Eu3+和Dy3+共掺单基质Ba2CaWO6白色荧光粉的合成与发光性质
2016-07-04易双萍冼洁强
黄 军,易双萍,冼洁强,张 璐
(广东工业大学 物理与光电工程学院,广东 广州 510006)
Eu3+和Dy3+共掺单基质Ba2CaWO6白色荧光粉的合成与发光性质
黄军,易双萍,冼洁强,张璐
(广东工业大学 物理与光电工程学院,广东 广州 510006)
摘要:通过高温固相法合成了Eu3+和Dy3+共掺杂的单一相Ba2CaWO6的荧光粉.通过XRD和扫描电镜分析了Ba2CaWO6荧光粉的晶相和形貌结构.Ba2CaWO6:Eu3+荧光粉因为5D0-7F1/7F2的能量跃迁发射很强的红光,而Ba2CaWO6:Dy3+荧光粉因为4F9/2-6H15/2和4F9/2-6H13/2的能量跃迁分别发蓝光和黄光.Eu3+/Dy3+共掺杂的Ba2CaWO6荧光粉发射暖白光.Dy3+到Eu3+的能量传递通过发光光谱强度来研究.通过调控Dy3+和Eu3+的掺杂比例可以对Ba2CaWO6:Dy3+, Eu3+样品的色坐标进行有效地调节,测试的色坐标表明Ba2CaWO6:Eu3+/Dy3+很适合紫外激发的白光LED荧光粉.
关键词:发光;能量传递;Dy3+-Eu3+;Ba2CaWO6
近几年来,荧光粉因为在发光二极管[1-3],有机发光二极管[4-6]、显示板[7-9],场发射显示屏[10-12]的广泛应用而吸引了大家广泛的关注.在这些应用中,白光LED因为其寿命长、环保、高光效而得到广泛的研究[13-15].制作LED通常有3种方法:(1) 紫外芯片结合红绿蓝3种荧光粉合成生成白光;(2) 蓝色芯片结合黄色荧光粉合成白光;(3) 红绿蓝3种芯片结合生成白光.现在越来越多的科研工作者开始研究单一相的荧光粉来产生白光[16-18],这种单一相荧光粉的优势就是可以产生从蓝色到红色的光谱.
有研究发现,WO6能够有效吸收紫外和近紫外光谱[19-20],比如Ba2CaWO6:Sm3+和Ba2CaWO6:Eu3+荧光粉的研究都有报道[21-22].对于稀土Dy3+离子有两个主要的发射带,一个是位于470~500nm的蓝光发射带,一个是位于560~600nm的黄光发射带,因此可以调节黄光和蓝光的比例来实现白光发射[23],但是Dy3+掺杂的单一相荧光粉缺少红光成分导致显示指数不够,为了克服这个缺陷,Dy3+可以和Eu3+共掺来实现单一相白光荧光粉.这样的研究有Gd2(MoO4)3:Dy3+-Eu3+[24]、Sr3AlO4F:Dy3+-Eu3+[25]、LiInW2O8:Dy3+-Eu3+[26]、CaMoO4:Dy3+-Eu3+[27]等.
根据查阅资料显示,关于Dy3+和Eu3+共掺Ba2CaWO6的荧光粉研究和报道还没有出现,本文开展研究了Ba2CaWO6:Dy3+-Eu3+的发光性质和能量传递,结果表明Ba2CaWO6:Dy3+-Eu3+可以用于紫外激发的单一相白光荧光粉.
1实验
1.1样品制备
通过高温固相法制备Ba2CaWO6:Dy3+-Eu3+荧光粉.分别用BaCO3、CaCO3、WO3,Dy2O3和Eu2O3作原料,按化学计量比称取BaCO3、CaCO3、WO3、Dy2O3和Eu2O3于研钵中,充分研磨使其混合均匀,装入坩埚中,在1 250 ℃烧结6h,冷却,研磨得到样品.
1.2样品测试
采用北京普析XD-2型X衍射仪(CuKα,36kV,20mA, λ=0.1 504nm)分析产物的物相结构;利用日本日立S-3400N-II扫描电子显微镜研究荧光粉的形貌和显微结构;利用日本日立HitachiF-7000 型荧光光谱仪测试荧光粉的激发光谱和发射光谱.
2结果与讨论
2.1样品XRD结构分析
图1为Ba2CaWO6:0.05Eu3+、Ba2CaWO6:0.06Dy3+、Ba2CaWO6:0.06Dy3+、0.05Eu3+的XRD图谱.将图谱的单一掺杂和共掺杂的图谱数据与标准卡片(JCPDS54-0188)进行对比,结果发现,所合成的样品衍射峰数据与标准卡片一致,表明所合成的样品均为纯的单一相,在此条件下Eu3+和Dy3+均可进入Ba2CaWO6晶格格位.
2.2样品的SEM分析
图2为Ba2CaWO6:0.06Dy3+、0.05Eu3+样品的扫描电镜图片,从图2中可以看出样品是由粒径大约为几微米的颗粒团聚组成,表面光滑.

图1 Dy3+/Eu3+单掺和共掺+Ba2CaWO6荧光粉的XRD图谱
Fig.1XRDpatternsofDy3+/Eu3+single-dopedandco-dopedBa2CaWO6phosphor

图2 Ba2CaWO6:0.06Dy3+、 0.05Eu3+的电子扫描显微镜图片
2.3Dy3+,Eu3+单掺Ba2CaWO6的荧光光谱分析
图3为Ba2CaWO6:Dy3+的激发和发射光谱,由图3曲线a可见,Ba2CaWO6:Dy3+的激发光谱是由250nm到350nm的宽带激发峰组成,最强峰位于314nm,宽带激发是由电荷迁移产生.另外在365nm处可以观察到由Dy3+的6H15/2-6P5/2跃迁产生的微弱激发峰.图3曲线b可见Ba2CaWO6:Dy3+的发射光谱在495nm和584nm处有两个Dy3+的特征峰,这两个峰是由Dy3+的4F9/2-6H15/2和4F9/2-6H13/2跃迁分别产生.当Dy3+占据晶格的高对称晶格位置时,Dy3+发射的光谱主要为蓝色,如果占据的是非对称位置时,Dy3+发射的主要是黄光[28].从图中可以看出发射光谱主要以蓝光为主,说明Dy3+在晶格中占据的是高对称晶格位置.
图4是Ba2CaWO6:Eu3+的激发和发射光谱,激发光谱是在595nm的监测波长下测得的,发射光谱Ba2CaWO6:0.06Dy3+是在314nm激发波长下测得.由图4曲线a可见Ba2CaWO6:Eu3+的激发光谱是由250nm到350nm的宽带激发组成,最强峰位于314nm,宽带激发是由电荷迁移产生的.例外可以观察到393nm处的激发峰,这是由于Eu3+的7F0-5L6跃迁产生.由图4曲线b可见,发射光谱为在595nm和612nm处Eu3+的特征峰,分别对应于Eu3+的5D0-7F1和5D0-7F2跃迁,由图可知,595nm的发射比612nm的发射更强,说明Eu3+占据的是晶格的对称位置.

图3 Ba2CaWO6:0.06Dy3+的激发光谱和发射光谱图
Fig.3Theexcitationandemissionspectrumof

图4 Ba2CaWO6:0.05Eu3+激发光谱和发射光谱图
Fig.4TheexcitationandemissionspectrumofBa2CaWO6:0.05Eu3+
图5曲线a是Ba2CaWO6:xDy3+发射峰相对强度随Dy3+浓度的变化而变化的曲线,从图中可见,当浓度从0增加到6%时,发射峰强度随浓度的增大而增大,当Dy3+浓度进一步增大时,发射峰强度开始下降,这是由于浓度淬灭引起的.图5曲线b是Ba2CaWO6:Eu3+不同Eu3+浓度样品在发射峰值为595nm(λex=314nm)激发下得到的样品发射强度与掺杂浓度的关系图.可以看出Eu3+的最佳掺杂浓度为5%.
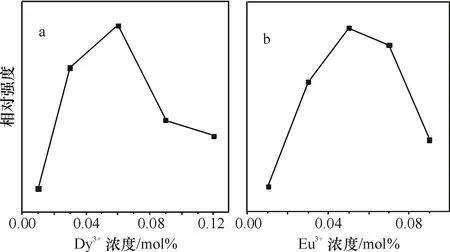
图5Ba2CaWO6:xDy3+发射光谱强度与Dy3+浓度关系曲线(a),Ba2CaWO6:aEu3+发射光谱强度与Eu3+浓度的关系曲线 (b)
Fig.5EmissionintensityofBa2CaWO6:xDy3+asfunctionofDy3+concentration(a),EmissionintensityofBa2CaWO6:aEu3+asfunctionofEu3+concentration(b)
2.4Dy3+,Eu3+共掺杂Ba2CaWO6的荧光光谱
图6是Ba2CaWO6:0.06Dy3+,aEu3+不同Eu3+浓度掺杂样品在314nm激发下的发射光谱图,由图6可见,发射光谱由3组发射峰组成,分别对应于Dy3+的蓝色发射峰(Dy3+:4F9/2-6H15/2),Dy3的黄色发射峰(Dy3+:4F9/2-6H13/2),Eu3+的红色发射(Eu3+:5D0-7F1和5D0-7F2)组成,随着Eu3+浓度的增加,红色区域的发射强度不断增强,蓝色区域的发射强度不断减弱,而黄色区域的发射强度影响不是很明显.从这个结果表明在Ba2CaWO6:Dy3+,Eu3+中存在能量从Dy3+到Eu3+的有效传递.这个结果表明也可以通过改变Eu3+和Dy3+的浓度比例来获取需要的白光.
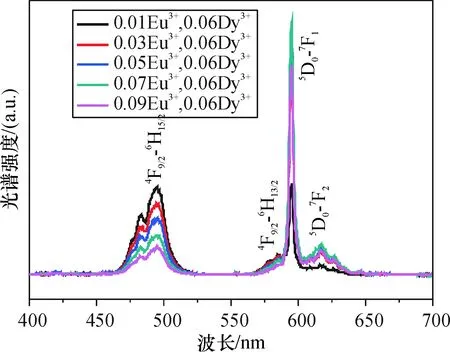
图6Ba2CaWO6:0.06Dy3+, aEu3+在不同Eu3+浓度下发射光谱的强度
Fig.6EmissionspectraofBa2CaWO6:0.06Dy3+, aEu3+phosphorswithdifferentEu3+ionsdopingconcentration(λex=314nm)
2.5Ba2CaWO6:0.06Dy3+, aEu3+的色度坐标
蓝、黄和红色成分之间的比例大小是影响材料发光色度坐标的最主要因素,为此,考察了Ba2CaWO6:0.06Dy3+, aEu3+的色度坐标与掺杂浓度之间的关系,表1列出了不同Eu3+掺杂浓度的色度坐标值,图7标出了不同Eu3+掺杂浓度的色坐标位置.从图7可以看出当Eu3+浓度增大时,它的色坐标向红色区域移动,这表明Eu3+的红色发射可以补偿Ba2CaWO6:Dy3+中缺少的红光组分,它的显色指数也必将提高,这也大大提高了其应用价值.
表1Ba2CaWO6单掺Eu3+,Dy3+和共掺Eu3+/Dy3+的色度坐标值
Tab.1CIEchromaticitycoordinatesoftheEu3+andDy3+ionssingle-doped,andtheEu3+/Dy3+ionsco-dopedBa2CaWO6phosphors
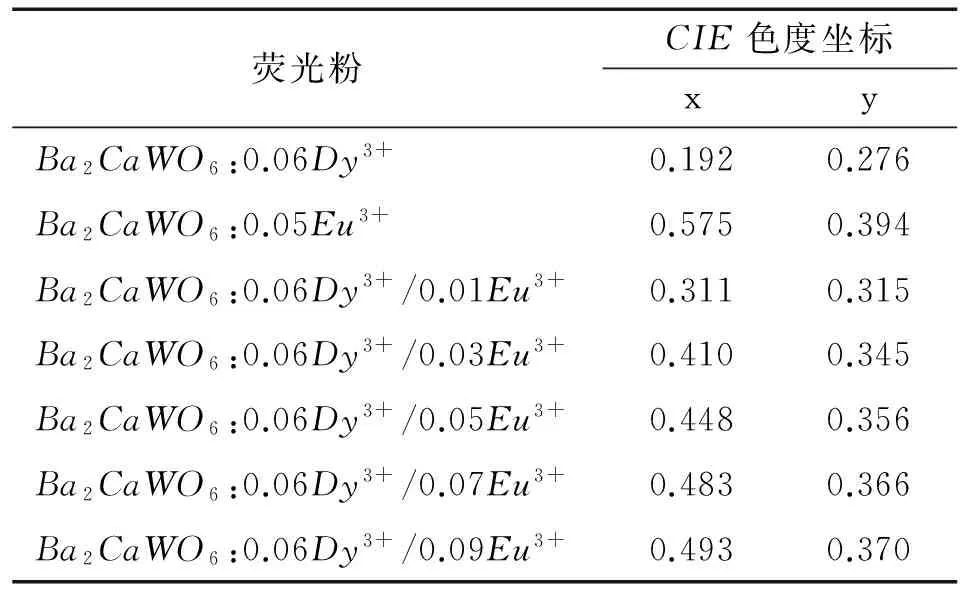
荧光粉CIE色度坐标xyBa2CaWO6:0.06Dy3+0.1920.276Ba2CaWO6:0.05Eu3+0.5750.394Ba2CaWO6:0.06Dy3+/0.01Eu3+0.3110.315Ba2CaWO6:0.06Dy3+/0.03Eu3+0.4100.345Ba2CaWO6:0.06Dy3+/0.05Eu3+0.4480.356Ba2CaWO6:0.06Dy3+/0.07Eu3+0.4830.366Ba2CaWO6:0.06Dy3+/0.09Eu3+0.4930.370

图7Ba2CaWO6:0.06Dy3+, aEu3+:(a) a=0, (b) a=0.01, (c) a=0.03, (d) a=0.05, (e) a=0.07, (f) a=0.09的色度坐标图
Fig.7CIEchromaticitycoordinatesoftheBa2CaWO6:0.06Dy3+, aEu3+:(a) a=0, (b) a=0.01, (c) a=0.03, (d) a=0.05, (e) a=0.07, (f) a=0.09
3结论
采用高温固相法,在1 250 ℃煅烧6h合成了Ba2CaWO6:Dy3+,Eu3+单一基质的白色荧光粉.在紫外光激发下Ba2CaWO6:Dy3+,Eu3+同时出现了属于Dy3+的495nm和584nm发射及属于Eu3+的595nm的发射,通过调控Dy3+和Eu3+的掺杂比例可以对Ba2CaWO6:Dy3+,Eu3+样品的色坐标进行有效的调节,其中Eu3+的加入,使得荧光粉的红色成分增加,提高了荧光粉的显色指数,使其更接近白光,从而制备出Dy3+,Eu3+共掺杂的Ba2CaWO6单一基质白光LED用荧光粉.
参考文献:
[1]JIAOM,JIAY,LYUW,etal.Sr3GdNa(PO4)3F:Eu2+,Mn2+:apotentialcolortunablephosphorforwhiteLEDs[J].JournalofMaterialsChemistryC, 2014, 2(1): 90-97.
[2]PARKJ,LEESJ,KIMYJ.EvolutionofLuminescenceofSr2-y-zCazSi(O1-xNx)4:yEu2+withN3-,Eu2+,andCa2+Substitutions[J].CrystalGrowth&Design, 2013, 13(12): 5204-5210.
[3]JIAY,LYUW,GUON,etal.UtilizingTb3+asanenergytransferbridgetoconnectEu2+-Sm3+luminescentcenters:realizationofefficientSm3+redemissionundernear-UVexcitation[J].ChemicalCommunications, 2013, 49(26): 2664-2666.
[4]HANC,ZHAOF,ZHANGZ,etal.Constructinglow-triplet-energyhostsforhighlyefficientbluepHOLEDs:controllingchargeandexcitoncaptureindopingsystems[J].ChemistryofMaterials, 2013, 25(24): 4966-4976.
[5]LIUXK,ZHENGCJ,LOMF,etal.Novelbluefluorophorwithhightripletenergylevelforhighperformancesingle-emitting-layerfluorescenceandphosphorescencehybridwhiteorganiclight-emittingdiodes[J].ChemistryofMaterials, 2013, 25(21): 4454-4459.
[6]TANGQ,LIUS,LIUY,etal.Colortuningandwhitelightemissionviainsitudopingofluminescentlanthanidemetal-organicframeworks[J].InorganicChemistry, 2013, 53(1): 289-293.
[7]ZHANGC,LIANGH,ZHANGS,etal.EfficientSensitizationofEu3+EmissionbyTb3+inBa3La(PO4)3underVUV-UVExcitation:EnergyTransferandTunableEmission[J].TheJournalofPhysicalChemistryC, 2012, 116(30): 15932-15937.
[8]LINH,ZHANGG,TANNERPA,etal.VUV-VisluminescentpropertiesofBaCaBO3FDopedwithCe3+andTb3+[J].TheJournalofPhysicalChemistryC, 2013, 117(24): 12769-12777.
[9]SHINDEKN,SINGHR,DHOBLESJ.Photoluminescencepropertiesof(Gd1-xYx)0.94Eu0.06PO4(0≤x≤1.0)phosphors[J].JournalofLuminescence, 2014, 145(15): 930-935.
[10]GENGD,LIG,SHANGM,etal.ColortuningviaenergytransferinSr3In(PO4)3:Ce3+/Tb3+/Mn2+phosphors[J].JournalofMaterialsChemistry, 2012, 22(28): 14262-14271.
[11]LIG,ZHANGY,GENGD,etal.Single-compositiontrichromaticwhite-emittingCa4Y6(SiO4)6O:Ce3+/Mn2+/Tb3+phosphor:luminescenceandenergytransfer[J].ACSAppliedMaterials&Interfaces, 2011, 4(1): 296-305.
[12]GENGD,SHANGM,ZHANGY,etal.TunableluminescenceandenergytransferpropertiesofCa5(PO4)2SiO4:Ce3+/Tb3+/Mn2+phosphors[J].JournalofMaterialsChemistryC, 2013, 1(12): 2345-2353.
[13]WANGZ,LIP,YANGZ,etal.LuminescenceandenergytransferofSm3+andEu3+inCa2PO4Cl[J].JournalofLuminescence, 2014, 151(6): 170-175.
[14]YES,XIAOF,PANYX,etal.Phosphorsinphosphor-convertedwhitelight-emittingdiodes:Recentadvancesinmaterials,techniquesandproperties[J].MaterialsScienceandEngineering:R:Reports, 2010, 71(1): 1-34.
[15]YES,XIAOF,PANYX,etal.Phosphorsinphosphor-convertedwhitelight-emittingdiodes:Recentadvancesinmaterials,techniquesandproperties[J].MaterialsScienceandEngineering:R:Reports, 2010, 71(1): 1-34.
[16]KUMARRD,KARUPPUCHAMYS.SynthesisandcharacterizationofnanostructuredZn-WO3andZnWO4bysimplesolutiongrowthtechnique[J].JournalofMaterialsScience:MaterialsinElectronics, 2015, 26(6):3256-3261.
[17]HANC,YANGMQ,WENGB,etal.Improvingthephotocatalyticactivityandanti-photocorrosionofsemiconductorZnObycouplingwithversatilecarbon[J].PhysicalChemistryChemicalPhysics, 2014, 16(32): 16891-16903.
[18]JEONYI,BHARATLK,YUJS.SynthesisandluminescencepropertiesofEu3+/Dy3+ionsco-dopedCa2La8(GeO4)6O2phosphorsforwhite-lightapplications[J].JournalofAlloysandCompounds, 2015, 620(1): 263-268.
[19]YUR,NOHHM,MOONBK,etal.PhotoluminescencecharacteristicsofSm3+-dopedBa2CaWO6asneworange-redemittingphosphors[J].JournalofLuminescence, 2014, 152(6): 133-137.
[20]LIH,YANGHK,MOONBK,etal.Color-conversionandphotoluminescencepropertiesofBa2MgW(Mo)O6:Euphosphor[J].JournalofAlloysandCompounds, 2011, 509(35): 8788-8793.
[21]YUR,NOHHM,MOONBK,etal.PhotoluminescencecharacteristicsofSm3+-dopedBa2CaWO6asneworange-redemittingphosphors[J].JournalofLuminescence, 2014, 152(32): 133-137.
[22]SUNX,HAOZ,LIC,etal.Enhancedorange-redemissionbyusingMocodopedinBa2CaWO6:Eu3+,Li+phosphorundernearUVexcitation[J].JournalofLuminescence, 2013, 134(22): 191-194.
[23]ZHANGJ,JINGY,PANPANL,etal.PreparationandluminescentpropertiesofMgTiO3:Eu3+phosphorforwhiteLEDs[J].JournalofRareEarths, 2012, 30(10): 1009-1012.
[24]WANJ,CHENGL,SUNJ,etal.EnergytransferandcolorimetricpropertiesofEu3+/Dy3+co-dopedGd2(MoO4)3phosphors[J].JournalofAlloysandCompounds, 2010, 496(1): 331-334.
[25]BANDIVR,GRANDHEBK,WOOHJ,etal.LuminescenceandenergytransferofEu3+or/andDy3+co-dopedinSr3Al4FphosphorswithNUVexcitationforWLEDs[J].JournalofAlloysandCompounds, 2012, 538(1): 85-90.
[26]NAIDUSA,BOUDINS,VARADARAJUUV,etal.Host-sensitizedemissionofLiInW2O8wolframites:Fromred-Eu3+towhite-Dy3+phosphors[J].JournalofSolidStateChemistry, 2011, 184(9): 2566-2570.
[27]KHANNAA,DUTTAPS.NarrowspectralemissionCaMoO4:Eu3+,Dy3+,Tb3+phosphorcrystalsforwhitelightemittingdiodes[J].JournalofSolidStateChemistry, 2013, 198(16): 93-100.
[28]YUM,LINJ,WANGZ,etal.Fabrication,patterning,andopticalpropertiesofnanocrystallineYVO4:A(A=Eu3+,Dy3+,Sm3+,Er3+)phosphorfilmsviasol-gelsoftlithography[J].ChemistryofMaterials, 2002, 14(5): 2224-2231.
Synthesis and Luminescence Properties of Eu3+/Dy3+Co-doped Single-phased Ba2CaWO6Phosphors for White-light Applications
Huang Jun, Yi Shuang-ping, Xian Jie-qiang, Zhang Lu
(SchoolofPhysicsandOptoelectronicEngineering,GuangdongUniversityofTechnology,Guangzhou510006,China)
Abstract:Dy3+, Eu3+, and Dy3+-Eu3+doped Ba2CaWO6 phosphors are synthesized by solid-state method. The structural and morphological studies of the Ba2CaWO6 phosphors are conducted by measuring X-ray diffraction pattern and scanning electron microscope images. The photoluminescence (PL) spectra of the Ba2CaWO6:Eu3+phosphor exhibit the intense red emission due to the transition of5D0-7F1/7F2 while the Ba2CaWO6:Dy3+phosphor shows the blue (4F9/2-6H15/2) and yellow (4F9/2-6H13/2) emissions. The Eu3+/Dy3+ions co-doped Ba2CaWO6 phosphor shows the improved white light by shifting towards the warm white region. The energy transfer from Dy3+to Eu3+ions is investigated by means of PL intensities. The luminescence intensities and chromaticity coordinates can be modified by controlling the concentration and the ration of doping Dy3+and Eu3+. The calculated chromaticity coordinates indicate that Ba2CaWO6:Eu3+/Dy3+phosphors may be suitable for the fabrication of near-UV excitation-based white light-emitting diodes.
Key words:luminescence; energy transfer; Dy3+-Eu3+; Ba2CaWO6
收稿日期:2015-03-08
基金项目:广东省产学研结合资助项目(2012B09110044);广东省自然科学基金资助项目(9151009001000052)
作者简介:黄军(1987-),男,硕士研究生,主要研究方向为发光功能材料.E-mail: huangjun99100@163.com
doi:10.3969/j.issn.1007-7162.2016.02.015
中图分类号:TQ586.1
文献标志码:A
文章编号:1007-7162(2016)02-0076-05
