Degradation of phenol in industrial wastewater over the F–Fe/TiO2 photocatalysts under visible light illumination☆
2016-06-12YandongLiuShijianZhouFuYangHuaQinYanKong
Yandong Liu ,Shijian Zhou ,2,*,Fu Yang ,Hua Qin ,Yan Kong ,*
1 State Key Laboratory of Materials-Oriented Chemical Engineering,College of Chemistry and Chemical Engineering,Nanjing Tech University,Nanjing 210009,China
2 Jiangsu National Synergetic Innovation Center for Advanced Materials(SICAM),Nanjing Tech University,Nanjing 210009,China
1.Introduction
The global environment has been seriously polluted by industrial wastewater with the development of world economy.Phenol,as a pollutant in wastewater with high content,has caused severe environmental problems due to its harmful toxicity,poor biodegradability and long remaining-ability[1–3].Therefore,the degradation of phenol in wastewater has attracted more and more attention.To resolve this problem,many technologies have been proposed involving physical adsorption,chemical coagulation and biological treatment[4–8].However,by these traditional techniques,phenol could not be decomposed completely,and even the secondary pollutants would be generated[9].Hence,it is still urgent for us to develop an advanced technique for the degradation of phenolic wastewater.
Recently,since it is able to decompose toxic chemicals into unharmful CO2and H2O completely,photocatalytic decomposition of organic contaminants with different semiconductor materials becomes an important technology[10].Among these semiconductor photocatalysts,titanium dioxide(TiO2)has been proved to be the mostpromising material in practical application due to its low-cost,nontoxicity and chemical stability.However,due to the wide band gap(3.0–3.2 eV)in TiO2,only UV portion of the solar spectrum could be absorbed,which leads to a poor solar energy utilization during the reaction process[6,11,12].Moreover,the high recombination efficiency of photogenerated electron–hole pairs is another reason for restricting their practical application in pollutant degradation[13].To overcome these drawbacks and improve the photocatalytic activity of TiO2in the visible light region,many efforts have been attempted.For instance,Chenget al.found that F and N co-doped TiO2(F–N-TiO2)nanoparticles exhibited an enhanced photoactivity with an apparent redshift to the region of visible light[11].Songet al.prepared a highly active g-C3N4/TiO2hybrid photocatalyst by using a simple sonication method.The apparent rate constant k of g-C3N4/TiO2–1.5 was 7.03 times higher than bare TiO2,which was mainly attributed to the extended visible light absorption[14].Agorku and coworkers demonstrated that S/Gd3+-codoping made a red shift in the absorption band of TiO2,resulting in the increase of photocatalytic activity under visible light[15].
In addition,many promoters have been applied to the further improvement of the photocatalytic activity in TiO2catalyst.Ferric species,as the important component of Fenton reagent,have been reported to be useful for the catalytic oxidation of phenol[16–18].Sun.et al.suggested that surface modified TiO2with iron oxide clusters(Fe/TiO2)through adsorption and decomposition of a large Fe(III)complex exhibiting an enhanced activity for phenol degradation in water under UVlight[19].Also,the surface fluorination of TiO2can lead to the reduction of the recombination of photogenerated electrons and holes by the formation of surface≡Ti--F groups[11,20,21].In this case,more mobile·OH radicals which are favorable to the oxidation reaction will be generated by F modification[21–23].Zhanget al.discovered that the F–Fe codoped TiO2exhibited an enhanced photodegradation rate(76%)at the best doping mass ratio of F:Fe:Ti=0.15:0.15:100,which is due to the large red shift in the light adsorption edge[24].Wang and co-workers also prepared the Fe(III)/F-TiO2photocatalyst by two wet-chemical method including Fe(III)(atomic percent of Fe to Ti=0.8%)ions impregnation and then F-ion(F/Ti=1%)adsorption on the TiO2surface[25].However,further research found that the catalyst with a relatively high iron loading(5%)displays the optimum photocatalytic properties for phenol degradation[26].Since the low iron content in these codoped catalysts,the synthesis of new F and Fe co-modified TiO2catalysts with a superior photoactivity and higher stability for phenoldegradation is still important.
In this work,we prepared F and Fe co-modified TiO2(F–Fe/TiO2)catalyst with high iron loading(4.5%)via a facile one-step hydrothermal method.The physical and chemical characterizations of F–Fe/TiO2photocatalysts were conducted,and the performance was evaluated in terms of phenol photodegradation under visible light irradiation.Then the simulated conditions of industrial wastewater including initial phenol concentration,visible light intensity,pH and different anions were investigated in the presence of F–Fe/TiO2photocatalyst.This research provides a promising practical approach in the efficient treatment of phenol in industrial wastewater.
2.Experimental Methods
2.1.Materials
Allchemicals except the tetrabutyltitanate(chemicalgrade)used in this study are analytical grade and used without further treatment.Tetrabutyl titanate and iron(III)chioride anhydrous were obtained from Sinopharm Chemical Reagent Co.Ltd.All the other reagents are supplied by Shanghai Jiuyi Chemical Reagent Co.Ltd.
2.2.Preparation of F-Fe/TiO2,F/TiO2,Fe/TiO2 and pure TiO2 samples
The F–Fe/TiO2photocatalyst was prepared by hydrothermal method.In a typical procedure,tetrabutyltitanate(20 ml)was added into absolute ethanol(80 ml)under vigorously stirring.After that,a certain amount of hydrofluoric acid solution(40 wt%)and FeCl3was added.The resulting solution was stirred for 30 min,subsequently transferred into a Teflon lined stainless-steel autoclave,which was reacted at 180°C for 24 h.After the reaction,the products were air-cooled to room temperature,then centrifuged,washed with absolute ethanol,and dried at60°C for severalhours.The final resulting samples were labeled as F–Fe/TiO2(atomic percent of F/Ti=0.8%,Fe/Ti=4.5%).The mono-modified F/TiO2or Fe/TiO2catalyst was prepared under the same conditions with only FeCl3or hydrofluoric acid,respectively.The pure TiO2catalyst was prepared by the same method using deionized water instead of hydrofluoric acid solution.
2.3.Preparation of F0.38-Fe0.13-TiO2 sample
The material of F and Fe co-doping TiO2(F0.38–Fe0.13–TiO2)was prepared by a stepwise sol–gel reaction and hydrogen peroxide oxidation according to the previous report[24].First of all,0.6 mol·L-1titanium tetrachloride solutions were prepared,and then appropriate amounts of NH4F and FeCl3were added to produce the solutions.The system was statically kept for 24 h,and then an ammonia solution was added slowly to reach pH value of 10.The obtained precipitate was washed with distilled water 6 times to remove impurities.Subsequently,distilled water was used to dilute the slurry to yield a suspension(about 10 g of sediment in 0.1 L of water),and saturated hydrogen peroxide was dropped using a drip funnel until an orange-red transparent solution was formed.The solution was heated to 80°C for 8 h to form the sol and then dried to produce powders of TiO2.The resulting sample is labeled as F0.38–Fe0.13–TiO2(F/Ti=0.38%,Fe/Ti=0.13%).
2.4.Preparation of Fe(III)/F-TiO2 sample
As Wanget al.reported[25],the Fe(III)/F-TiO2photocatalysts was prepared with impregnation method as follows.The P25 TiO2powder calcined at550°C for 2 h was used as the TiO2precursor.Firstly,TiO2(0.5 g)was dispersed into 5 ml Fe(NO3)3solution with a pH of 2(adjusted by 1 mol·L-1HCl solution)under stirring.After 15 min,the suspension solution was heated to 60°C and maintained for 2 h.The resultant powders were filtrated,rinsed with distilled water,and dried at 60°C to obtain the Fe(III)/TiO2photocatalysts.And then F ions were impregnated onto the Fe(III)/TiO2sample to form the Fe(III)/F-TiO2(F/Ti=1.0%,Fe/Ti=0.8%)photocatalyst based on above method,wherein,the aforementioned TiO2and Fe(NO3)3were replaced with Fe(III)/TiO2and NH4F,respectively.
2.5.Characterization
Powder X-ray diffraction(XRD)patterns were recorded on a Bruker D8 Advance X-ray diffractometer to study the crystal structure and crystallinity,using Ni- filtered CuKαradiation(λ =0.154178 nm)at 40 kV and 40 mA in the 2θ range from 20°to 60°.The elemental composition and electronic structure of the synthesized materials were carried out with an X-ray photoelectron spectroscopy(XPS,PHI 5000 Versa Probe),using AlKαradiation(1486.6 eV)and characteristic C 1s peak centered at 284.6 eV as the standard bond energy of the samples.The UV–visible diffuse reflection spectra were determined by a UV–Vis spectrometer(Perkin–Elmer Lambda 950)with a light path length of 1 cm.
2.6.Photodegradation of phenol under visible light
The photocatalytic degradation of phenol was carried out in a quartz column with water circulation jacket at room temperature.The pH of experiments involved was adjusted with NaOH(0.1 mol·L-1)and H2SO4(0.1 mol·L-1).In a typical photocatalytic experiment,a given amount of catalyst was added to the quartz column containing 50 ml aqueous solution of phenol with the desired initial concentration at pH=4.The prepared suspension was stirred in the dark for 1 h to ensure the establishment of an adsorption–desorption equilibrium of phenol on the catalyst.Then,the suspension was irradiated under a 700 W xenon lamp equipped with 400 nm cutoff filters(PCS-400)to remove the UV lights.At given time intervals(every 60 min),4 ml suspensions were sampled and centrifuged to remove catalyst powders,and the filtrates were analyzed by UV–Vis spectrophotometer(BLV-GHX-V,Shanghai Bilang Instrument Co.Ltd.)to determine the photodegradation rate of phenol.The simulated conditions of industrial wastewater including phenol such as initial phenol concentrations(50–400 mg·L-1),visible light intensity(500–900 W),pH(4,7 and 10)and several different sodium salts(NaCl,Na2SO4,NaNO3and Na2CO3)at 0.1 mol·L-1on the degradation reaction were investigated.The photocatalytic stability of catalysts was examined by recycling runs.After the completion of the reaction,the separated photocatalysts were gathered,washed by water and ethanol alternatively,and dried for the subsequent cycling test.
3.Results and Discussion
3.1.Catalyst characterization
Fig.1 demonstrates the XRD patterns of pure TiO2,F/TiO2,Fe/TiO2and F–Fe/TiO2photocatalysts.In Fig.1,only diffraction peaks of anatase TiO2(JCPDS no.21-1272)are observed in all samples,indicating that the introduction of F and Fe species has no effect on the phase of TiO2.Meanwhile,there is no diffraction peak corresponding to Fe-like compounds detected,suggesting that Fe species should be well-dispersed in the prepared samples.Apparently,compared with pure TiO2,the peak intensity of F-TiO2is strengthened,manifesting the addition of F is beneficial for the crystallinity of the catalyst.According to previous reports[27],the good anatase crystallization is beneficial to the promotion of photocatalytic activity TiO2catalysts.

Fig.1.XRD patterns of different samples.
The XPS spectra were used to investigate the elemental chemical states of various modified samples.As shown in Fig.2,all the XPS spectra were calibrated with the C1s peak at284.6 eV.In the F 1s spectra of F/TiO2and F–Fe/TiO2,only one peak with binding energy located at about 684.1 eV can be observed,which assigned to the surface fluoride(≡Ti--F)formed by ligand exchange between F-and surface hydroxyl group on TiO2surface[20,28].Furthermore,it can be seen that there is no significant difference between the F/TiO2and F–Fe/TiO2,indicating that the introduction of Fe species almost has no influence on the content and chemical states of F.Based on calculated results from the XPS spectra,the surface content of F in F/TiO2and F–Fe/TiO2is 0.91 and 0.89%,respectively.In the Fe 2p spectra of Fe/TiO2and F–Fe/TiO2,the peaks at around 711.0 and 724.3 eV correspond to the 2p3/2and 2p1/2of Fe3+[29,30].Meanwhile,the satellite ‘shoulder’at 717.6 eV confirms the presence of FeOx[30,31].Comparing the Fe 2p peaks of Fe-TiO2and F–Fe/TiO2,the positions and intensities almost be the same,indicating that F modification does not affect the concentration and states of Fe species in the TiO2catalysts.The XPS spectra reveal that the Fe species are adsorbed on the surface of TiO2in the form of FeOx.As calculated from the XPS spectra,the surface content of Fe accounts for Fe/TiO2and F–Fe/TiO2is 4.38%and 4.42%,respectively,which is close to the theoretical value.
3.2.Optical property
The UV–Vis DRS spectra(Fig.3)were recorded to investigate the optical properties of various samples.The bandgaps of samples were determined by a plot(αhυ)1/2vsthe photon energyhυ.The adsorption coefficient α and Eg is related by the following equation:

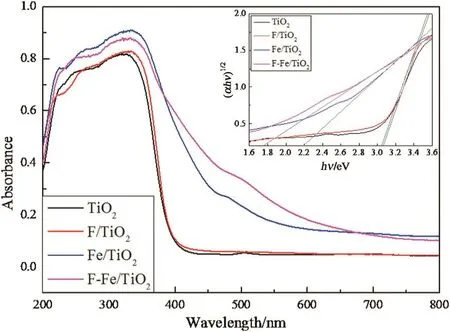
Fig.3.UV–Vis DRS spectra of pure TiO2,F/TiO2,Fe/TiO2 and F–Fe/TiO2 catalysts.
whereEg(eV)is the bandgap,hand υ represent the Planck constant,and frequency,respectively.As shown in the inset of Fig.3,the bandgaps of TiO2,F/TiO2,Fe/TiO2and F–Fe/TiO2were calculated to be 3.08,3.05,2.20 and 1.76 eV,respectively.Compared to the pure TiO2,the F/TiO2catalyst exhibits similar bandgap absorption with a slight decrease of bandgap of 0.03 eV.This observation suggests that the electronic absorption spectroscopy properties between F/TiO2and pure TiO2is similar,which further demonstrates that the F just replaces the--OH on TiO2crystal surface rather than enters into the TiO2crystal lattice to replace the oxygen.Therefore,the UV–Vis DRS results are in good accordance with the XPS results.After introducing Fe species,Fe/TiO2and F–Fe/TiO2show a stronger absorption in the visible light region with a bandgap of 2.20 and 1.76 eV,which is due to the excitation of 3d electrons of Fe3+to TiO2conduction band[29]and the charge transition between interacting iron ions(Fe3++Fe3+→Fe4++Fe2+)[32,33].The red shift of the absorption wavelength indicates that the comodified F–Fe/TiO2catalyst can absorb visible light and be further applied for the visible-light photocatalysis.
3.3.Photocatalytic performance of catalysts
The photocatalytic performances of various prepared samples were evaluated by the degradation of phenol under visible light irradiation for 300 min.As shown in Fig.4(a),the F–Fe/TiO2photocatalyst exhibits the highest photocatalytic activity for the degradation of phenol in all samples,while the photocatalytic activities of F/TiO2and Fe/TiO2are higher than pure TiO2.Moreover,it should be noted that,as compared with F0.38–Fe0.13–TiO2and Fe(III)/F-TiO2,the prepared F–Fe/TiO2catalyst from the hydrothermal process in this study exhibit a higher photoactivity.To further investigate the reaction kinetics process,the reaction rate constant of phenol degradation over various samples were measured by the slop of following equation:
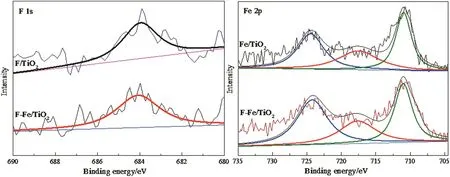
Fig.2.XPS spectra of F 1s and Fe 2p for modified TiO2 catalysts.
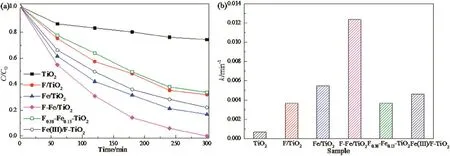
Fig.4.(a)Potodegradation of phenol over various samples and(b)apparent rate constants under visible light irradiation([phenol]=100 mg·L-1,[catalyst]=1 g·L-1,[visible light intensity]=700 W,pH=4).

wherein,k(min-1)is the apparent reaction rate constant,C0andC(mg·L-1)represent the concentration of phenol at initial and time t(min),respectively.Fig.4(b)shows the reaction rate constant of phenol degradation under visible light irradiation on various TiO2photocatalysts.The reaction rate constant of F–Fe/TiO2is improved to be 0.012 min-1,which is 18.96,3.37 and 2.25 times higher than pure TiO2,F/TiO2and Fe/TiO2,respectively.This observation confirms that the co-modification of F and Fe on TiO2can improve the photodegradation rate of phenol significantly.Also,the reaction rate constant for F–Fe/TiO2is higher than that of F0.38–Fe0.13–TiO2and Fe(III)/F-TiO2.Also,Table 1 lists several reported modified TiO2catalysts,and their photocatalytic results in the degradation of phenol.It is found that,as compared with these studies,the catalysts synthesized in this study not only exhibit superior photoactivity under visible light irradiation,but also could degrade phenol with high concentration completely.
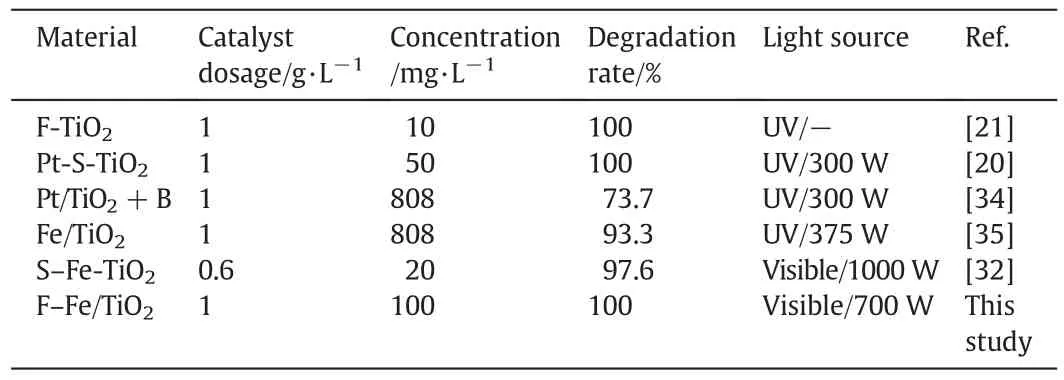
Table 1Comparison on catalytic activity for phenol degradation over some modified TiO2 catalysts
To further verify the industrial application value of F–Fe/TiO2catalyst,we simulated the industrial phenolic wastewater to investigate the influence of different initial parameters on photocatalytic degradation of phenol,including initial phenol concentration,visible light intensity,pH and various anions.
3.3.1.Effect of initial phenol concentration
It is well known that,the initial concentration has a great influence on photodegradation of organic compounds.The effect of initial phenol concentration(50–400 mg·L-1)on the photodegradation was demonstrated in Fig.5.It is revealed that,with the decreasing initial phenol concentration from 400 to 100 mg·L-1,the degradation rate significantly increases from 35.73%to 100%in 300 min.Moreover,100%phenol is removed within 180 min with the concentration of 50 mg·L-1.Zhanget al.reported that,in a typical photocatalytic reaction,molecular oxygen is activated by photogenerated electrons to produce·O2-or H2O2,while holes are trapped by surface-absorbed hydroxyls(OH-)to generate·OH,which would participate in the subsequent degradation reaction[36].At higher phenol concentration,due to the growth of the equilibrium adsorption of phenol on the active sites,a large amount of phenol molecules get adsorbed.Therefore,competitive adsorption of OH-on the same site decreases,and consequently the amount of·OH and ·O2-on the same site decreases[37].Furthermore,in the different initial phenol concentration,the probability of phenol molecules to react with·OH would be decreased and then the removal efficiency is accordingly dropped[38].In addition,with progress in degradation reaction especially at high initial concentration,some intermediates,such as 2-hydroxy-propaldehyde,hydroxyacetic acid,3-hydroxy-propyl acid,glycerol etc.[39],are formed and competitively adsorbed on the catalyst surface and also competitively react with oxidant species[37].All of these factors finally cause a decrease of the degradation rate of phenol.Since the catalyst dosage is 1 g·L-1for different initial phenol concentration,the present results indicate that photocatalytic degradation is rather promising when the ratio of phenol to catalyst is below 1:10.3.3.2.Effect of visible light intensity
The effect of visible light intensity(500–900 W)on phenol photodegradation was examined for F–Fe/TiO2photocatalyst.As shown in Fig.6,the degradation rate with a light intensity of 500,600 and 700 W is 71.48%,84.76%and 100%within 300 min,respectively.When the visible light intensity increases from 700 to 900 W,the time required for complete degradation reduces from 300 to 180 min.This can be attributed to the fact that the enhancement of visible light intensity can provide more energy for the production of photogenerated electron–hole pairs and hence,promoting the formation of·OH radicals which are beneficial to the degradation of phenol[40].Therefore,it is suitable for the complete degradation of phenol when the visible light intensity is higher than 700 W.
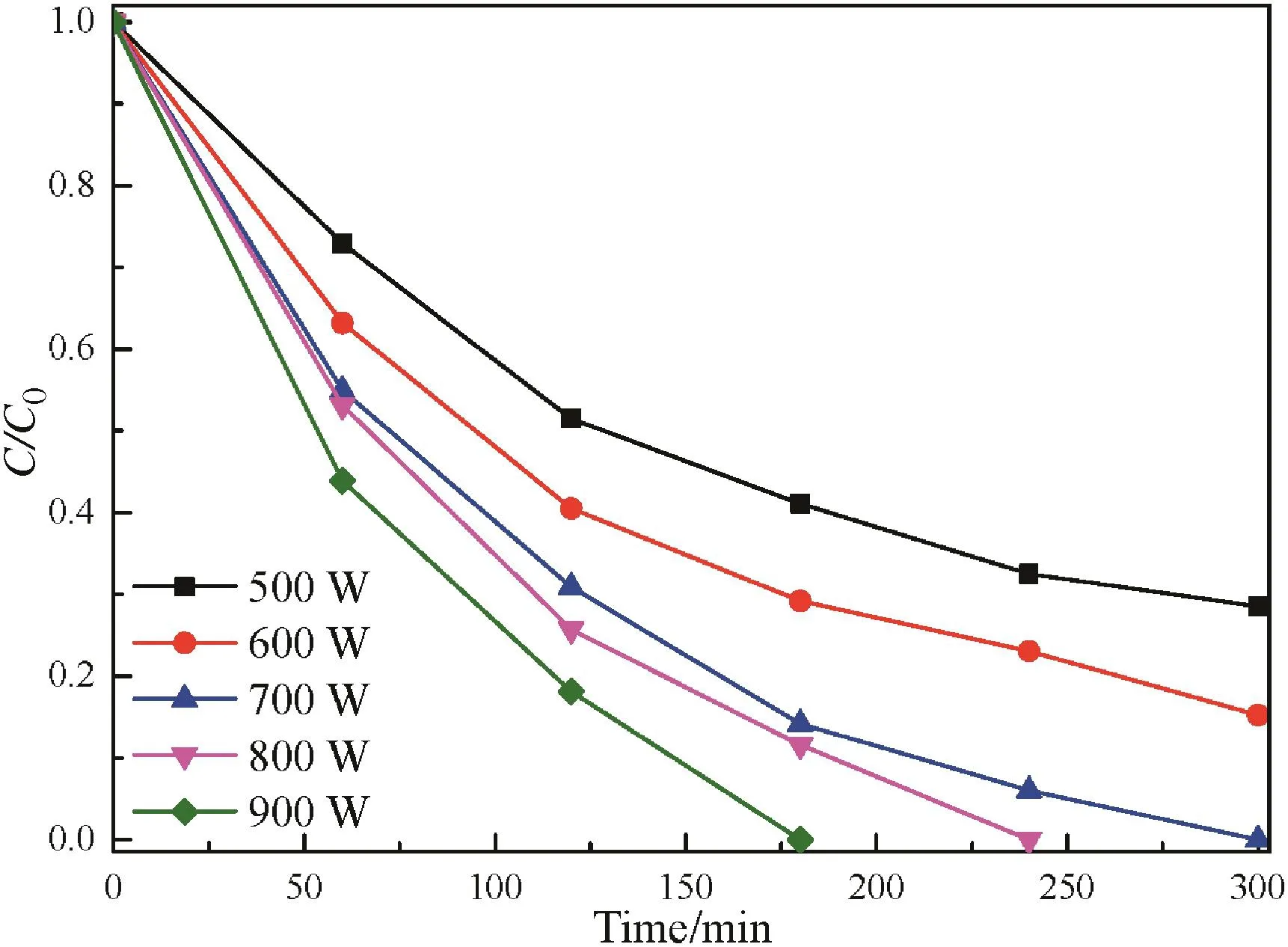
Fig.6.Influence of visible light intensity on the photocatalytic degradation of phenol over F–Fe/TiO2 catalyst under visible light irradiation.([catalyst]=1 g·L-1,[phenol concentration]=100 mg·L-1,pH=4).
3.3.3.Effect of pH
Due to the complex acid–base properties of industrial wastewater including phenol,the pH effect needs to be investigated.Also,the pH of an aqueous mediumis an important factor that may influence the uptake of the adsorbate on the photocatalyst surface[41].According to previous reports[42],the point of zero charge(pHpzc)of TiO2is about 6.8,thus below this value the TiO2surface is positively charged and above it is negatively charged.The results in Fig.7 indicate that the degradation is favored in an acidic solution,in contrary,it is inhibited in a basic solution as compared with that of in neutral solution.In the acidic solution,phenol is primarily in its nonionic form,water solubility is minimized and the adsorption onto the catalyst is maximized[40].It is supposed that the density of·OH radicals is highest near the surface of F–Fe/TiO2and decreases rapidly with distance from the surface[40].At higher pH,phenol tends to exist as negatively charged phenolate species,which have extremely strong solubility in solution and will not be adsorbed on TiO2surface significantly[42].Also,the coulombic repulsion between the negatively charged surface of particles and the OH-could avoid the formation of·OH radicals,hence reducing the photodegradation rate of phenol.Moreover,high pH favors the formation of carbonate ions which are effective scavengers of OH-ions and can cause the less degradation of phenol[37,42].Through above results,we conclude that the degradation efficiency is favorable in the acidic solution.
3.3.4.Effect of different anions
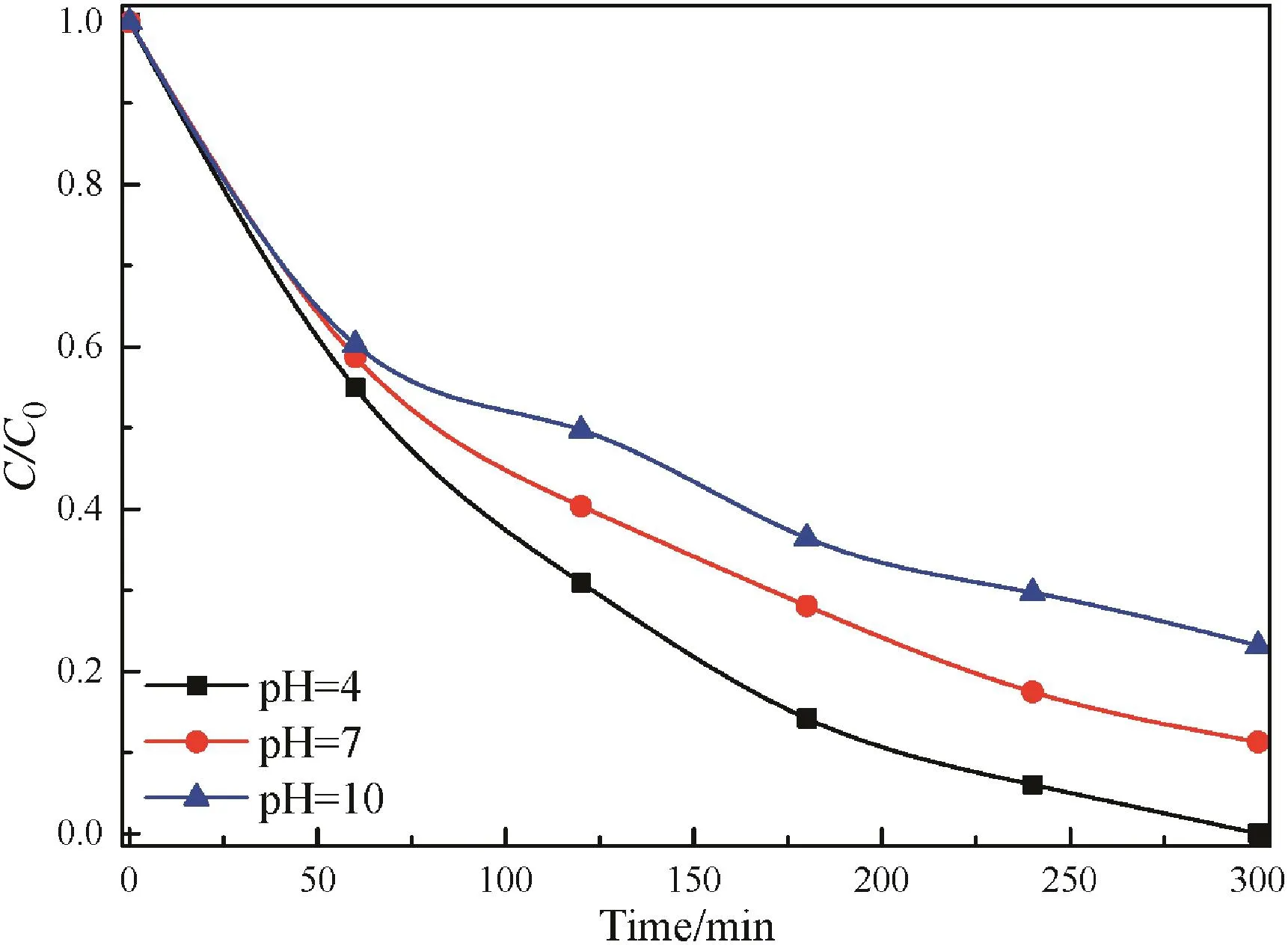
Fig.7.Influence of pH on the photocatalytic degradation of phenolover F–Fe/TiO2 catalyst under visible light irradiation.([catalyst]=1 g·L-1,[phenol concentration]=100 mg·L-1,[visible light intensity]=700 W).
To further assess the process,the influences of several anions,such as Cl-,andwhich are common in phenolic wastewater,were investigated.In the presence of Cl-,,andthe phenol degradation rate is 74.04%,82.13%,91.76%and 97.07%,respectively within 300 min(Fig.8).Kormann et al.found that the adsorption of organic molecules is poor because the chloride can be easily adsorbed on the positively charged TiO2surface at low pH.Also,chloride is considered to deactivate·OH radicals according to following equations[42]:

Therefore,the decrease in the phenol degradation rate in the case of chloride is due to the combined effect of both poor adsorption on catalyst surface and lower·OH radical concentration.Carbonate ion has also been reported to scavenge·OH radicals via a mechanism similar to that of Cl-[43].Nitrate and sulfate ions have comparatively weaker effect on adsorption and consequently on the photocatalytic degradation.In conclusion,the adsorbed anions compete with organic molecules for the photo-oxidizing species on the surface of catalyst and inhibit the degradation of phenol with the order of Cl->>>
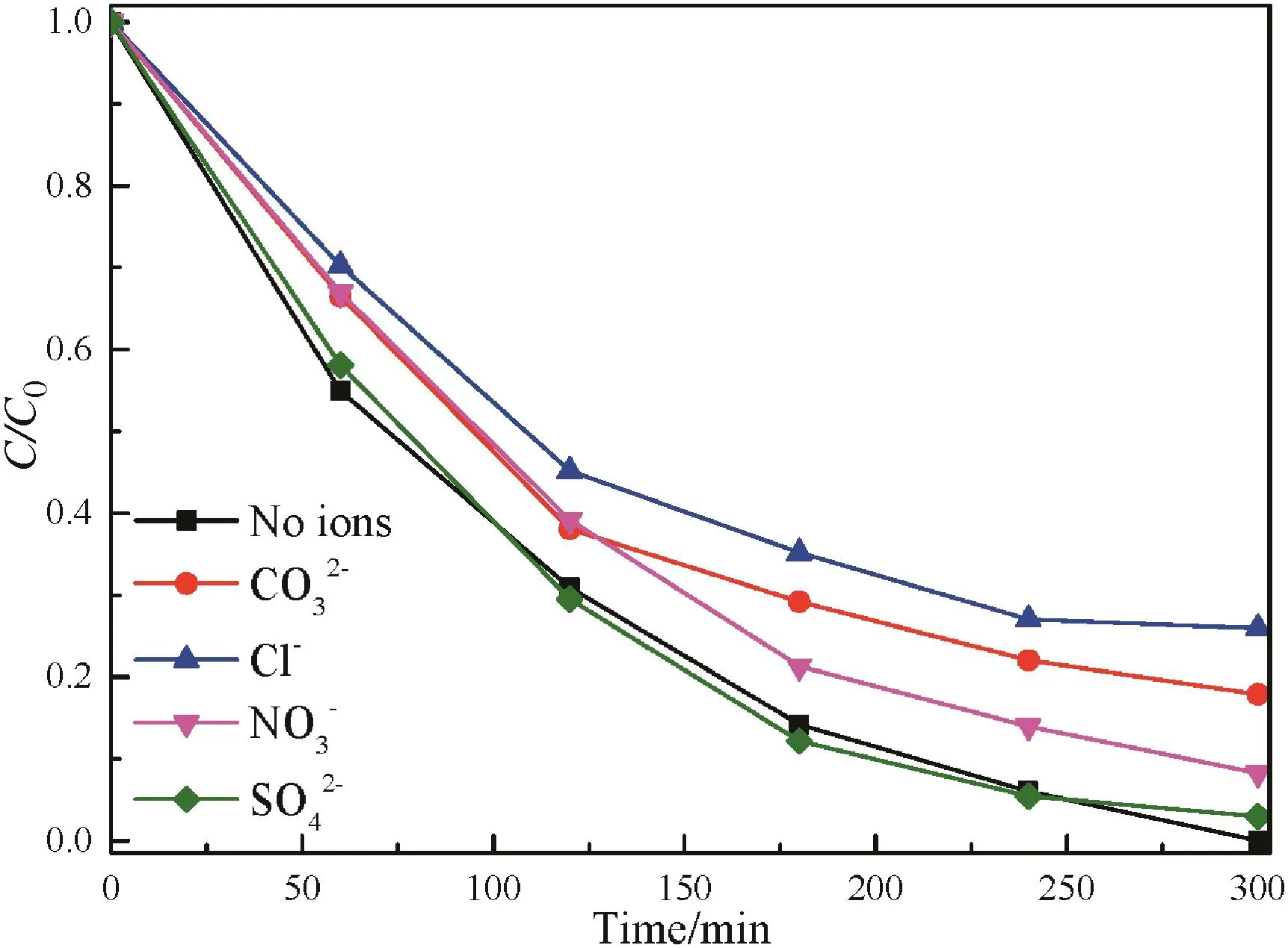
Fig.8.Influence of the presence of various anions on photocatalytic degradation of phenol over F–Fe/TiO2 catalyst under visible light irradiation.([catalyst]=1 g·L-1,[phenol concentration]=100 mg·L-1,[visible light intensity]=700 W,pH=4).
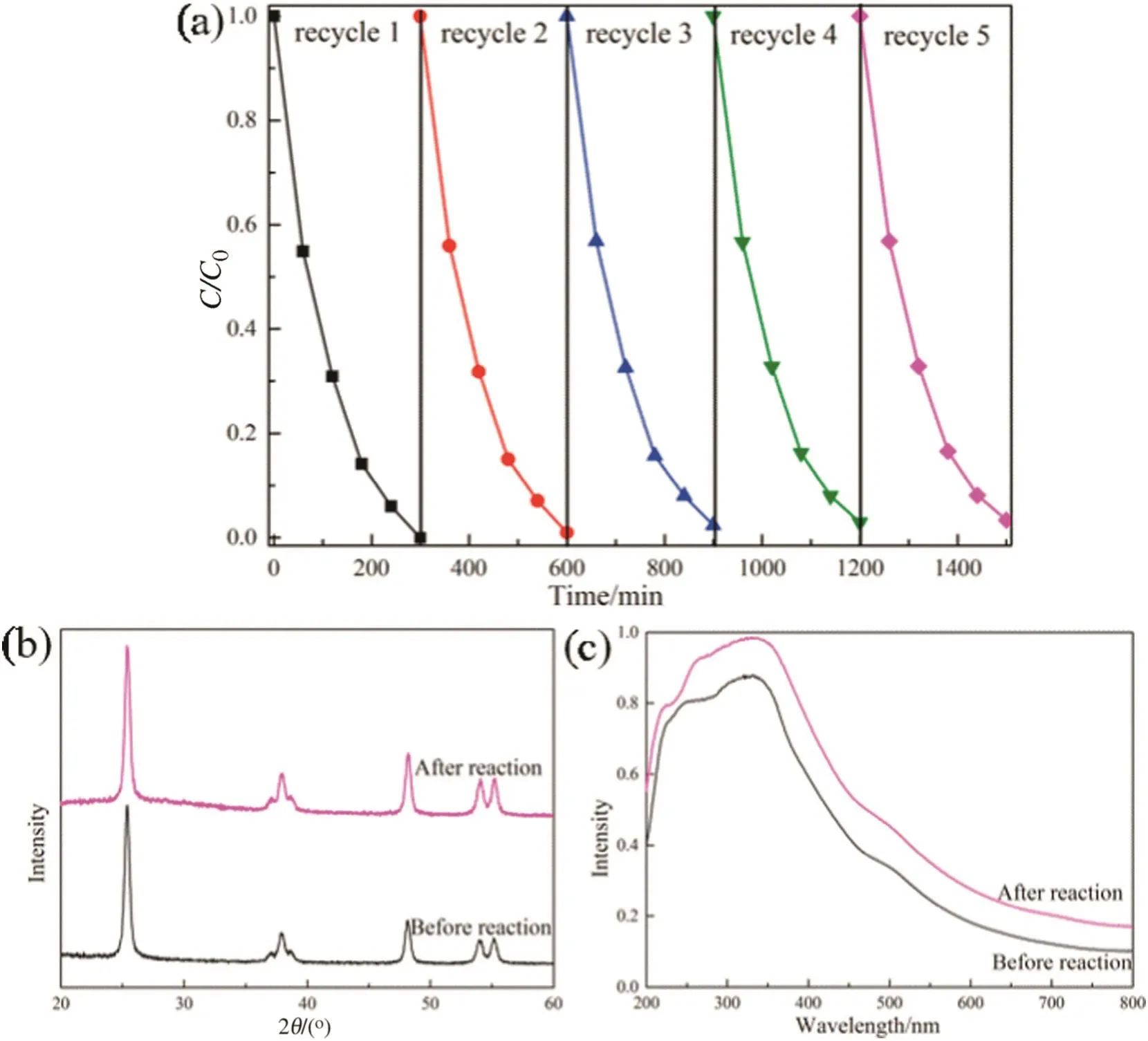
Fig.9.(a)Cycling of phenol degradation over F–Fe/TiO2 under visible light irradiation,(b)XRD patterns and(c)UV–Vis DRS of F–Fe/TiO2 before and after the reaction.
3.4.Stability of catalysts
To evaluate the catalytic stability of F–Fe/TiO2catalyst,the recycling of photodegradation of phenol under visible light was conducted,as shown in Fig.9(a).It is found that,after 5 times recycle,the photoactivity of F–Fe/TiO2catalyst still almost be the same,indicating the good performance of reuse during the reaction.In order to well understand the physiochemical properties ofF–Fe/TiO2catalyst before and after reaction,the XRD and UV–Vis DRS experiments have been carried out and the results are shown in Fig.9(b,c).As observed,there is not any obvious change between the F–Fe/TiO2catalyst before and after reaction,demonstrating the high stability of the catalyst.In short,with the high catalytic stability and reuse rate,the catalyst of F–Fe/TiO2has a promising potential industrial application in the treatment of phenolic wastewater.
4.Conclusions
Photocatalytic degradation of phenol has been carried out over F–Fe/TiO2(synthesized by hydrothermal method)under visible light irradiation.Results showed that the F–Fe/TiO2(F:0.89%;Fe:4.42%)catalyst possesses a superior photocatalytic activity as compared with pure TiO2,F/TiO2,Fe/TiO2,F0.38–Fe0.13–TiO2and Fe(III)/F-TiO2.The simulated conditions of industrial phenolic wastewater including initial phenol concentration,visible light intensities,pH and anions types were investigated in the presence of F–Fe/TiO2photocatalyst.It is found that,in acidic solution,phenol can be degraded completely when the ratio of phenol to catalyst is below 1:10 and visible light intensity is higher than 700 W.The presence of various anions inhibited the photodegradation efficiency with the order of Cl-Moreover,the F–Fe/TiO2photocatalyst exhibited excellent stability.The involving investigations demonstrated that the as prepared F–Fe/TiO2could act as an efficient potential catalyst for the treatment of phenol in industrial wastewater.
[1]Q.L.Ge,X.P.Yue,G.Y.Wang,Simultaneous heterotrophic nitrification and aerobic denitrification at high initial phenol concentration by isolated bacterium Diaphorobacter sp PD-7,Chin.J.Chem.Eng.23(5)(2015)835–841.
[2]F.Shahrezaei,Y.Mansouri,A.A.L.Zinatizadeh,A.Akhbari,Process modeling and kinetic evaluation of petroleum refinery wastewater treatment in a photocatalytic reactor using TiO2nanoparticles,Powder Technol.221(2012)203–212.
[3]P.Górska,A.Zaleska,J.Hupka,Photodegradation of phenol by UV/TiO2and Vis/N,CTiO2processes:Comparative mechanistic and kinetic studies,Sep.Purif.Technol.68(1)(2009)90–96.
[4]J.Wang,H.Ruan,W.Li,D.Li,Y.Hu,J.Chen,Y.Shao,Y.Zheng,Highly efficient oxidation of gaseous benzene on novel Ag3VO4/TiO2nanocomposite photocatalysts under visible and simulated solar light irradiation,J.Phys.Chem.C116(26)(2012)13935–13943.
[5]J.A.O.Méndez,J.A.H.Melián,J.Araña,J.M.D.Rodríguez,O.G.Díaz,J.P.Peña,Detoxification of waters contaminated with phenol,formaldehyde and phenol–formaldehyde mixtures using a combination of biological treatments and advanced oxidation techniques,Appl.Catal.B Environ.163(2015)63–73.
[6]J.Lim,D.Monllor-Satoca,J.S.Jang,S.Lee,W.Choi,Visible light photocatalysis of fullerol-complexed TiO2enhanced by Nb doping,Appl.Catal.B Environ.152-153(2014)233–240.
[7]B.Li,K.Sun,Y.Guo,J.Tian,Y.Xue,D.Sun,Adsorption kinetics of phenol from water on Fe/AC,Fuel110(2013)99–106.
[8]M.Eiroa,A.Vilar,C.Kennes,M.C.Veiga,Effect of phenol on the biological treatment of waste waters from a resin producing industry,Bioresour.Technol.99(9)(2008)3507–3512.
[9]H.Ling,K.Kim,Z.Liu,J.Shi,X.Zhu,J.Huang,Photocatalytic degradation of phenol in water on as-prepared and surface modified TiO2nanoparticles,Catal.Today258(2015)96–102.
[10]F.He,J.Li,T.Li,G.Li,Solvothermal synthesis of mesoporous TiO2:The effect of morphology,size and calcination progress on photocatalytic activity in the degradation of gaseous benzene,Chem.Eng.J.237(2014)312–321.
[11]J.Cheng,J.Chen,W.Lin,Y.Liu,Y.Kong,Improved visible light photocatalytic activity of fluorine and nitrogen co-doped TiO2with tunable nanoparticle size,Appl.Surf.Sci.332(2015)573–580.
[12]Y.Fang,D.Cheng,W.Wu,Understanding electronic and optical properties of N–Sn codoped anatase TiO2,Comput.Mater.Sci.85(2014)264–268.
[13]J.Wang,Q.Meng,J.Huang,Q.Li,J.Yang,Band structure engineering of anatase TiO2by metal-assisted P–O coupling,J.Chem.Phys.140(17)(2014)174705.
[14]G.Song,Z.Chu,W.Jin,H.Sun,Enhanced performance of g-C3N4/TiO2photocatalysts for degradation of organic pollutants under visible light,Chin.J.Chem.Eng.23(8)(2015)1326–1334.
[15]E.S.Agorku,B.B.Mamba,A.C.Pandey,A.K.Mishra,Sulfur/gadolinium-codoped TiO2nanoparticles for enhanced visible-light photocatalytic performance,J.Nanomater.(2014).
[16]H.Q.Wang,X.M.Li,C.R.Xiong,S.Y.Gao,J.Wang,Y.Kong,One-pot synthesis of ironcontaining nanoreactors with controllable catalytic activity based on multichannel mesoporous silica,ChemCatChem7(23)(2015)3855–3864.
[17]B.Han,X.Shi,Y.Zhang,Q.Kong,Q.Sun,Y.Kong,Influences of pore sizes on the catalytic activity of Fe-MCM-41 in hydroxylation of phenol,Asian J.Chem.25(16)(2013)9087–9091.
[18]C.Wu,Y.Kong,F.Gao,Y.Wu,Y.Lu,J.Wang,L.Dong,Synthesis,characterization and catalytic performance for phenol hydroxylation of Fe-MCM41 with high iron content,Microporous Mesoporous Mater.113(1–3)(2008)163–170.
[19]Q.Sun,W.Leng,Z.Li,Y.Xu,Effect of surface Fe2O3clusters on the photocatalytic activity of TiO2for phenol degradation in water,J.Hazard.Mater.229(2012)224–232.
[20]J.J.Murcia,M.C.Hidalgo,J.A.Navío,J.Araña,J.M.Doña-Rodríguez,Study of the phenol photocatalytic degradation over TiO2modified by sulfation, fluorination,and platinum nanoparticles photodeposition,Appl.Catal.B Environ.179(2015)305–312.
[21]Y.N.Tan,C.L.Wong,A.R.Mohamed,Hydrothermal treatment of fluorinated titanium dioxide:Photocatalytic degradation of phenol,Asia Pac.J.Chem.Eng.7(6)(2012)877–885.
[22]J.K.Zhou,L.Lv,J.Q.Yu,H.L.Li,P.Z.Guo,H.Sun,X.S.Zhao,Synthesis of self-organized polycrystalline F-doped TiO2hollow microspheres and their photocatalytic activity under visible light,J.Phys.Chem.C112(14)(2008)5316–5321.
[23]H.Kim,W.Choi,Effects of surface fluorination of TiO2on photocatalytic oxidation of gaseous acetaldehyde,Appl.Catal.B Environ.69(3–4)(2007)127–132.
[24]Y.Zhang,F.Lv,T.Wu,L.Yu,R.Zhang,B.Shen,X.Meng,Z.Ye,P.K.Chu,F and Fe codoped TiO2with enhanced visible light photocatalytic activity,J.Sol-Gel Sci.Technol.59(2)(2011)387–391.
[25]X.Wang,R.Yu,P.Wang,F.Chen,H.Yu,Co-modification of F-and Fe(III)ions as a facile strategy towards effective separation of photogenerated electrons and holes,Appl.Surf.Sci.351(2015)66–73.
[26]C.Adana,A.Bahamonde,I.Oller,S.Malato,A.Martinez-Arias,Influence of iron leaching and oxidizing agent employed on solar photodegradation of phenol over nanostructured iron-doped titania catalysts,Appl.Catal.B Environ.144(2014)269–276.
[27]J.Li,J.Xu,W.-L.Dai,H.Li,K.Fan,One-pot synthesis of twist-like helix tungsten–nitrogen-codoped titania photocatalysts with highly improved visible light activity in the abatement of phenol,Appl.Catal.B Environ.82(3–4)(2008)233–243.
[28]W.Yu,X.Liu,L.Pan,J.Li,J.Liu,J.Zhang,P.Li,C.Chen,Z.Sun,Enhanced visible light photocatalytic degradation of methylene blue by F-doped TiO2,Appl.Surf.Sci.319(2014)107–112.
[29]N.R.Mathews,M.A.Cortes Jacome,C.Angeles-Chavez,J.A.Toledo Antonio,Fe doped TiO2powder synthesized by sol gel method:Structural and photocatalytic characterization,J.Mater.Sci.Mater.Electron.26(8)(2014)5574–5584.
[30]T.Yamashita,P.Hayes,Analysis of XPS spectra of Fe2+and Fe3+ions in oxide materials,Appl.Surf.Sci.254(8)(2008)2441–2449.
[31]Y.Wang,S.Wang,H.Zhang,X.Gao,J.Yang,L.Wang,Brookite TiO2decorated α-Fe2O3nanoheterostructures with rod morphologies for gas sensor application,J.Mater.Chem.A2(21)(2014)7935.
[32]Y.Niu,M.Xing,J.Zhang,B.Tian,Visible light activated sulfur and iron co-doped TiO2photocatalyst for the photocatalytic degradation of phenol,Catal.Today201(2013)159–166.
[33]J.-Q.Li,D.-F.Wang,Z.-Y.Guo,Z.-F.Zhu,Preparation,characterization and visible light-driven photocatalytic activity of Fe-incorporated TiO2microspheres photocatalysts,Appl.Surf.Sci.263(2012)382–388.
[34]X.Xiong,Y.Xu,Synergetic effect of Pt and borate on the TiO2-photocatalyzed degradation of phenol in water,J.Phys.Chem.C120(7)(2016)3906–3912.
[35]Q.Sun,W.Leng,Z.Li,Y.Xu,Effect of surface Fe2O3clusters on the photocatalytic activity of TiO2for phenol degradation in water,J.Hazard.Mater.229-230(2012)224–232.
[36]H.Zhang,L.-H.Guo,D.Wang,L.Zhao,B.Wan,Light-induced efficient molecular oxygen activation on a Cu(II)-grafted TiO2/graphene photocatalyst for phenol degradation,ACS Appl.Mater.Interfaces7(3)(2015)1816–1823.
[37]S.H.Borji,S.Nasseri,A.H.Mahvi,R.Nabizadeh,A.H.Javadi,Investigation of photocatalytic degradation of phenol by Fe(III)-doped TiO2and TiO2nanoparticles,J.Environ.Health Sci.Eng.12(2014).
[38]Y.Yao,F.Lu,Y.Zhu,F.Wei,X.Liu,C.Lian,S.Wang,Magnetic core-shell CuFe2O4@C3N4hybrids for visible light photocatalysis of Orange II,J.Hazard.Mater.297(2015)224–233.
[39]Z.Guo,R.Ma,G.Li,Degradation of phenol by nanomaterial TiO2in wastewater,Chem.Eng.J.119(1)(2006)55–59.
[40]C.-H.Chiou,C.-Y.Wu,R.-S.Juang,Influence of operating parameters on photocatalytic degradation of phenol in UV/TiO2process,Chem.Eng.J.139(2)(2008)322–329.
[41]A.Adak,A.Pal,M.Bandyopadhyay,Removal of phenol from water environment by surfactant-modified alumina through adsolubilization,Colloids Surf.A Physicochem.Eng.Asp.277(1–3)(2006)63–68.
[42]N.Kashif,F.Ouyang,Parameters effect on heterogeneous photocatalysed degradation of phenol in aqueous dispersion of TiO2,J.Environ.Sci.21(4)(2009)527–533.
[43]A.A.Yawalkar,D.S.Bhatkhande,V.G.Pangarkar,A.Beenackers,Solar-assisted photochemical and photocatalytic degradation of phenol,J.Chem.Technol.Biotechnol.76(4)(2001)363–370.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Statistical mechanics and artificial intelligence to model the thermodynamic properties of pure and mixture of ionic liquids☆
- Modification and sequential treatment of EU-1 zeolite in mild alkali and alkaline-acid conditions
- Catalytic kinetics of dimethyl ether one-step synthesis over CeO2–CaO–Pd/HZSM-5 catalyst in sulfur-containing syngas process☆
- Development of a bifurcation analysis approach based on gPROMS platform☆
- A comprehensive fractal char combustion model☆
- Molar volume of eutectic solvents as a function of molar composition and temperature☆
