cycle arrest at G0/G1phase
- Potentiating activity of luteolin on membrane permeabilizing agent and ATPase inhibitor against methicillin-resistant Staphylococcus aureus
- Evaluation of activity of triclabendazole against Taenia solium metacestode in naturally infected pigs
- Enterobacteria and Vibrio from Macrobrachium amazonicum prawn farming in Fortaleza, Ceará, Brazil
- Bio-ecology of malaria vectors in an endemic area, Southeast of Iran
