Potentiating activity of luteolin on membrane permeabilizing agent and ATPase inhibitor against methicillin-resistant Staphylococcus aureus
2016-07-07DaeKiJoungYoungSeobLeeSinHeeHanSangWonLeeSeonWooChaSuHyunMunRyongKongOkHwaKangHoJunSongDongWonShinDongYeulKwonBKPlusTeamProfessionalGraduateSchoolofOrientalMedicineWonkwangUniversityIksanJeonbuk57079Republic
Dae-Ki Joung, Young-Seob Lee, Sin-Hee Han, Sang-Won Lee, Seon-Woo Cha, Su-Hyun Mun, Ryong Kong, Ok-Hwa Kang, Ho-Jun Song, Dong-Won Shin, Dong-Yeul Kwon,*BK Plus Team, Professional Graduate School of Oriental Medicine, Wonkwang University, Iksan, Jeonbuk 570-79, Republic of KoreaDepartment of Herbal Crop Research, National Institute of Horticultural & Herbal Science, RDA, 9 Bisanro, Eumsung, Chungbuk 69-87, Republic of KoreaDepartment of Oriental Pharmacy, College of Pharmacy and Wonkwang-Oriental Medicines Research Institute, Wonkwang University, Iksan, Jeonbuk 570-79, Republic of KoreaDepartment of Oriental Medicine Resources, Sunchon National University, Sunchon, Jeonnam 50-7, Republic of Korea
Potentiating activity of luteolin on membrane permeabilizing agent and ATPase inhibitor against methicillin-resistant Staphylococcus aureus
Dae-Ki Joung3, Young-Seob Lee2, Sin-Hee Han2, Sang-Won Lee2, Seon-Woo Cha2, Su-Hyun Mun1, Ryong Kong1, Ok-Hwa Kang3, Ho-Jun Song1, Dong-Won Shin4, Dong-Yeul Kwon1,3*
1BK21 Plus Team, Professional Graduate School of Oriental Medicine, Wonkwang University, Iksan, Jeonbuk 570-749, Republic of Korea
2Department of Herbal Crop Research, National Institute of Horticultural & Herbal Science, RDA, 92 Bisanro, Eumsung, Chungbuk 369-873, Republic of Korea
3Department of Oriental Pharmacy, College of Pharmacy and Wonkwang-Oriental Medicines Research Institute, Wonkwang University, Iksan, Jeonbuk 570-749, Republic of Korea
4Department of Oriental Medicine Resources, Sunchon National University, Sunchon, Jeonnam 540-742, Republic of Korea
ABSTRACT
Objective: To investigate the mechanism of antibacterial activity of luteolin (LUT) against methicillin-resistant Staphylococcus aureus (MRSA). Methods: The mechanism of anti-MRSA activity of LUT was analyzed by the viability assay in membrane permeabilizing agent, ATPase inhibitors, and peptidoglycan (PGN) derived from Staphylococcus aureus (S. aureus). Also, transmission electron microscopy was used to monitor survival characteristics and changes in S. aureus morphology. Results: Compared to the LUT alone, the optical density of suspensions treated with the combination of 125 μg/mL Tris and 250 μg/mL DCCD were reduced to 60% and 46%, respectively. PGN (15.6 μg/mL) gradually impeded the activity of LUT, and PGN (62.5 μg/mL) completely blocked the activity of LUT on S. aureus. Conclusions: Increased susceptibility to LUT with the Tris and DCCD combinations is evident in all tested MRSA isolates. The results indicate LUT synergy in increasing cytoplasmic membrane permeability and inhibiting ATPase. S. aureus PGN directly blocks the antibacterial activity of LUT, suggesting the direct binding of LUT with PGN. These findings may be validated for the development of antibacterial agent for low MRSA resistance.
ARTICLE INFO
Article history:
Received 15 October 2015
Received in revised form 20 November 2015
Accepted 15 December 2015
Available online 20 January 2016
Keywords:
Iuteolin
Methicillin-resistant Staphylococcus aureus
Membrane permeabilizing agent
ATPase inhibitor
Peptidoglycan.
Tel: +82-63-850-6802
Fax: 82-63-850-6802
E-mail: sssimi@wku.ac.kr
Foundation project: This study is supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Educatio(2013060380), the Korea governmen(MSIP)(2008-0062484), and Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ00962201), Rural Development Administration, Republic of Korea.
1. Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) was first reported in 1961, soon after the introduction of methicillin into clinical use. MRSA is resistant to most β-lactam antibiotics including penicillins and cephalosporins[1]. MRSA accounts for nearly 70 percent of Staphylococcus aureus (S. aureus) infections, and is the main cause of community-acquired and healthcareassociated infections[2]. The mechanisms of bacterial resistance against antibiotics are inactivation of antibiotics by enzymes, change in the target site, change of membrane permeability, and antibiotic efflux out of cells[3,4]. Therefore, alternative therapeutic strategies involving effective antimicrobial agents that minimize bacterial resistance to antibiotics are needed.
Luteolin (LUT), a well-known flavonoid polyphenolic compound (Figure 1), is found in many plant groups including Bryophyta, Pteridophyta, Pinophyta, and Magnoliophyta. LUT has diverse biological benefits that include cardioprotection, antioxidantion, anti-inflammation, anti-cancer, and antimicrobial effects[5-9]. Qio et al reported the impact of LUT on the alpha-toxin producedby S. aureus; LUT decreased production of the toxin[10]. In the present study, we investigated the anti-MRSA activity of LUT on the membrane-binding agent and ATPase-inhibiting agent. We also confirmed that binding effect of LUT on the bacterial cell wall. Bacterial ultrastructural changes following treatment with LUT were assessed by transmission electron microscopy (TEM).

Figure 1. Structure of LUT.
2. Materials and methods
2.1. Reagents
N,N’-dicyclohexylcarbodiimide (DCCD) and lipopolysaccharide (LPS) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Mueller-Hinton broth (MHB) was purchased from Difco (Baltimore, MD, USA). Tris-(hydroxymethyl) aminomethane (Tris) was obtained from AMRESCO (San Francisco, CA, USA). Peptidoglycan (PGN) was purchased from Fluka (Basel, Switzerland).
2.2. Bacterial strains and growth conditions
S. aureus ATCC 33591 (methicillin-resistant strain) and S. aureus ATCC 25923 (methicillin-susceptible strain) were stored in 30% glycerol and frozen at −-70 ℃. They were cultured in MHA and MHB, and incubated at 37 ℃ for 24 h for each experiment.
2.3. Minimum inhibitory concentration (MIC)
MIC was determined using the broth microdilution method according to the Clinical and Laboratory Standards Institute 2006 guideline[11]. LUT was diluted in MHB in 96-well plate (0.5% w/v stock concentration). Preparation of the microorganism suspension was prepared by growing S. aureus in broth for 24 h, and adjusting the suspensions to 0.5 McFarland standard turbidity (approximately 1.5×108CFU/mL). The final inoculum was adjusted to 1.5×106CFU/mL. Inoculated broth in wells was incubated at 37 ℃for 18 h. At the end of each incubation period, turbidity indicated that bacterial growth had not been inhibited by the concentration of antimicrobial agent in the medium. MIC was defined at the lowest concentration of antibiotics and LUT that inhibited growth.
2.4. Effect of LUT on membrane-permeabilizing agent or ATPase inhibitor
To elucidate whether the antibacterial activity of LUT was associated with either altered membrane permeability or ATP synthase inhibit, the antibacterial activity of LUT was examined in the presence of membrane-permeabilizing agent, Tris and adenosine triphosphatase (ATPase)-inhibiting agent, N,N-DCCD respectively. Tris was used to increase membrane permeability of cell membranes[1]. DCCD, a metabolic inhibitor that could decrease ATP levels by disrupting electrochemical proton gradients in a bacterial environment, was used as an inhibitor of ATPase[12]. The effect of LUT on the membrane-permeabilizing agent (125 μg/mL Tris) or ATPase inhibitor (250 μg/mL DCCD) was determined.
2.5. Effect of PGN on LUT activity
To determine the activity of PGN in the presence of LUT, a LUT+PGN combination assay was performed[13]. This assay indicated whether LUT bound to PGN, the major constituent of the S. aureus cell wall. LUT (31.25 μg/mL) was added to PGN by serial dilution. LPS, a constituent of the outer membrane of Gram-negative bacteria[14], was used as negative control.
2.6. TEM
MRSA exponential-phase cultures were prepared by diluting cultures into MHB overnight, which was continued at 37 ℃ until the cultures reached the mid-logarithmic phase of growth. MHB-grown exponential-phase MRSA was treated with 1/2 MIC and the MIC of LUT for 10 h. Following the treatment, 2 mL of the culture was collected by centrifugation at 10 000 g for 10 min. After removal of the supernatant, pellets were fixed with modified Karnovsky’s fixative. The specimens were examined with an energyfiltering LIBRA 120 TEM (CarlZeiss, Oberkochen, Germany) at an accelerating voltage of 120 kV. Transmitted electron signals were recorded using anUltrascan4000 SP 4 k×4 k slow-scan chargecoupled device camera (Gatan, Pleasanton, CA, USA) attached to the electron microscope.
2.7. Statistical analyses
All experiments were performed more than three times. Data from the experiments are presented as the mean ± standard error of the mean. Statistical analyses were performed using one-way analysis of variance followed by Dunnett's t test (SPSS software version 19.0; IBM SPSS, Armonk, NY, USA). P<0.01 was considered statistically significant difference.
3. Results
3.1. Potentiated effects of LUT by Tris and DCCD
MIC values of LUT against two strains of S. aureus were presented in Table 1. Compared to the optical density at 600 nm (OD600) value of LUT alone (3.9 μg/mL), the OD600of suspensions treatedwith the combination of 125 μg/mL Tris was reduced to 60%. Bacterial viability in the presence of LUT with 250 μg/mL DCCD, a metabolic inhibitor, as reduced to 46% compared to LUT alone (15.6 μg/mL) (Figure 2).

Table 1 MIC of LUT against two strains of S. aureus used in this study.
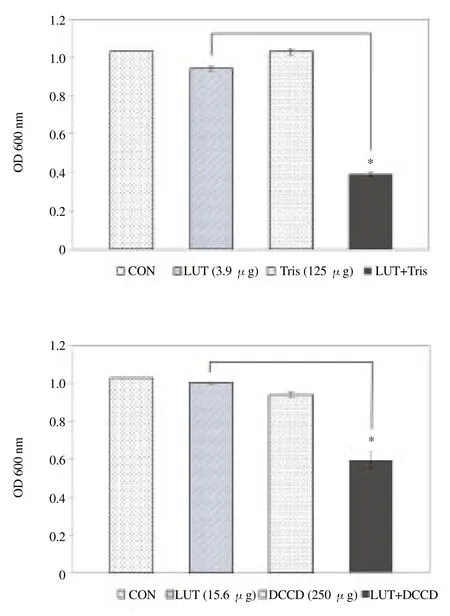
Figure 2. Effect of membrane-permeabilizing agent Tris and ATPaseinhibitor N,N'-DCCD on susceptibility of S. aureus (ATCC 33591) to LUT. The viability of bacteria was determined spectrophotometrically (optical density at 600 nm, OD 600 nm) after incubation for 36 h with LUT with 125 μg/mL Tris and 250 μg/mL DCCD. The data was the average of three independent experiments. *P<0.01 as compared to LUT alone, were determined.
3.2. Binding of LUT andPGN
The binding of LUT with PGN was confirmed by the addition of PGN (0.0–62.5 μg/mL) derived from S. aureus into MHB containing LUT. As shown in Figure 3, LUT (31.25 μg/mL) inhibited S. aureus growth by over 25%. A 62.5 μg/mL concentration of PGN disturbed the activity of LUT and 15.6 μg/mL concentration of PGN impeded the activity of LUT on S. aureus, while 62.5 μg/mL PGN completely blocked the antibacterial activity of LUT.

Figure 3. Direct binding of LUT with PGN in cell wall of S. aureus (ATCC 25923).
3.3. TEM
Antibiotic drugs induced other cellular changes such as separation of cytoplasmic contents[15]. The antibacterial activity of LUT on MRSA might be due to the ability of LUT to disrupt the cell wall of MRSA. LUT induced membrane disruption and cell lysis. LUT-free cultured cells had a normal morphology of S. aureus with distinct septa and smooth surfaces (Figure 4A). Cells treated with LUT (31.25 μg/mL) appeared to have damaged cytoplasmic membrane and had a rougher surface (Figure 4B). MRSA cells treated with LUT (62.5 μg/mL) were disrupted with reduced intracellular contents (Figure 4C).

Figure 4. TEM images of MRSA (ATCC 33591) after 10 h of LUT treatment.
4. Discussion
There is a clear need for development of new antibiotic that are widely effective against multidrug-resistant pathogens. We examined the effects of the membrane permeabilizing agent and ATP-binding cassette (ABC) transporter inhibiting agent on antibacterial activity of LUT. Most bacteria produce ABC transporter that is an essential uptake system for amino acids in the bacterial membrane. This is a determinant of bacterial antibiotic resistance[15-17]. ABC transporters have ATP-dependent transporting activity; DCCD inhibits the H+translocation activity of the F0domain of F0F1-ATPase[14]. In MRSA, LUT showed synergistic activity by increasing cytoplasmic membrane permeability and inhibiting ATPase. Gram-positive bacteria including S. aureus contain numerous layers (up to 30) of PGN. In S. aureus and other Gram-positive and Gram-negative bacteria, PGN is essential in osmotic protection and cell division[18]. Wall teichoic acids of S. aureus are anionic glycopolymers crosslinked to the thick PGN network, serving as a primer for cell wall biosynthesis[14,15]. The direct binding of PGN and LUT completely interrupts LUT-induced damage of the bacterial cell wall. The focus of the present study was on the development of natural antimicrobial agents to directly address the multidrug resistant of S. aureus. These findings can be important indication in study on mechanism of antimicrobial activity against MRSA in vitro. Further, in vivo experiments are needed for clinical application in MRSA-infection.
Conflict of interest statement
We declare that we have no conflict of interest.
References
[1]Mun SH, Joung DK, Kim SB, Park SJ, Seo YS, Gong R, et al. The mechanism of antimicrobial activity of sophoraflavanone B against methicillin-resistant Staphylococcus aureus. Foodborne Pathog Dis 2014; 11(3): 234-239.
[2]MuthaiyanA, Martin EM,Natesan S, Crandall PG, Wilkinson BJ, Ricke SC. Antimicrobial effect and mode of action of terpeneless cold-pressed Valencia orange essential oil on methicillin-resistant Staphylococcus aureus. J ApplMicrobiol 2012; 112(5): 1020-1033.
[3]Crowley B, Benedi VJ, Domenech-Sanchez A. Expression of SHV-2 beta-lactamase and of reduced amounts of OmpK36 porin in Klebsiella pneumoniae results in increased resistance to cephalosporins and carbapenems. Antimicrob Agents Chemother 2002; 46(11): 3679-3682.
[4]Mun SH, Kang OH, Joung DK, Kim SB, Seo YS, Choi JG, et al. Combination therapy of sophora flavanone B against MRSA: in vitro synergy testing. Evid Based Complement Alternat Med 2013; 2013: 823794.
[5]Lopez-Lazaro M. Distribution and biological activities of the flavonoid luteolin. Mini Rev Med Chem 2009; 9(1): 31-59.
[6]Lin Y, Shi R, Wang X, Shen HM. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets 2008; 8(7): 634-646.
[7]Seelinger G, Merfort I, Schempp CM. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Med 2008; 74(14): 1667-1677.
[8]Seelinger G, Merfort I, Wölfle U, Schempp CM. Anti-carcinogenic effects of the flavonoid luteolin. Molecules 2008; 13(10): 2628-2651.
[9]Su Y, Ma L, Wen Y, Wang H, Zhang S. Studies of the in vitro antibacterial activities of several polyphenols against clinical isolates of methicillinresistant Staphylococcus aureus. Molecules 2014; 19(8): 12630-12639.
[10]Qiu J, Li H, Meng H, Hu C, Li J, Luo M, et al. Impact of luteolin on the production of alpha-toxin by Staphylococcus aureus. Lett Appl Microbiol 2011; 53(2): 238-243.
[11]Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standards M7-A7.Wayne: CLSI.; 2006.
[12]Jung HJ, Lee DG. Synergistic antibacterial effect between silybin and N,N'-dicyclohexylcarbodiimide in clinical Pseudomonas aeruginosa isolates. J Microbiol 2008; 46(4): 462-467.
[13]Zhao WH, Hu ZQ, Okubo S, Hara Y, Shimamura T. Mechanism of synergy between epigallocatechin gallate and β-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2001; 45(6): 1737-1742.
[14]Mun SH, Kim SB, Kong R, Choi JG, Kim YC, Shin DW, et al. Curcumin reverse methicillin resistance in Staphylococcus aureus. Molecules 2014; 19(11): 18283-18295.
[15]Lehrer RI, Andrew Tincu J, Taylor SW, Menzel LP, Waring AJ. Natural peptide antibiotics from tunicates: structures, functions and potential uses. Integr Comp Biol 2003; 43(2): 313-322.
[16]Fulyani F, Schuurman-Wolters G, Zagar A, Guskov A, Slotboom DJ, Poolman B. Functional diversity of tandemsubstrate-binding domains in ABC transporters frompathogenic bacteria. Structure 2013; 21(10): 1879-1888.
[17]Steinfels E, Orelle C, Fantino JR, Dalmas O, Rigaud JL, Denizot F, et al. Characterizationof YvcC (BmrA), a multidrug ABC transporter constitutively expressed in Bacillus subtilis. Biochemistry 2004; 43(23): 7491-7502.
[18]Sobhanifar S, Worrall LJ, Gruninger RJ, Wasney GA, Blaukopf M, Baumann L, et al. Structure and mechanism of Staphylococcus aureus TarM, the wall teichoic acid α-glycosyltransferase. Proc Natl Acad Sci U S A 2015; 112(6): E576-E585.
Contents lists available at ScienceDirect IF: 1.062
Asian Pacific Journal of Tropical Medicine
journal homepage:www.elsevier.com/locate/apjtm
doi:Document heading 10.1016/j.apjtm.2015.12.004
*Corresponding author:Dong-Yeul Kwon, PhD, Department of Oriental Pharmacy, College of Pharmacy, Wonkwang University, Iksan, Jeonbuk 570-749, Republic of Korea.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Evaluation of antiviral activities of Houttuynia cordata Thunb. extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection
- Non-aqueous extracts of Curcuma mangga rhizomes induced cell death in human colorectal adenocarcinoma cell line (HT29) via induction of apoptosis and cell cycle arrest at G0/G1phase
- Evaluation of activity of triclabendazole against Taenia solium metacestode in naturally infected pigs
- Enterobacteria and Vibrio from Macrobrachium amazonicum prawn farming in Fortaleza, Ceará, Brazil
- Bio-ecology of malaria vectors in an endemic area, Southeast of Iran
- Surveillance of dengue and chikungunya infection in Dong Thap, Vietnam: A 13-month study
