Non-catalytic conversion of wheat straw,walnut shell and almond shell into hydrogen rich gas in supercritical water media
2016-06-01FaridSafariMohammadSalimiAhmadTavasoliAbtinAtaei
Farid Safari,Mohammad Salimi,Ahmad Tavasoli,*,Abtin Ataei
1 Department of Energy Engineering,Science and Research Branch,Islamic Azad University,Tehran,Iran
2 School of Chemistry,College of science,University of Tehran,Tehran,Iran
1.Introduction
Energy security is one of the most important concerns of modern era.Using renewable energy resources,maximizing energy efficiency and improving energy conservation with novel methods are strategies for achieving energy security and sustainable energy development[1].Technologies,which convert primary energy resources into energy carriers,have always been received lots of attention during last decades.Biomass as a renewable energy resource can be converted into solid,liquid and gaseous bio products via different technologies.This valuable energy resource is currently supplying about10%of world's energy consumption[2].Millions tons of agricultural residues are wasted or burned annually in the world.According to the statistic report of food and agriculture organization of the united nation,Iran produced 13800000 tons of wheat,450000 tons of walnut and 100000 tons of almond in 2012 which were placed in the 12th,2nd and 4th rank in the world,respectively.Considering that almost 50%of these products are being wasted annually in the form of wheat straw,walnut shell and almond shell in the lack of conversional industries,it is necessary to develop promising methods for conversion of these valuable resources into useful fuels and chemicals which is a sustainable way for supplying future's energy demand[3,4].Different methods such as biochemical and thermo-chemical and recently advanced hydrothermal processes have been studied[5].Hydrogen considered by many scientists as a key energy carrier of the future.The amount of energy released during hydrogen combustion is higher than any other fossil fuels on a mass basis,with a lower heating value(LHV)of 120.21 MJ·kg-1which is 2.5,2.8 times higher than that of natural gas and crude oil,respectively[6,7].Also hydrogen's combustion is very clean so that its only burning product is water.Currently,the large scale hydrogen production is what mostly based on electrolysis of water or steam reforming of methane,But production of hydrogen from biomass as a renewable resource offers the possibility of sustainable production without any considerable emission[8,9].Generally,we have 2 main routes for hydrogen production from biomass namely thermo chemical conversions[pyrolysis,gasification,supercritical water gasification(SCWG)]and biochemical conversions,along with electrolytic and photolytic processes[10].Supercritical water gasification is a kind of gasification which occurs in supercritical water media(temperatures and pressures above 374°C and 22.1 MPa,respectively)and it is one of the most advanced thermo-chemical conversion technologies for converting biomass into hydrogen-rich syngas which includes mainly CO2,CO,CH4,H2and small amounts of longer chain hydrocarbons[11,12].Water in its supercritical condition has unique twofold roles of reaction medium and reactantalong with its low density,dielectric constant and ionic product[13,14].These variations in properties make water act as a non-pol arsol vent with high diffusivity and high transport properties[15].Moreover,at near-critical and supercritical conditions,water is very miscible with organic compounds,enabling supercritical water to solve the major part of biomass and reform it through a homogeneous mixture[1,16].
The structures of Agricultural wastes are mostly lingo-cellulosic which includes lignin,cellulose and hemicellulose.gasification of these feed stocks into syngas in supercritical water media have been studied by many researchers.However,Yaniket al.,found that,biomass type and the location of plant growth affect the gas yield and its composition[17].Conversion of fruit shells into hydrogen rich gas under low temperature,but supercritical condition studied by demirbas[18].It was shown that the biomass with higher cellulose and hemicellulose content were better gasified and feed stocks with more cellulose content had higher hydrogen yield.D'Jesu'set al.[19]studied the noncatalytic SCWG of corn silage.It was observed that the hydrogen and methane gas yields decreased after a given resident time.Madenogluet al.[20],studied the catalytic and non-catalytic gasification of cotton and tobacco stalk in supercritical water condition using stainless steel batch reactor.Noncatalytic gasification of tobacco stalk with higher cellulose and hemicellulose and lower lignin resulted in higher gaseous products than that of cotton stalk.The effect of reaction time on gaseous product and its composition was also studied by some researchers.Sricharoenchaikul[21]showed that an increase in resident time significantly improved the gaseous product yield during non-catalytic SCWG of black liquor in a batch reactor.Baratiet al.reported that the total amount of gas yield in SCWG of bagasse in a batch micro reactor increased until 45 min and then remained constant as reaction time increased.It was also observed that the hydrogen yield first increased and then decreased by increasing the reaction time[13].
This study aims to perform the non-catalytic gasification of wheat straw,walnut shell and almond shell as the main agricultural residues in Kurdistan province of Iran within supercritical water media using a tubular batch micro reactor.The optimum resident time which maximizes the total gas and hydrogen yields for each biomass will be investigated.The reactor system is efficiently designed for sufficient reacting condition,considering the latest experiments and research literature.
2.Materials and Methods
2.1.Materials
The biomass particles were supplied from gardens and agriculture farms around Sanandaj,located in Kurdistan province,Iran.According to the statistics of agricultural products of this province,wheat straw was selected as the most abundant feedstock in the province and walnut shell and almond shell were chosen as abundant hard nut shells with higher amount of lignin.Pretreatment for all these feed stocks including washing,drying,grounding and sieving were performed to achieve clean and dry biomass particle sizes up to 150 μm.The elemental analyses of biomass samples were done by a CHNS analyzer(Vario EL III by Elementar,Germany)for further investigations.Also,the components in the structure of each biomass were analyzed by the method of P.J.Van Soest[22].
2.2.Experimental setup
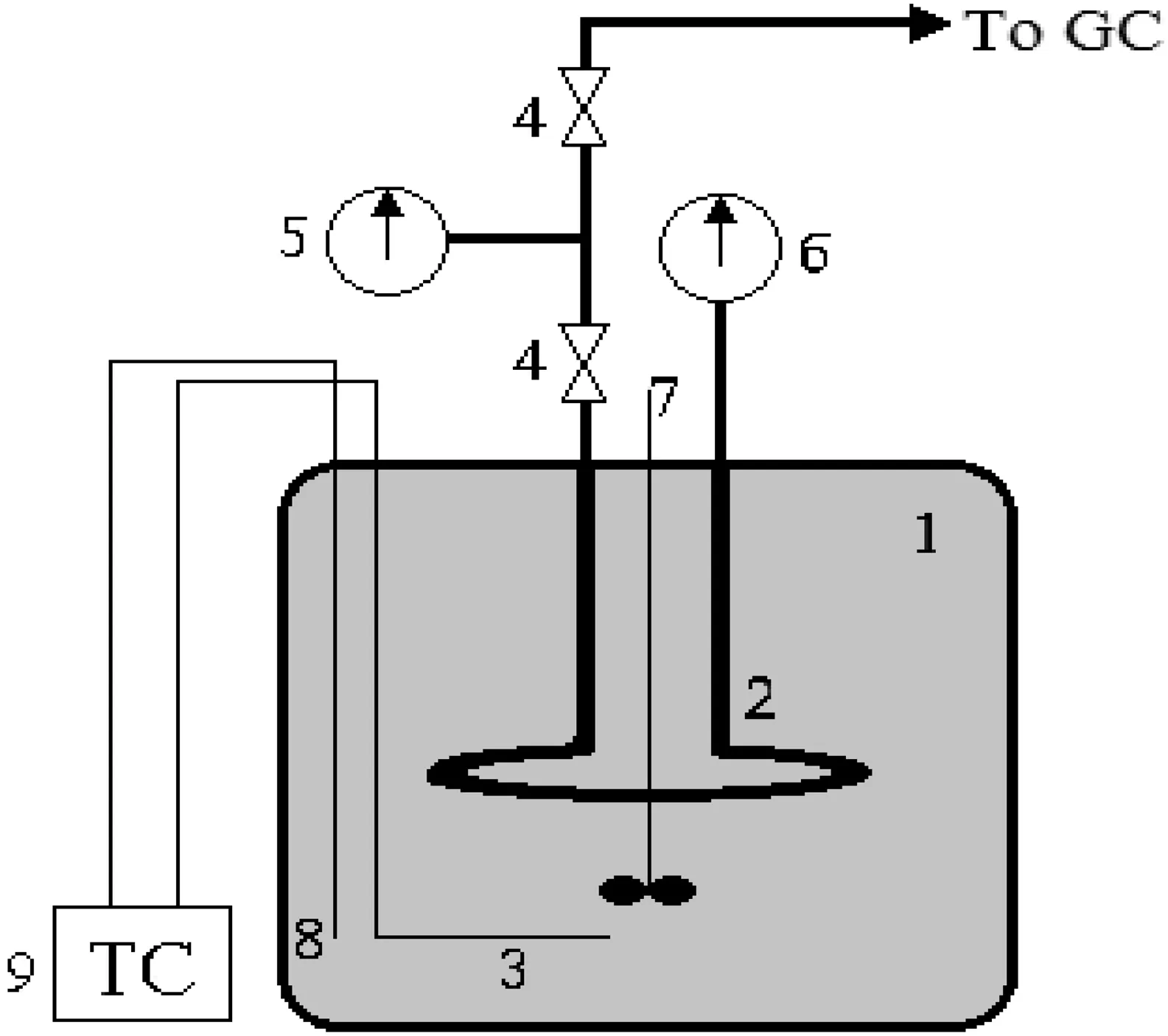
Fig.1.Schematic of the batch micro reactor system:1)molten salt bath,2)tubular batch reactor,3)electrical heater,4)high pressure valves,5)low-pressure gauge,6)high pressure gauge,7)mixer,8)k-type thermocouple,9)temperature controller.
At the end of each experiment,reactor's free volume, final pressure and temperature were used to calculate the total gas yield.Syngas composition and the amount of each component were measured by a gas chromatograph(Varian 3400 and Teyfgostar-Compact)using Argon as carrier gas to determine the product gas composition.
2.3.Reactions

Fig.2.A typical variation of reactor pressure with time(T:440°C,0.06 g wheat,6 g water).
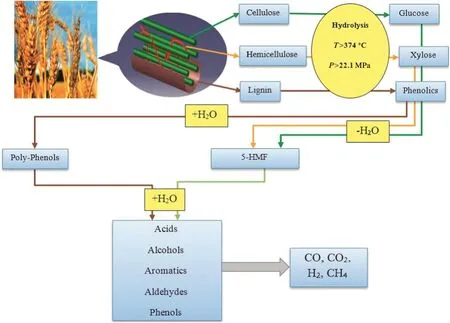
Fig.3.Schematic of typical pathway for supercritical water gasification of wheat straw model compounds.
Decomposition of the lingo-cellulosic biomass in supercritical water media should be reviewed.It is necessary to investigate the degradation of biomass model compounds.Fig.3,represents the schematic pathway for supercritical water gasification of wheat straw.Cellulose is an insoluble polymer in the water and consists of glucose subunits.These subunits are linked with each other by β-1,4-glycosidic bonds[24].As mentioned in reaction(1),cellulose is hydrolyzed through the rupture of β-1,4-glycosidic linkages to produce glucose[25,26].Hemicellulose is a branched amorphous polymer consists of C5and C6sugars which are linked by various forms of glycosidic bonds.Connections between cellulose and hemicellulose make networks to stabilize the plant cell wall while Lignin covers them.The sugars obtained from cellulose and hemicellulose,will be further dehydrated into 5-HMF(5-hydroxymethylfurfural).This 5-HMF can be converted into acids,alcohols and aldehydes in a suitable condition[27].Lignin is a three-dimensional phenyl propane polymer with ester bond links.It holds cellulose and hemicellulose together in a matrix to form primary cell wall and prevent plant from damages[10,24,28].
Lignin's hydrolysis in supercritical water,which is followed by dealkylation,is promoting the decomposition of lignin.As mentioned in reaction(3),this process leads to the formation of phenolic compounds such as syringols,guaiacols which will be further formpolyphenols[26,29].These products are fur there formed to syngas by reactions(5)and(6)[30].
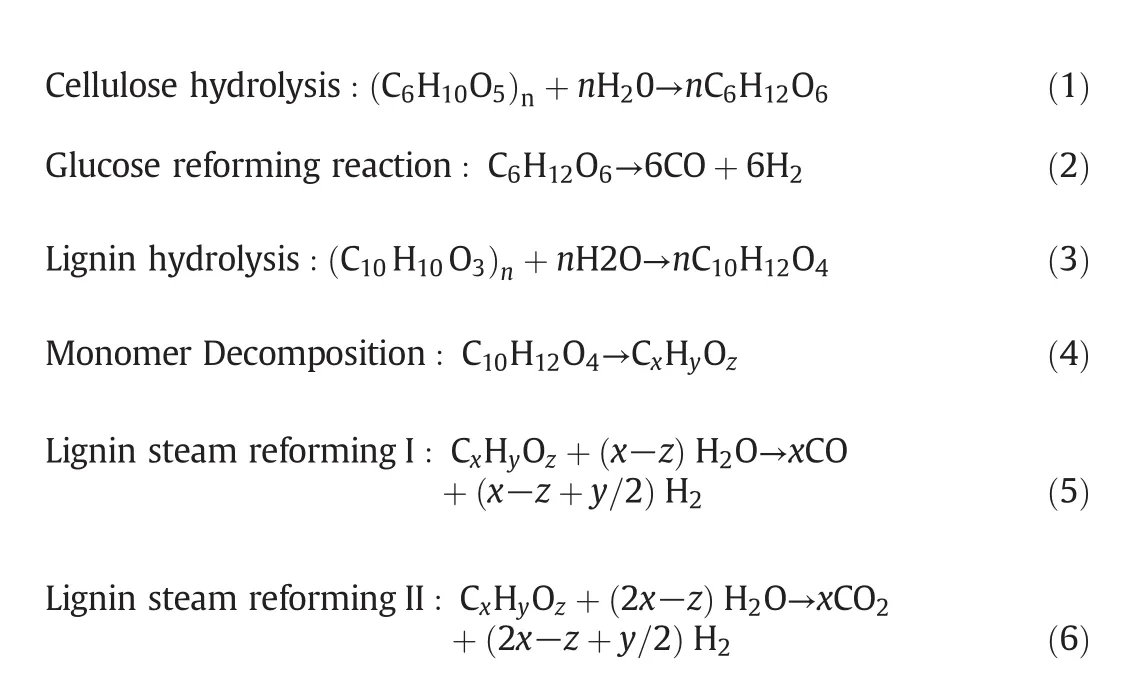

In addition to above reactions,some intermediate reactions can be occurred in order to complete the successful gasification process which are given below[13,31].

The reforming reactions produce CO,CO2and H2,whereas CH4is produced by methanation via reaction(7).Water-gas shift reaction(WGS)is another significant reaction of SCWG of biomass which is the reforming of CO with water to produce CO2and H2via reaction(8).At high CO2and H2partial pressures,formation of CO may be occurred via the reverse water gas shiftreaction[32].As mentioned,methanation consumes hydrogen and water-gas shift produces hydrogen.So,it is obvious that we should advance the reactions in order to avoid methanation and accelerate water-gas shift reaction when hydrogen-rich gas is required[1-13].
3.Results and Discussion
3.1.Feedstock analysis
Lingo-cellulosic biomasses used in this study are mainly made of carbon,oxygen,hydrogen and slight amount of sulfur and nitrogen.The CHNS analysis of the used biomasses are given in Table 1.This table shows that Almond shell has the highest percentage of lignin and wheat straw contains the highest percentage of cellulose.The mass fraction(y)of oxygen in biomass is determined by Eq.(9).
The components in the structure of agricultural wastes are mainly consist of cellulose,hemicellulose and lignin which are related to the nature of biomass.Structural analysis for three prepared agricultural wastes in this study are given in Table 2.
[4]赵铁军、惠怀海:《文化节的营销策略研究——以庆阳香包文化节的营销策略为例》,《陇东学院学报》2012年第4期。
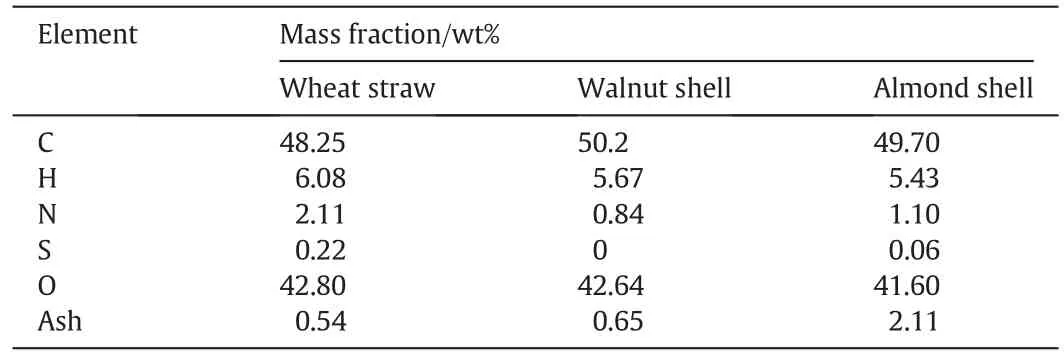
Table 1Elemental analysis of biomass samples
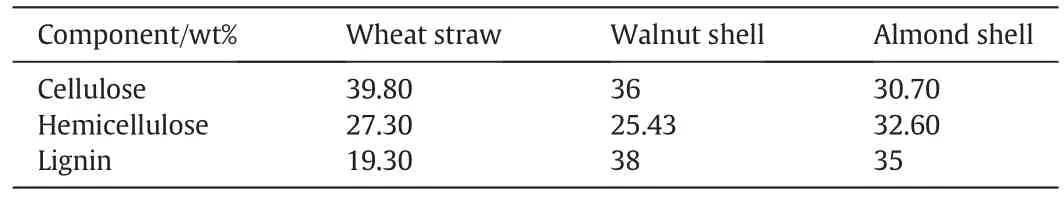
Table 2Structural analysis of biomass samples
3.2.Base case results
Results for the base case are presented first and then the effects of resident time on gaseous products will be discussed.Fig.4 shows the variation of some properties of water with temperature in 25 MPa[14].This figure shows that,the properties of water in supercritical condition at 25 MPa changes sharply up until about 420-440°C but after that they don't show significant changes.So,by considering these conditions and according to Fig.2,loadings and temperature were adjusted to reach the pressure of 25 MPa in the reactor which could be the sufficient condition for experiments according to the Fig.4.So,experiments were performed the at the temperature of 440°C,water loading of 6 g,biomass loading of 0.06 g,pressure of 25 MPa and the reaction time of 15 min.
3.2.1.Gaseous product analysis

Fig.4.The variation of some properties of water with temperature in 25 MPa[14].
Fig.5.gasification yields for different feed stocks.(T:440°C,Biomass loading:0.06 g,Water loading:6 g,Reaction Time:15 min).
Total gas yield and its composition for supercritical water gasification of 0.06 g of each biomass in 6 ml water in the temperature of 440°C with reaction time of 15 min have been shown in Fig.5.This figure demonstrates that,wheat straw was better gasified and its hydrogen yield(6.52 mmol per gram of biomass)was more than that of other biomasses.It may be because of higher amount of cellulose and hemicellulose in the structure of wheat straw.Also,higher hydrogen content in the structure of wheat straw can be another reason for the higher hydrogen yield.In accordance with the hydrogen yield,for wheat straw,the total gas yield showed the maximum of 24.24 mmol per gram of biomass.The results on this figure show that lower content of hydrogen and lower content of cellulose cause the minimum hydrogen yield and minimum total gas yield for Almond Shell.The carbon gasification efficiency(CGE)which is the ratio of the amount of carbon in the gas phase products to the amount of carbon in the feedstock and hydrogen gasification efficiency(HGE)which is the ratio of the hydrogen in the gas phase products to the amount of hydrogen in the feedstock were measured[33].Mathematically,CGE and HGE are de fined by Eqs.(10),(11)as follows[27]:
As indicated in Fig.6,Carbon and hydrogen gasification efficiency have also been calculated from elemental analysis and the yields of gaseous products.Hydrogen gasification efficiencies for wheat straw,walnut shell and almond shell were equal to 23%,13%and 19%,respectively.Also,the calculated CGE for wheat straw,walnut shell and almond shell were 44.92%,38.68%and 40.68%,respectively.

Fig.6.Hydrogen and carbon gasification efficiency for the used feed stock.(T:440°C,Biomass loading:0.06 g,Water loading:6 g,Reaction time:15 min).
3.2.2.Carbon balance
Total carbon distribution in the gas phase,liquid effluent,and solid residue were analyzed to calculate the carbon balance.Carbon balance for each experiment was calculated based on the mass percentage of converted carbon[34].Fig.7 depicts the mass percentage of converted carbon among gaseous,aqueous and solid phase products.The aqueous phase was analyzed by a total organic carbon(TOC)analyzer(Shimadzu,TOC-L)which has been presented in Fig.8.Moreover,the solid residue was characterized using CHNS analyzer.As shown in Fig.7,SCWG of wheat straw with lower lignin content in its initial form,results in lower carbon in solid residue and higher carbon in gaseous products.Each product analyzed 3 times for more accuracy and the average considered for carbon balance which closed in 93.8%,96.7%and 96%for walnut shell,almond shell and wheat straw respectively.The missing carbon in the balance may be because of the hydrocarbons sticking the wall of reactor and can't be collected after experiment.
3.3.Effect of reaction time
Effect of reaction time on gaseous product is studied in this part.The system conditions were fixed in base case condition and reaction time changed from 5 to 30 min and the gaseous products was measured each time.The amount of C2+components was assumed to be negligible and only the main gaseous components including CO2,CO,CH4and H2were considered.Fig.9,demonstrates the variation of total gas yields of 3 different feed stocks with reaction time.It is indicated that the total gas yields of different feed stocks used in this study increased with increase of resident time but they approximately reach a certain amount after 30 min and remain constant after that.Reaction time has a bounded effect on the gas yield and after a certain reaction time the conversion of biomass stops and only the composition will be changed.It is also can be perceived that biomass with higher amount of lignin(walnut shell)gasified less in shorter resident times and it takes more to be gasified than biomass with higher cellulose content(wheat straw).
Gaseous composition and its variation with reaction time were investigated for each biomass used in this study.Fig.10,indicate that how the composition of produced gas made by SCWG of wheat straw,are affected by reaction time.The hydrogen yield increases slightly by increasing the reaction time from 5 to 10 min and reaches maximum at10 min with 7.25 mmolH2per one gram of wheat straw and then decreases.It is due to methanation reaction that occurs simultaneously.According to the reaction(7),3 mol of H2and one mole of CO are needed for producing one mol of CH4while in reaction(8),one mole of CO and one mol of H2O is consumed for production of one mol CO2and one mol H2.So,considering these reactions and steam reforming reaction we can infer that water-gas shift and methanation occurin reaction times above 10 min and CO slightly decreases while CO2increases sharply and H2decreases sharply.

Fig.7.Mass percentage of converted carbon in gaseous,aqueous and solid phase products for SCWG of used feed stocks.(T:440°C,Biomass loading:0.06 g,Water loading:6 g,Reaction time:15 min).

Fig.8.TOC results of aqueous products for SCWG of used feed stocks.(T:440°C,Biomass loading:0.06 g,Water loading:6 g,Reaction time:15 min).
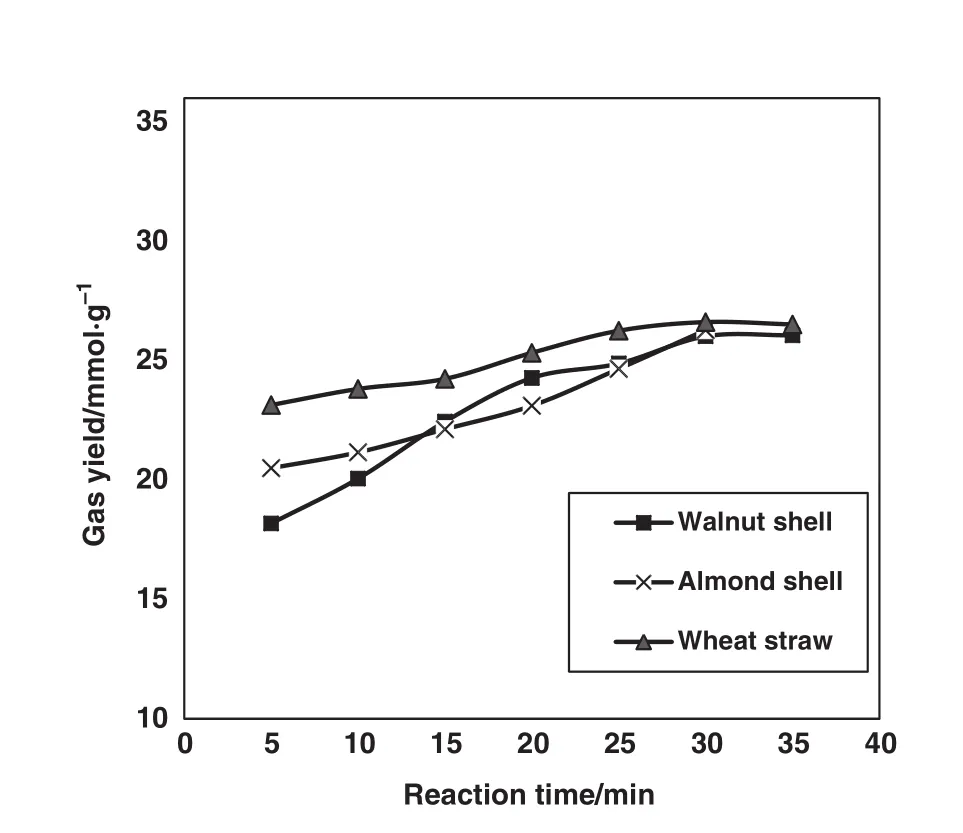
Fig.9.Temporal variation of total gas yields of different feed stocks.(T:440°C,Biomass loading:0.06 g,Water loading:6 g).

Fig.10.Variation of gas components forwheatstraw with reaction time.(T:440°C,Wheat loading:0.06 g,Water loading:6 g).
Fig.11,demonstrates the variation of the produced gas components of walnut shell with reaction time.Here,we have steam reforming and decomposition ofbiomass compounds at the first15 to 20 min and then intermediate reactions take place with water-gas shiftand methanation reactions.As shown in Fig.11,hydrogen yield reaches its maximum at 20 min with 4.63 mmol of H2per one gram of walnut shell and then decreases.The higher amount of CO2could be the result of higher percentage of carbon in elemental analysis.Also methanation happens after 20 min.As a result,hydrogen is consumed and CH4is produced so that the yield of methane reaches a maximum of 2.57 mmol·g-1in 30 min.
Fig.12,indicates the temporal variation of gas components of almond shell.The process for this one is approximately the same as the two previous feed stocks but the amount of CO2increases after 15 min and it may be because of steam reforming of glucose and lignin that are happened later in this process.As shown.The slight decrease of hydrogen after this time is due to the slight methanation that happens simultaneously.
Figs.13,14 depict the temporal variation of carbon and hydrogen gasification efficiencies for three used feed stocks.As seen in the Fig.13,the CGE of all feed stocks increased gradually within the first 30 min and after that,walnut shell,wheat straw and almond shell reached the carbon gasification efficiencies equal to 59%,58%and 56%,respectively.Furthermore,as shown in Fig.14,wheat straw had much higher hydrogen gasification efficiency at the shorter resident times but no significant temporal change was observed for HGE of almond shell.For walnut shell,the HGE raised up sharply after 15 min and reached 25%in 30 min.This could be because of the presence of more lignin in its structure that does not allow cellulose and hemicellulose to be completely gasified.
However,with a look at these three figures we can conclude that the percentage of elements in elemental analysis has a direct relation with gaseous products.Also,lignin has a complex structure and the hydrolysis of lignin into phenolic and conversion of those poly-phenolics into synthesis gas components is very hard in the absence of catalyst.The presence of lignin in biomass results in difficult conversion of cellulose and hemicellulose into sugars[28-35].Lignin in the cell wall of biomass postpones hydrolysis of the carbohydrates.This can be perceive from Fig.12,which hydrogen gasification efficiency of walnut shell with more lignin content remains low until 15 min and then goes up.

Fig.11.Variation of gas components for walnut shell with reaction time.(T:440°C,Walnut shell loading:0.06 g,Water loading:6 g).

Fig.12.Variation of gas components for almond shell with reaction time.(T:440°C,Almond shell loading:0.06 g,Water loading:6 g).
4.Conclusions
The gasification of wheat straw,walnut shell and almond shellin supercritical water media was studied using a stainless steel batch micro reactor with the volume of 26 ml.The gas yields and their compositions was compared first in the base case condition with the temperature of 440°C,0.06 g biomass loading and 6 g water loading at resident time of 15 min.The hydrogen gas yields of 6.52,4.26 and 4.1 mmol per gram and hydrogen gasification efficiencies of 23%,13%and 19%were observed for wheat straw,walnut shell and almond shell respectively.It was also specified that total gas yields increased with increase of reaction time up to 30 min and then approximately remained constant.Feed stocks with higher cellulose and hemicellulose and lower lignin in their structures were better gasified in shorter resident times.CO2increased gradually with increase of reaction time and methanation was occurred mainly after 15 min.Hydrogen yields were maximum at 10,15 and 20 min of reaction times for wheat straw,almond shell and walnut shell respectively.HGE and CGE of used feed stocks increased with increase of the reaction time.

Fig.13.Temporal variation of carbon gasification efficiencies.(T:440°C,Biomass loading:0.06 g,Water loading:6 g).

Fig.14.Temporal variation of hydrogen gasification efficiencies.(T:440°C,Biomass loading:0.06 g,Water loading:6 g).
[1]F.Safari,A.Tavasoli,A.Ataei,J.K.Choi,Hydrogen and syngas production from gasification of lignocellulosic biomass in supercritical water media,Int.J.Recycl.Org.Waste.Agric.4(2)(2015)121-125.
[2]IEA Key World Energy Statistics,OECD Publishing,Paris,France,2014.
[3]Faostat annual report 2012,available on(http://faostat3.fao.org/)[Accessed April 2015].
[4]M.Shoja,M.A.Babatabar,A.Tavasoli,A.Ataei,Production of hydrogen and syngas via pyrolysis of bagasse in a dual bed reactor,J.Energy Chem.22(2013)639-644.
[5]S.Yilmaz,H.Selim,A review on the methods for biomass to energy conversion systems design,Renew.Sustain.Energ.Rev.25(2013)420-430.
[6]GREET Transportation Fuel Cycle Analysis Model,GREET 1.8b,developed by US department of energy,Argonne National Laboratory,Argonne,IL,Released September 5,2008.Available at(https://greet.es.anl.gov)[Accessed April 2015].
[7]Y.Kalinci,A.Hepbasli,I.Dincer,Biomass-based hydrogen production:A review and analysis,Int.J.Hydrog.Energy34(2009)8799-8817.
[8]K.Kang,R.Azargohar,A.K.Dalai,H.Wang,Non-catalytic gasification of lignin in supercritical water using a batch reactor for hydrogen production:An experimental and modeling study,Energy Fuel29(2015)1776-1784.
[9]D.B.Levin,L.Pitt,M.Love,Biohydrogen production:prospects and limitations to practical application,Int.J.Hydrog.Energy29(2004)173-185.
[10]P.Mohanty,K.K.Pant,R.Mittal,Hydrogen generation from biomass materials:Challenges and opportunities,WIREs Energy Environ.4(2014)139-155.
[11]M.Rashidi,A.Tavasoli,Hydrogen rich gas production via supercritical water gasi fication of sugarcane bagasse using unpromoted and copper promoted Ni/CNT nanocatalysts,J.Supercrit.Fluids98(2015)111-118.
[12]R.Mehrani,M.Barati,A.Tavasoli,A.Karimi,Hydrogen production via supercritical water gasification of bagasse using Ni-Cu/γ-Al2O3 nano-catalysts,Environ.Technol.36(2015)1265-1272.
[13]M.Barati,M.Babatabar,A.Tavasoli,A.K.Dalai,U.Das,Hydrogen production via supercritical water gasification of bagasse using unpromoted and zinc promoted Ru/γ-Al2O3 nanocatalysts,Fuel Process.Technol.123(2014)140-148.
[14]W.Wagner,A.Pruss,The IAPWS formulation for the thermodynamic properties of ordinary water substance for general and Scientific use,J.Phys.Chem.Ref.Data31(2002)387-535.
[15]Y.Calzavara,C.J.Dubien,G.Boissonnet,S.Sarrade,Evaluation of biomass gasification in supercritical water process for hydrogen production,Energy Convers.Manag.46(2005)615-631.
[16]G.Brunner,Hydrothermal and supercritical Water processes,5,Elsevier,Amsterdam,2014 395-509.
[17]J.Yanik,S.Ebale,A.Kruse,M.Salgam,M.Yuksel,Biomass gasification in supercritical water:part 1.effect of the nature of biomass,Fuel86(2007)2410-2415.
[18]A.Demirbas,Hydrogen-rich gas from fruit shells via supercritical water extraction,Int.J.Hydrog.Energy29(2004)1237-1243.
[19]P.D'Jesu's,A.Boukis,B.Kraushaar-Czarnetzk,E.Dinjus,influence of Process variables on gasification of corn silage in supercritical water,Ind.Eng.Chem.Res.45(2006)1622-1630.
[20]T.G.Madenoglu,S.Kurt,M.Saglam,M.Yuksel,L.Ballice,Hydrogen production from some agricultural residues by catalytic subcritical and supercritical water gasification,J.Supercrit.Fluids67(2012)22-28.
[21]V.Sricharoenchaikul,Assessment of black liquor gasification in supercritical water,Bioresour.Technol.100(2009)638-643.
[22]H.K.Goering,P.J.Van Soest,Forage fiber analysis in:Agriculture Handbook No:379,Superintendent of Documents,US Government Printing Office,Washington,1970.
[23]P.Azadi,S.Khan,F.Stroble,F.Azadi,R.Farnood,Hydrogen production from cellulose,lignin,bark and model carbohydrates in supercritical water using nickel and ruthenium catalysts,Appl.Catal.B Environ.117-118(2012)330-338.
[24]H.Kobayashi,A.Fukuoka,Synthesis and utilisation of sugar compounds derived from lignocellulosic biomass,Green Chem.15(2013)1740-1763.
[25]P.Béguin,J.P.Aubert,The biological degradation of cellulose,FEMS Microbiol.Rev.13(1994)25-58.
[26]S.N.Reddy,S.Nanda,A.K.Dalai,J.A.Kozinski,Supercritical water gasification of biomass for hydrogen production,Int.J.Hydrog.Energy39(2014)6912-6926.
[27]M.H.Waldner,F.Vogel,Renewable production of methane from woody biomass by catalytic hydrothermal gasification,Ind.Eng.Chem.Res.44(2005)4543-4551.
[28]N.Mosier,C.Wyman,B.Dale,R.Elander,Y.Y.Lee,M.Holtzapple,M.Laadisch,Features of promising technologies for pretreatment of lignocellulosic biomass,Bioresour.Technol.97(2005)673-686.
[29]M.Brebu,C.Vasile,Thermaldegradation oflignin-A review,Cellul.Chem.Technol.44(2010)353-363.
[30]F.L.P.Resende,S.A.Fraley,M.J.Berger,P.E.Savage,Noncatalytic gasification of lignin in supercritical water,Energy Fuel22(2008)1328-1334.
[31]Q.Guan,C.Wei,X.Chai,P.Ning,S.Tian,J.Gu,Q.Chen,R.Miao,Energetic analysis of gasification of biomass by partial oxidation in supercritical water,Chin.J.Chem.Eng.23(2015)205-212.
[32]F.L.P.Resende,P.E.Savage,Kinetic model for noncatalytic supercritical water gasi fication of cellulose and lignin,AICHE J.6(2015)2412-2420.
[33]M.Rashidi,A.Tavasoli,Hydrogen rich gas production via noncatalytic gasification of sugarcane bagasse in supercritical water media,Pet.Coal56(2014)311-319.
[34]I.Behnia,Treatment of Aqueous Biomass and Waste via Supercritical Water gasification for the Production of CH4 and H2(M.E.Sc Thesis)The University of Western Ontario,2013.
[35]P.Kumar,D.M.Barrett,M.J.Delwiche,P.Stroeve,Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production,Ind.Eng.Chem.Res.48(2009)3713-3729.
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Computational chemical engineering - Towards thorough understanding and precise application☆
- A review of control loop monitoring and diagnosis:Prospects of controller maintenance in big data era☆
- Experimental and numerical investigations of scale-up effects on the hydrodynamics of slurry bubble columns☆
- The heat transfer optimization of conical fin by shape modification
- The steady-state and dynamic simulation of cascade distillation system for the production of oxygen-18 isotope from water☆
- Experimental mass transfer coefficients in a pilot plant multistage column extractor
