离体条件下外源茉莉酸甲酯对人参锈腐病菌的影响
2016-05-27孙嘉曼傅俊范
孙嘉曼, 傅俊范, 张 禹
( 1. 广西作物遗传改良生物技术重点开放实验室, 广西农业科学院, 南宁 530007; 2. 沈阳农业大学 植物保护学院, 沈阳 110866; 3. 龙邦出入境检验检疫局, 广西 百色 533800 )
离体条件下外源茉莉酸甲酯对人参锈腐病菌的影响
孙嘉曼1,2, 傅俊范2*, 张禹3
( 1. 广西作物遗传改良生物技术重点开放实验室, 广西农业科学院, 南宁 530007; 2. 沈阳农业大学 植物保护学院, 沈阳 110866; 3. 龙邦出入境检验检疫局, 广西 百色 533800 )
摘要:人参(Panax ginseng)是我国传统的名贵药材,由毁灭柱孢(Cylindrocarpon destructans)引起的人参锈腐病是严重影响人参产量和品质的重要根部病害之一,在人参生产中会造成严重的经济损失。茉莉酸甲酯(methyl jasmonate, MeJA)是一类新型的生长调节物质,既可以参与植物对病原菌及其他逆境胁迫做出的应答并进行信号传递,又可用来诱导植物的抗病反应。为了明确MeJA对人参锈腐病菌的影响并解析MeJA与病原菌致病因子之间的相互关系,该文研究了外源MeJA在不同浓度下对C. destructans的直接影响,包括对菌落生长、孢子萌发、菌丝生长量、病菌分泌水解酶的影响。结果表明:MeJA能够强烈抑制病原菌的生长和孢子萌发,而对病原菌致病酶的活性则表现出促进作用;人参锈腐病菌在PDA平板上的菌落直径从(8.23±0.15) cm (对照) 减少到(0.71±0.00) cm (800 μg·mL-1MeJA),在MeJA浓度达到最高时,菌落生长几乎完全被抑制;MeJA的浓度大于400 μg·mL-1时,病原菌的生物量减少了65.3%~100%,孢子萌发率和芽管长度减少了100%;MeJA在浓度大于200 μg·mL-1时,果胶酶、纤维素酶和淀粉酶活性升高而蛋白酶的活性却没有变化。综上表明,MeJA对病原菌产生抑制作用的临界浓度为200 μg·mL-1。该研究结果为后续使用MeJA处理人参植株进行诱导抗病性的研究奠定了基础,同时也有助于进一步了解人参锈腐病的致病机理,并为病害防控提供了理论参考。
关键词:茉莉酸甲酯, 生物量, 人参锈腐病菌, 致病酶
Ginseng (Panaxginseng) widely cultivated as a medicinal herb is an economically important cash crop in Northeast China(Wang,2001). The dried root is highly valued for its medicinal properties and is widely used in Chinese traditional medicine (Rahman & Punja 2005a; Ali et al,2006).Cylindrocarpondestructans(teleomorph:Nectriaradicicola),a pathogenic fungus responsible for Cylindrocarpon root rot of ginseng,is difficult to be eliminated from soil (Reeleder & Brammall,1994; Punja,1997). Cylindrocarpon root rot is one of the major threats to stable ginseng production (Reeleder & Brammall,1994; Punja,1997; Ahn & Lee,2001; Rahman & Punja,2005b; Kim et al,2009),which can result in yield losses of up to 25%-30% (Seifert et al,2003; Kernaghan et al,2007). The pathogenC.destructansis the most important soil-borne pathogen that caused root rot of ginseng,limiting the re-use of fields for successive ginseng crops (Reeleder & Brammall,1994; Reeleder et al,2002).
Much attention has been paid to the effects ofC.destructanson ginseng and other plant hosts,but much less to the effects of host plants on the pathogen in the plant-microbe interactions. In fact,pathogen invasion is closely related to host aspects. Jasmonate (JA) is widely distributed in the plant kingdom with multiple physiological functions during plant development,growth,and defense responses (Creelman & Mullet,1997). Methyl jasmonate (MeJA),one of the major physiological active forms of jasmonates,is a vital cellular regulator that mediates diverse developmental processes in plants. It has been demonstrated to alter defense responses against biotic and abiotic stresses in various plant species (Penninckx et al,1998). Large amounts of work has been done on the ability of MeJA to elicit plant defenses against necrotrophic fungi. Previous results show that MeJA can protect spruce seedlings against the soil-borne pathogenPythiumultimum(Kozlowski et al,1999),and MeJA applied to potato leaves can induce systemic resistance againstPhytophthorainfestans(Cohen,1993). Gaige (2010) suggested that MeJA and ethylene could induce partial resistance inMedicagotruncatulaagainst the charcoal rot pathogenMacrophominaphaseolina. The effects of MeJA on the control of Monosporascus root rot and vine decline of melon have also been studied (Aleandri et al,2010). However,little research has been devoted to the direct effects of MeJA on specific pathogen itself e.g. on colony growth,spore germination,germ tube lengths,mycelial mass production,activities of pectinase,cellulase,amylase and protease ofinvitrosoil-borne pathogen,C.destructans.
The aim of this work is to assess the effects of MeJA onCylindrocarpondestructansand to investigateinvitrowhether there is a relationship between MeJA and the pathogenic factors ofC.destructans.
1Materials and Methods
1.1 Experimental materials
Cylindrocarpondestructanswas isolated from infected ginseng roots from the major ginseng cultivation areas,by the laboratory of plant disease epidemiology,Shenyang Agricultural University,China. Colonies were cultured on potato dextrose agar (PDA) plates and grown at 20 ℃ in the dark in an incubator for 2 weeks (Rahman & Punja,2005b,2006). MeJA used in the experiment was obtained from the Sigma Co. (St. Louis,MO,USA).
1.2 Experimental methods
To determine the effects of MeJA on colony growth,MeJA was added to PDA to achieve the desired concentrations. Petri dishes containing PDA were inoculated with a 7 mm diameter mycelial plug from a 14-day-old culture ofC.destructansand incubated at 20 ℃ for 2 weeks. Colony diameter was measured at 3-day intervals by taking two perpendicular measurements on each colony. Three replicate dishes of each treatment were carried out and the experiment conducted twice.
Percent germination and germ tube lengths were determined for spores ofC.destructansin MeJA solution,following methods described by He & Wolyn (2005). Spores (1×106spores·mL-1) were harvested from the plates by rubbing the surface mycelium gently with a rubber swab and collecting the spores in distilled water. Spore suspension (4 mL) was diluted with 4 mL MeJA solution for each treatment and the resulting suspensions were incubated at 20 ℃ for 8 h. At least 100 spores per treatment replicate were measured microscopically for percent spore germination and germ tube length. The experiment was repeated twice with three replications and the data averaged.
The mycelial mass production was assessed by adapting the method of Rahman & Punja (2006) with minor modifications. Briefly,flasks containing 100 mL of potato dextrose broth were inoculated with a 7 mm diameter mycelial plug from a 14-day-old colony ofC.destructansand incubated on a rotary shaker (130 r·min-1) at 20 ℃ for 2 weeks. The mycelial mass (dry weight) from three replicate flasks was determined after filtration and drying at 80 ℃ for 12 h. The experiment was performed twice. Culture filtrate was centrifuged at 8 000 r·min-1for 10 min at 4 ℃ and the supernatant was used for enzyme assays.
Pectinase activity (mainly polygalacturonase) was determined described by Silva et al(2005). One unit of enzyme activity was defined as the amount of β-galacturonic acid hydrolyzed from pectin per minute under the assay condition. Cellulase activity was assayed using the DNS (3,5-dinitrosalicylic acid) method (Berlin et al,2005). One unit of cellulase activity was defined as the amount of enzyme that produced 1 μmol reduced sugar per minute. Amylase activity was determined by the procedure according to Murado et al(1997). One unit of amylase activity was defined as the amount of enzyme releasing 1 μmol of glucose per minute. The gelatin assay of Tseng & Mount (1974) was used to quantify protease activity. One unit of protease activity was defined as the amount of enzyme causing an increase in absorbance of 0.01 in 1 min at 280 nm. The protein concentration in enzyme preparations was measured by the method of Lowry et al(1951) following precipitation with trichloroacetic acid.
1.3 Data analysis
Experiments were carried out using eight concentrations of MeJA: 0,1,10,50,100,200,400 and 800 μg·mL-1. Data on the colony growth were analyzed by analysis of variance (ANOVA). Means of the treatments were compared by Duncan’s multiple range tests atP<0.05. All statistical analyses were conducted with SPSS Base Version 11.5 statistical software (SPSS Inc. Chicago,IL).
2Results and Analysis
2.1 Effects of MeJA on colony growth and mycelial mass production of Cylindrocarpon destructans
MeJA,a methyl ester of JA,plays an important role in the defense of plants against pathogens (Preston et al,2001; Aleandri et al,2010; Gaige et al,2010). It can serve as a signal molecule bridging pathogen and plant host,particularly in the ginseng-C.destructansinteractions. In the present study,the growth ofC.destructanswas strikingly suppressed by MeJA both in a potato dextrose liquid culture and on PDA plates. The dry weight of mycelia decreased from (74.00±9.54) mg (control) to 0 (800 μg·mL-1MeJA) (Table 1).
A severe repression of colony growth on PDA was observed at a high concentration of MeJA,in which the colony diameter was found to be (5.54±0.23) cm at a concentration of 400 μg·mL-1and (0.71±0.00) cm at a concentration of 800 μg·mL-1,although the diameter had no difference compared with the untreated control [(8.23±0.15) cm] at lower concentrations (1-50 μg·mL-1MeJA) (Table 1). This was in agreement with the report that MeJA inhibited mycelial growth of Phytophthora infestansinvitro(Cohen,1993). MeJA was not significantly inhibitory toC.destructansat lower concentrations,but a potent suppression of colony growth was observed at high concentrations of MeJA (Table 1).
2.2 Effects of MeJA on spore germination and germ tube lengths
Dramatic inhibition of spore germination and germ tube growth by MeJA were obtained in a concentration-dependent manner. The percent of spore germination was strongly suppressed,with a reduction of 7.9%-100.0% compared with the control (Table 2). Potent suppression of the growth of germ-tubes was observed at all concentrations (1-800 μg·mL-1),especially at 400-800 μg·mL-1,where the growth of germ-tubes was inhibited completely (Table 2).
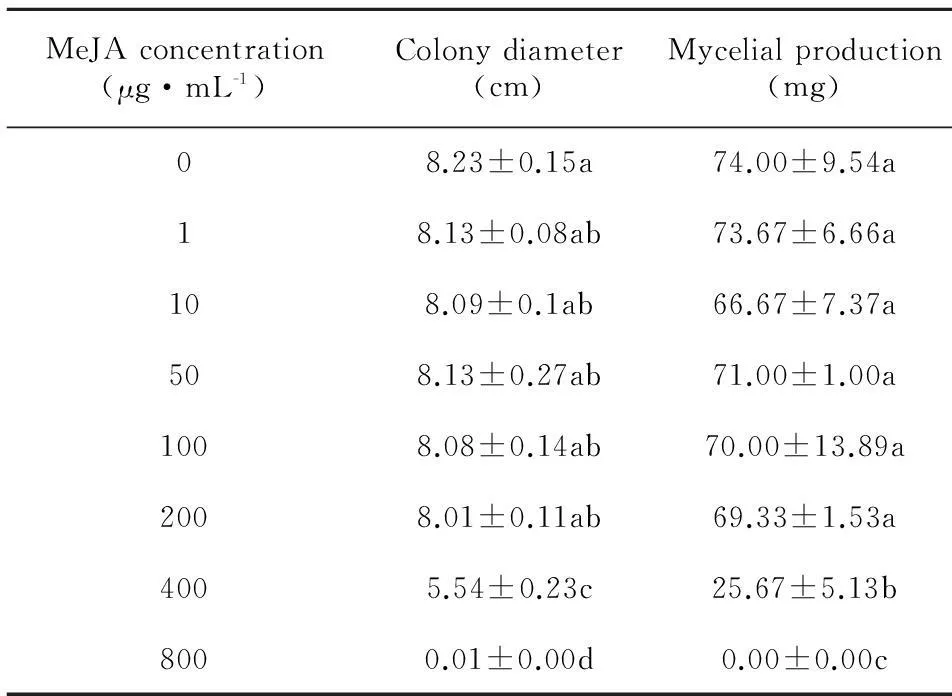
Table 1 Effects of exogenous MeJA on mycelial growth

Table 2 Effects of exogenous MeJA on spore germination
2.3 Effects of MeJA on the activities of enzymes related to pathogenesis
Increase of the pectinase activity was observed with treatment by MeJA. The activity of pectinase was increased by MeJA depending on its concentration,with the maximum value [(0.61±0.05) U·mL-1·min-1] at the concentration of 800 μg·mL-1(Table 3). The activity of cellulase was stimulated at high concentrations of MeJA (200-800 μg·mL-1) in liquid culture,while it was suppressed at low concentrations (1-50 μg·mL-1). The activity of cellulase was (0.31±0.02) μmol-1·min-1at the highest concentration (800 μg·mL-1) of MeJA (Table 3). At lower concentrations of MeJA (1-100 μg·mL-1),amylase activity little changed,but substantial increase of the activity was found at high concentrations of 200-800 μg·mL-1,which was (0.45±0.02) μmol-1·min-1at the concentration of 800 μg·mL-1(Table 3). Protease activity byC.destructanshad scarcely influenced by MeJA in liquid culture,although small amounts of fall tendency was observed,which the activity was almost no difference compared to control (Table 3).
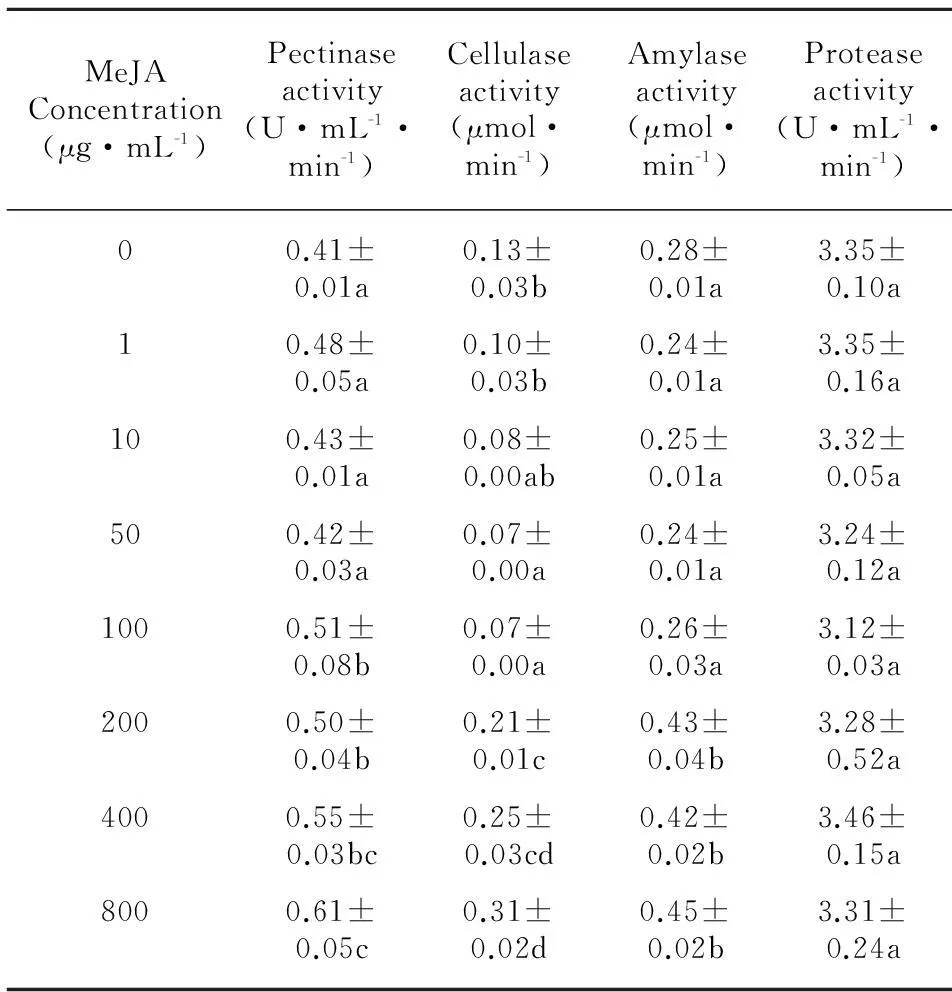
Table 3 Effects of MeJA at different concentrations
3Discussion
It is well known that spore germination and mycelial growth ofC.destructansplay an important part in the infection process in plant diseases. We believed that decreased germination and mycelial growth ofC.destructansby MeJA would be one of the mechanisms on plant resistance to pathogens. From the present study,MeJA not only enhances the plant resistance to pathogens but also directly inhibits the growth of the pathogens.
Enzymes related to pathogenesis secreted byC.destructans,such as pectinase,cellulase,amylase and protease,were important pathogenic factors in the progression of the infection. Pectinases and cellulases facilitate the penetration of the fungus into the plant by the hydrolytic cleavage of polymers (pectic substances,cellulose) which constitute the plant cell walls (Fuchs et al,1965). It has been proposed that proteases may be required for nutritional purposes or to degrade protein in the plant cell wall to allow spread of the pathogens or overcome host defenses (Dow et al,1990). Increase of amylase activity from the fungi contributes to the deposition and utilization of host carbon source. In the current study,pectinase,cellulase and amylase activity ofC.destructanswas stimulated by MeJA. Pectinase activity at the highest concentration of MeJA increased by 47.7%. Cellulase activity was repressed by MeJA at concentrations lower than 100 μg·mL-1,while was stimulated at high concentrations (200-800 μg·mL-1). A great increase of amylase activity was obtained treated with MeJA at concentrations higher than 200 μg·mL-1,which was increased by 63% at the concentration of 800 μg·mL-1. Little effect of MeJA onC.destructansprotease activity was found (Table 3). The findings meant that excessive MeJA artificially added in practice would have adverse effect on the plant,which needs to be further studied in the future.
In conclusion,MeJA inhibited the colony growth and spore germination ofCylindrocarpondestructans,while at the same time stimulated the production of phytopathogenic enzymes. The critical concentration of MeJA inhibitory effects onC.destructanswas 200 μg·mL-1. The results lays a foundation for the subsequent experiment using MeJA to induct disease resistance.
References:
AHN IP,LEE YH, 2001. A viral double-stranded RNA up regulates the fungal virulence ofNectriaradicicola[J]. Mol Plant-microl Inter,14(4): 496-507.ALEANDRI MP,REDA R,TAGLIAVENTO V,et al, 2010. Effect of chemical resistance inducers on the control ofMonosporascusroot rot and vine decline of melon [J]. Phytop Med,49(1): 18-26.ALI MB,YU KW,HAHN EJ,et al, 2006. Methyl jasmonate and salicylic acid elicitation induces ginsenosides accumulation,enzymatic and non-enzymatic antioxidant in suspension culturePanaxginsengroots in bioreactors [J]. Plant Cell Rep,25(6): 613-620.BERLIN A,GILKES N,KILBURN D,et al, 2005. Evaluation of novel fungal cellulase preparations for ability to hydrolyze softwood substrates-evidence for the role of accessory enzymes [J]. Enz Microl Technol,37(2): 175-184.
COHEN YGU,NIEDERMAN T, 1993. Local and systemic protection againstPhytopthorainfestansinduced in potato and tomato plants by jasmonic acid and jasmonic methyl ester [J]. Phytopathology,83: 1 054-1 062.
CREELMAN RA,MULLET JE, 1997. Biosynthesis and action of jasmonates in plants [J]. Ann Rev Plant Physiol Plant Mol Biol,48: 355-381.
DOW JM,CLARKE BR,MILLIGAN DE,et al, 1990. Extracellular proteases fromXanthomonascampestrispv.campestris,the black rot pathogen [J]. Appl Environ Microl,56(10): 2 994-2 998.
FUCHS A,JOBSEN JA,WOUTS W, 1965. Arabanases in phytopathogenic fungi [J]. Nature,206: 714-715.
GAIGE AR,AYELLA A,SHUAI B, 2010. Methyl jasmonate and ethylene induce partial resistance inMedicagotruncatulaagainst the charcoal rot pathogenMacrophominaphaseolina[J]. Physiol Mol Plant Pathol,74(5-6): 412-418.
HE CY,WOLYN DJ, 2005. Potential role for salicylic acid in induced resistance of asparagus roots toFusariumoxysporumf. spasparagi[J]. Plant Pathol,54(2):227-232.
KERNAGHAN G,REELEDER RD,HOKE SMT, 2007. Quantification ofCylindrocarpondestructansf. sppanacisin soils by real-time PCR [J]. Plant Pathol,56(3): 508-516.KIM JH,KIM SG,KIM MS,et al, 2009. Different structural modifications associated with development of ginseng root rot caused byCylindrocarpondestructans[J]. Plant Pathol J,25(1):1-5.
KOZLOWSKI G,BUCHALA A,METRAUX JP, 1999. Methyl jasmonate protects Norway sprucePiceaabies(L.) Karst. seedlings againstPythiumultimumTrow [J]. Physiol Mol Plant Pathol,55(1):53-58.
LOWRY OH,ROSEBROUGH NJ,FARR AL,et al, 1951. Protein measurement with the Folin phenol reagent [J]. J Biol Chem,193(1):265-275.
MURADO MA,GONZALEZ MP,TORRADO A,et al, 1997. Amylase production by solid state culture ofAspergillusoryzaeon polyurethane foams. Some mechanistic approaches from an empirical model [J]. Proc Biochem,32(1): 35-42.
PENNINCKX I,THOMMA B,BUCHALA A,et al, 1998. Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene inArabidopsis[J]. Plant Cell,10(12): 2 103-2 113.
PRESTON CA,LAUE G,BALDWIN IT, 2001. Methyl jasmonate is blowing in the wind,but can it act as a plant-plant airborne signal? [J]. Biochem Syst Ecol,29(10): 1 007-1 023.
PUNJA ZK, 1997. Fungal pathogens of American ginseng (Panaxquinquefolium) in British Columbia [J]. Can J Plant Pathol,19(3): 301-306.
RAHMAN M,PUNJA ZK, 2005a. Biochemistry of ginseng root tissues affected by rusty root symptoms [J]. Plant Physiol Biochem,43(12): 1 103-1 114.
RAHMAN M,PUNJA ZK, 2005b. Factors influencing development of root rot on ginseng caused byCylindrocarpondestructans[J]. Phytopathology,95(12): 1 381-1 390.RAHMAN M,PUNJA ZK, 2006. Influence of iron onCylindrocarponroot rot development on ginseng [J]. Phytopathology,96(11):1 179-1 187.REELEDER RD,BRAMMALL RA, 1994. Pathogenicity ofPythiumspecies,Cylindrocarpondestructans,andRhizoctoniasolanito ginseng seedlings in Ontario [J]. Can J Plant Pathol,16(4): 311-316.
REELEDER RD,ROY R,CAPELL B, 2002. Seed and root rots of ginseng (Panaxquinquefolius) caused byCylindrocarpondestructansandFusariumspp. [J]. J Ginseng Res,26: 151-158.
SEIFERT KA,MCMULLEN CR,YEE D,et al, 2003. Molecular differentiation and detection of ginseng-adapted isolates of the root rot fungusCylindrocarpondestructans[J]. Phytopathology,93(12): 1 533-1 542.
SILVA D,TOKUIOSHI K,MARTINS ED,et al, 2005. Production of pectinase by solid-state fermentation withPenicilliumviridicatumRFC3 [J]. Proc Biochem,40(8): 2 885-2 889.
TSENG TC,MOUNT MS, 1974. Toxicity of endopolygalacturonate trans-eliminase,phosphatidase and protease to potato and cucumber tissue [J]. Phytopathology,64: 229-236.
WANG TS,2001. China ginseng [M]. Shenyang: Liaoning Science and Technology Press.
