温室多壁碳纳米管芒硝基相变材料储能性能
2016-05-17铁生年
柳 馨,铁 健,铁生年
(青海大学新能源光伏产业研究中心,西宁810016)
温室多壁碳纳米管芒硝基相变材料储能性能
柳 馨,铁 健,铁生年※
(青海大学新能源光伏产业研究中心,西宁810016)
该文对比不同温度下强酸处理多壁碳纳米管(multi-walled carbon nanotubes,MWCNTs)对其表面结构改性,并以物理分散制备出含MWCNTs的十水硫酸钠基复合相变储能材料。探讨不同酸化温度下MWCNTs对十水硫酸钠基复合相变储能材料的过冷和相分层影响。并对其比热,导热系数及相变潜热特征进行分析。结果表明:酸化处理后MWCNTs产生羧基;添加质量分数1%的120℃酸化后的MWCNTs的A、B两种十水硫酸钠基复合相变储能材料过冷度降低最大;添加酸化处理后的MWCNTs的十水硫酸钠基复合相变储能材料相容性较好;含120℃酸化后的1%的MWCNTs的A、B两种十水硫酸钠基复合相变储能材料比热及导热系数在相变温度点附近都达到最大,分别为5.095 mm2/s和0.932 5 w/mk、4.235 6 mm2/s和0.941 3 w/mk;含质量分数1%的120℃酸化处理的MWCNTs的B类复合相变储能材料较A类的潜热值大,其分别为143.6 J/g,97.42 J/g;该试验表明含1%MWCNTs-B相变储能材料更适合应用于温室。
温室;相变材料;十水硫酸钠;储热性能
柳 馨,铁 健,铁生年.温室多壁碳纳米管芒硝基相变材料储能性能[J].农业工程学报,2016,32(6):226-231.doi:10.11975/j.issn.1002-6819.2016.06.031 http://www.tcsae.org
Liu Xin,Tie Jian,Tie Shengnian.Energy storage properties of mans nitro phase transition materials of multi-walled carbon nanotubes of greenhouse[J].Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE),2016,32(6): 226-231.(in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2016.06.031 http://www.tcsae.org
0 引言
为克服恶劣多变的天气,温室大棚在青藏地区的农产品开发与规模生产起着举足轻重的作用,但温室大棚温差较大,白天温室内温度较高,夜间温室温度又较低。传统形式的升降温方式如烟道加热,蒸汽加热及喷雾降温等措施普遍存在高能耗及高运营成本,尤其是升温过程产生煤,电等的能源消耗,同时燃烧产生的废气又污染环境。中、低温相变材料作为一种高效储能材料,在农业上的应用主要体现在温室大棚的温度环境调控上,相变储能材料可以通过相变过程对太阳能进行存储,解决能源在时间和空间上的不匹配问题[1-3]。
青藏地区是芒硝(Na2SO4.10H2O)资源的聚集地,其作为一种中、低温相变储能材料,具有相变体积变化小,潜热密度大,导热系数大等优点,因而具有实际应用前景[4-6]。实际应用中,其存在过冷和相分层问题,又制约其使用[7-9]。本研究拟解决十水硫酸钠钠基复合相变储能材料的过冷和相分层问题。
基于温室大棚应用,常选择适宜温室大棚植物生长温度(15~25℃)相变储能材料,通常满足以下条件[10-11]:1)相变材料的相变温度应为植物适宜生长温度;2)相变材料相变潜热大,体积膨胀率小;3)不产生对植物生长有害的物质;4)导热系数大,密度大,比热容大;5)价格低廉,来源丰富。例如,韩丽蓉[12]研究表明在Na2SO4.10H2O加入10%KCl可以将相变温度由32.4℃降低到23.77℃左右,本课题组[13]研究Na2SO4.10H2O与Na2CO3.10H2O以质量分数9:1再添加4%NaCl可将想变温度降到25℃以下。虽然其相变温度适于应用温室内,但是材料本身仍具有过冷和相分层现象,制约其使用。
多壁碳纳米管(multi-walled carbon nano-tubes,MWCNTs)具有极高的导热系数,碳纳米强化传热已有较多的研究报道[14-17],而将其应用于相变储能材料领域中也有良好效果[18-19]。导热材料添加于无机相变储能材料中可以增大材料的导热系数,使材料内部传热增强,在外界温度变化时,内部材料的温度变化均匀,过饱和度就均匀,从而减少材料的相分层现象[20]。为此本文将探讨MWCNTs的添加对Na2SO4.10H2O基复合相变储能材料的影响。
本文将以物理分散方法制备MWCNTs与Na2SO4.10H2O基复合相变储能材料混合的复合相变储能材料。对比不同温度下强酸处理的多壁碳纳米管(MWCNTs)的表面结构的改性;探讨MWCNTs酸化温度与添加质量分数对Na2SO4.10H2O基复合相变储能材料的过冷和相分层现象影响;同时对复合相变储能材料的热常数及相变潜热特征进行分析,制备出适宜青藏地区温室大棚应用的相变储能材料。
1 试验
1.1 样品制备
在40 ml混酸(VH2SO4∶VHNO3=3∶1)加入1 g MWCNTs(孔径60~100 nm,长度1~5 μm,徐州捷创新材料科技有限公司),然后控制温度分别在90、120、140℃反应20 min停止加热,待其冷却至室温后,将上述所得反应物用去离子水反复清洗至中性,然后在100℃恒温箱中烘干,分别记为0-MWCNTs(未氧化处理MWCNTs)、90-MWCNTs、120-MWCNTs、140-MWCNTs。取Na2SO4.10H2O研磨,称取一定量Na2SO4.10H2O再添加10%KCl制成复合相变储能材料并记为A,同时称取一定量Na2SO4.10H2O和Na2CO3.10H2O以质量比9∶1混合再添加总质量的4%的NaCl制成复合相变储能材料并记为B。使A、B复合相变储能材料在30℃变成融化状态下分别加入质量分数为0.5%,1%,3%,5%的0-MWCNTs,90-MWCNTs,120-MWCNTs,140-MWCNTs超声振荡 20 min并加以搅拌,制成MWCNTs复合的十水硫酸钠基复合相变储能材料,分别记为A-0-MWCNTs、A-90-MWCNTs、A-120-MWCNTs、A-140-MWCNTs以及B-0-MWCNTs、B-90-MWCNTs、B-120-MWCNTs、B-140-MWCNTs。
1.2 测试与表征
采用美国Keysight公司产的34970A数据采集仪记录复合相变储能材料的温度随时间变化情况;采用美国热电公司产的Nexus傅里叶变换红外光谱仪对MWCNTs进行红外检测;采用日本株式会社产JSM-5610LV低真空扫描电子显微镜(scanning electron microscope,SEM)观察酸化处理后的MWCNTs表面形貌;采用瑞典HOT DISK产的TPS2200型号热常数分析仪(thermal constants analyzer,TCA)对MWCNTs复合后的十水硫酸钠相变储能材料的热常数进行分析,从0℃升温到50℃(5℃/次)进行测量;采用德国NETZSCH公司产的200F3差示扫描热量仪(different scanning calorimetry,DSC)分析复合相变储能材料的相变潜热,氮气气氛(50 mL/min),升温速度1℃/min。
2 结果与讨论
2.1 酸化MWCNTs的红外分析
图1给出了0-MWCNTs,90-MWCNTs,120-MWCNTs,140-MWCNTs的红外光谱图。结果表明:0-MWCNTs除在3 400.56 cm-1处有特征峰外,其余无明显特征峰,此处为羟基的振动峰。经过混酸处理后,90-MWCNTs在1703.6、1 145.34 cm-1处出现了-C=O的振动峰及C-O的伸缩振动峰;120-MWCNTs多壁碳纳米管在2 412.13、1 705.17、1 158.27 cm-1出现了新的峰值,它是羧基中的-O-H的振动峰,-C=O的振动峰及C-O的伸缩振动峰;及140-MWCNTs在2 415.6、1 704.21、1 132.16 cm-1同样出现了羧基中的-O-H的振动峰,-C=O的振动峰及C-O的伸缩振动峰。试验表明经过混酸氧化后,多壁碳纳米管上产生了羧基基团。

图1 碳纳米管红外光谱图Fig.1 Fourier transform infrared spectroscopy analysis of MWCNTs
2.2 酸化MWCNTs的形貌分析
图2中的a,b,c,d分别为0-MWCNTs,90-MWCNTs,120-MWCNTs,140-MWCNTs的扫描电镜照片。结果表明:0-MWCNTs及90-MWCNTs变化不明显;120-MWCNTs有相当一部分变短,同时有略微的团聚现象;140-MWCNTs的管长较其它更短,并且呈现团聚状态。这是因为混酸具有强氧化性,破坏并且剪短了MWCNTs,而羧基正出现在这些破坏处。
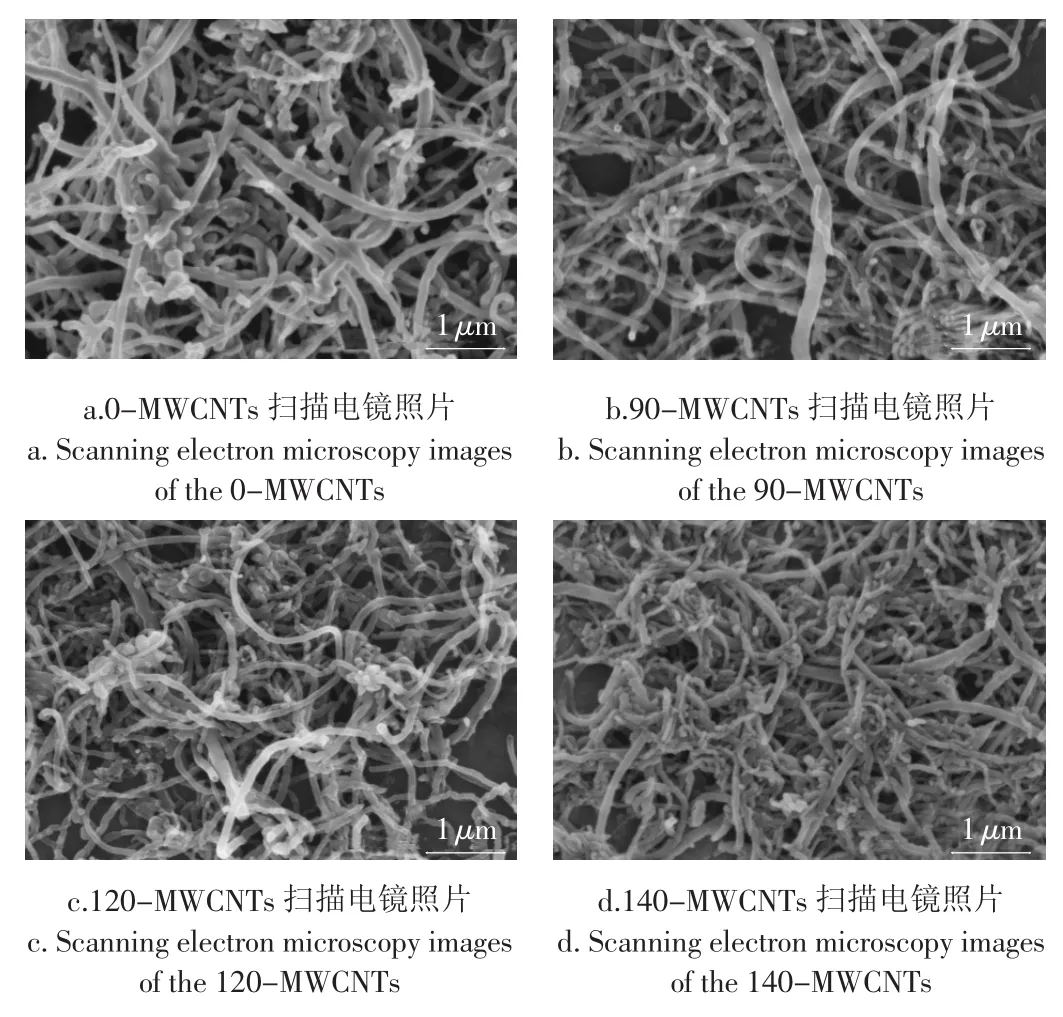
图2 MWCNTs酸化前后的扫描电镜照片Fig.2 Scanning electron micrographs of MWCNTs before and after acid treatment
2.3 复合相变储能材料过冷现象
图3中的a,b,c,d和图4中的a,b,c,d分别为添加质量分数0.5%~5%的MWCNTs的A和B复合相变储能材料。结果表明:A、B相变储能材料本身具有一定的过冷度,过冷度现象严重时会影响水合盐形成晶体物质,导致相变潜热储热能力下降;由图3a及图4a看出,未氧化的MWCNTs对复合储能材料的过冷度没有明显影响,由于MWCNTs的亲水性较差,所以与材料的相容性较差,以至于材料的添加对复合相变储能材料没有明显影响;由图3b及4b看出,90-MWCNTs的添加对复合相变储能材料都有一定的影响,过冷度略小于A种复合相变储能材料,说明酸化后的MWCNTs产生的亲水基团羧基起到了一定作用,使得复合相变储能材料发生非均匀成核降低过冷度;由图3c及4c看出复合相变储能材料的过冷度降低较为明显,说明120-MWCNTs的添加对复合相变储能材料的过冷度有明显影响,可能MWCNTs经过120℃高温氧化后,MWCNTs上形成的羧基较多,亲水性较好,所以过冷度降低较多;由图3d和4d看出,140-MWCNTs的A、B复合相变储能材料过冷度较120-MWCNTs降低较少,可能是140℃酸化后的MWCNTs团聚现象较为严重,对复合相变储能材料的过冷度降低有一定影响。
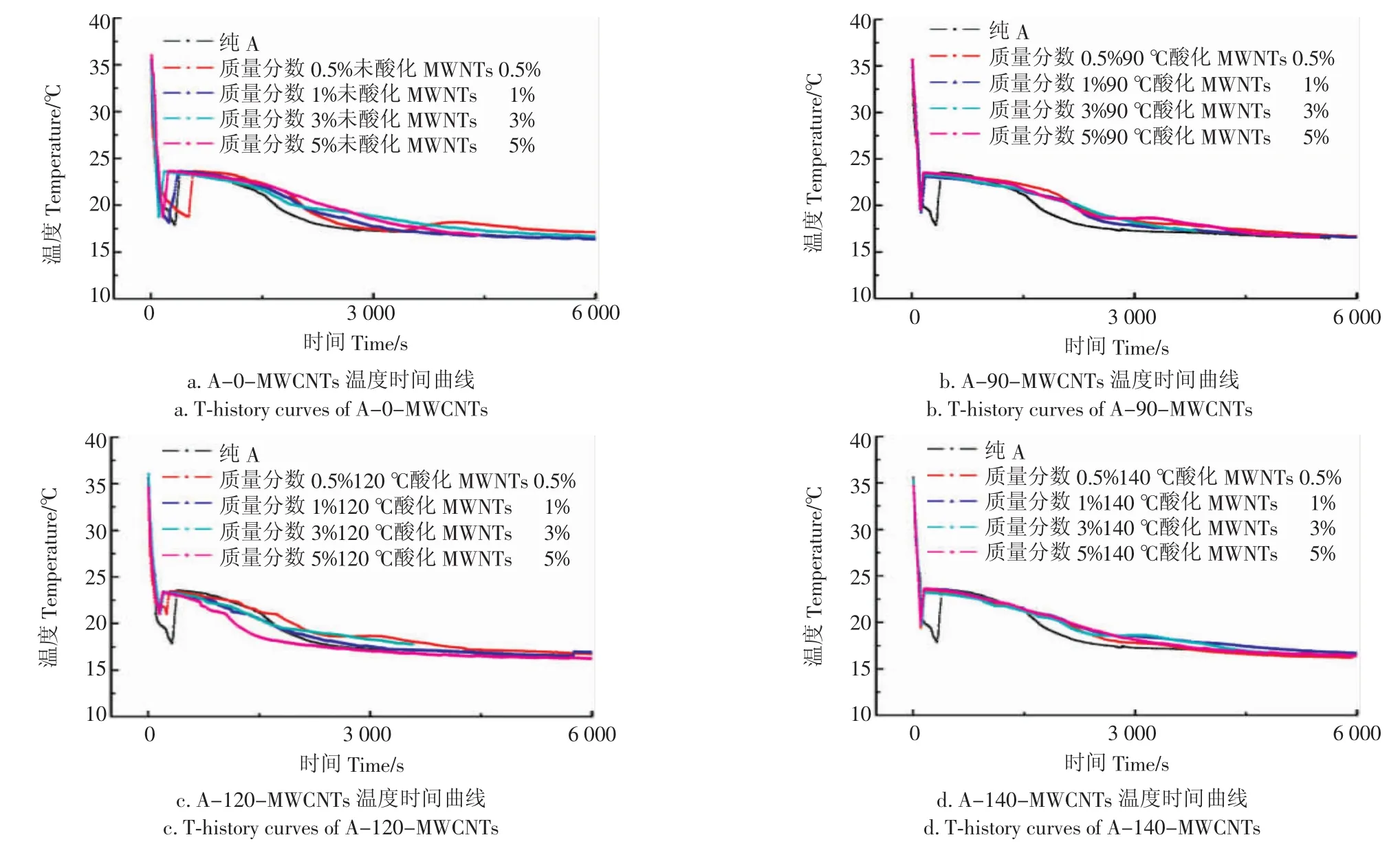
图3 A类复合相变储能材料的T-history曲线Fig.3 T-history curves of class A composites phase change materials
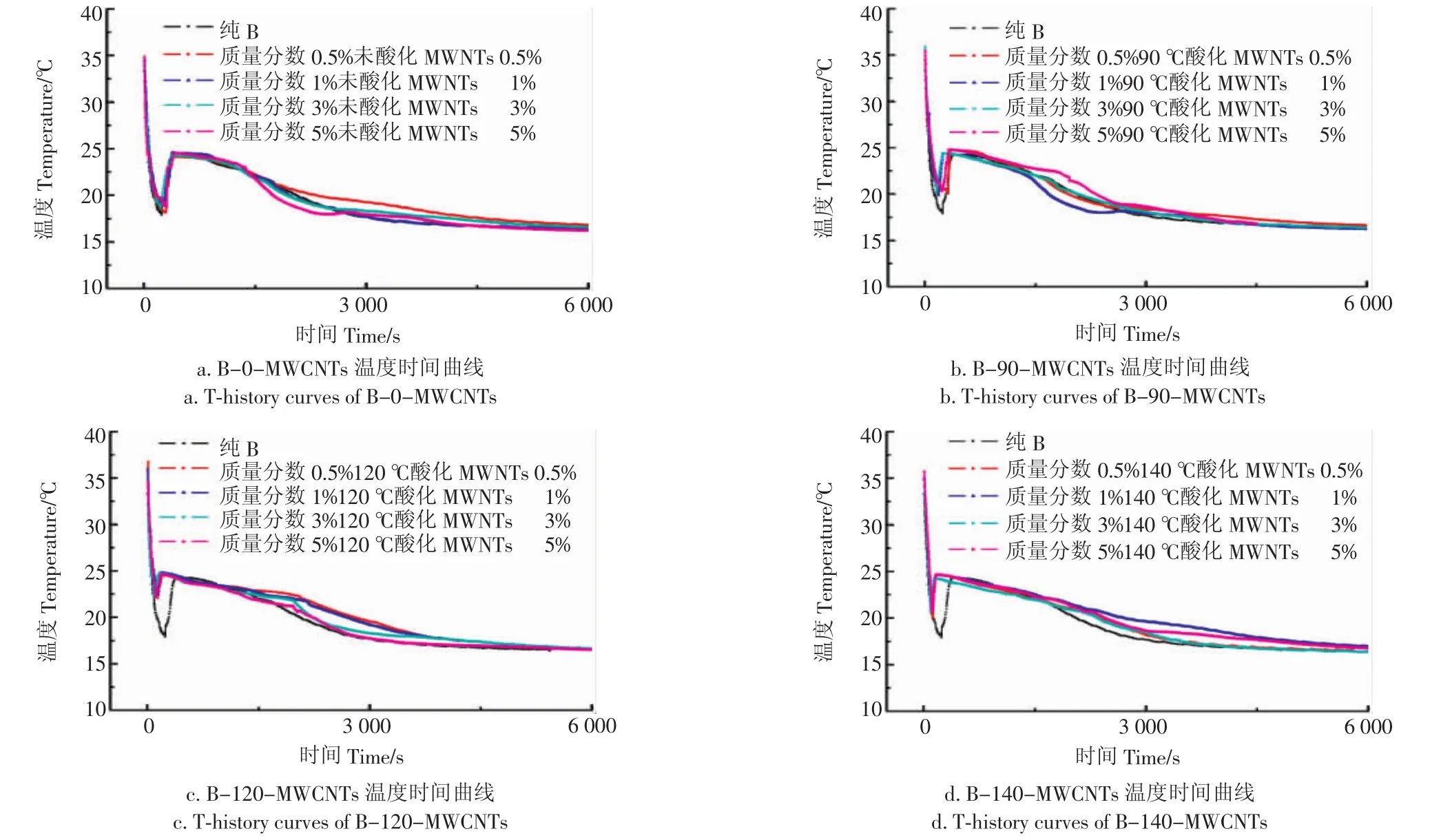
图4 B类复合相变储能材料的T-history曲线Fig.4 T-history curves of class B composites phase change materials
2.4 复合相变储能材料相分层现象
图5为复合相变储能材料相容性照片,a图为含1%A-0-MWCNTs的相容性照片。结果表明:MWCNTs多数含量漂浮于水合盐的上层,其余一部分融入水合盐中,相容性较差,这表明未氧化的MWCNTs难溶于此体系中;然而经过酸化处理后MWCNTs由于表面羧酸化,易溶于无机相变储能材料中,以120℃酸化后MWCNTs为添加剂,b,c图依次为不同质量分数的A-120-MWCNTs和B-120-MWCNTs的复合相变储能材料,从图中可以看出,纯A、B相变储能材料为白色,试管底层为白色固体盐,中层为结晶的水合盐,上层有部分溶液未结晶,其本身有一定的相分层现象,添加120-MWCNTs后与相变储能水合盐相容性较好,混为一体呈现黑色,同时水合盐本身无明显相分层现象,说明酸化后的MWCNTs易溶于此体系中,以减少复合相变储能材料的相分层现象。
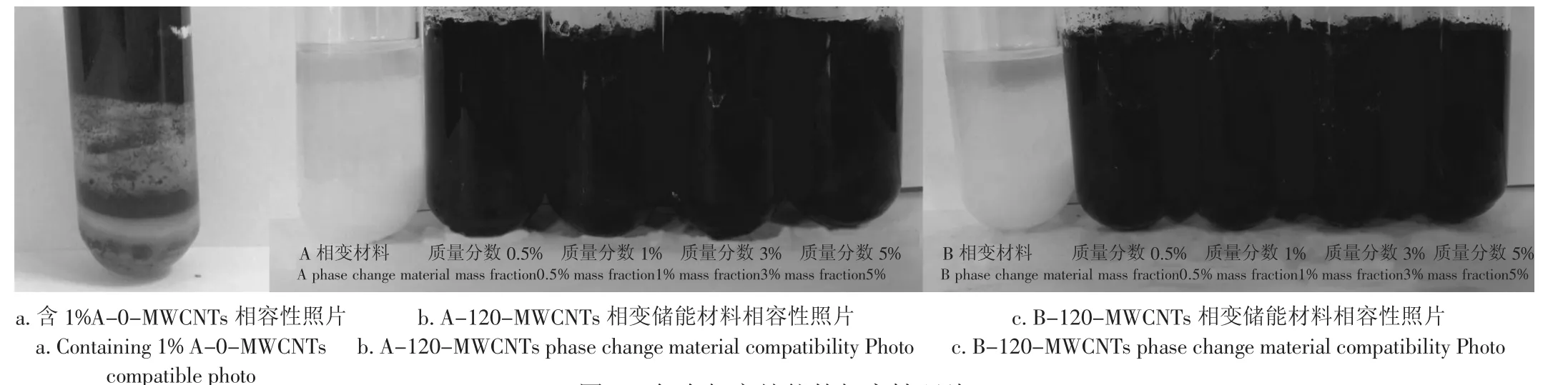
图5 复合相变储能的相容性照片Fig.5 Composite phase change energy storage compatibility photo
2.5 复合相变储能材料热常数
图6为含1%120-MWCNTs复合相变储能材料的热常数图。图a为含1%120-MWCNTs的A,B复合相变储能材料比热随温度变化曲线。结果表明:复合相变储能材料比热在相变温度点附近出现了反常现象,即出现了一个峰,复合相变储能材料的比热达到最大,分别为5.095、4.235 6 mm2/s,这表明材料内部发生了从有序到无序的转变,说明在该相变温度点材料有吸热现象[21-24]。图b为含1%120-MWCNTs的A、B复合相变储能材料的导热系数随温度的变化的曲线。结果表明:在相变温度点附近含1%120-MWCNTs的A、B复合相变储能材料的导热系数最高,分别为0.932 5、0.941 3 w/mk,并且在相变点的左右两侧导热系数都随着温度的升高而增加。
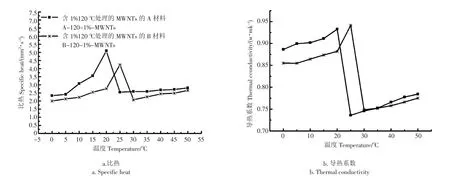
图6 复合相变储能材料的热常数图Fig.6 Thermal constant of composite phase change energy storage materials
2.6 复合相变储能材料的相变潜热
图7为复合相变储能材料DSC图。结果表明:添加1%的120-MWCNTs的A,B复合相变储能材料较纯A,B的相变温度点都有所提前,A类相变储能复合材料由相变点21.1提前到20.8,B类相变储能复合材料由相变点23.4提前到21.5,分别提前,0.3℃,1.9℃,由于120-MWCNTs的添加,使得材料的导热性提高,材料内部传热效果增强,材料随温度变化的能力增强,所以易使相变点提前;同时添加1%的120-MWCNTs的A,B复合相变储能材料较纯A,B的潜热减小,分别减小14.48 J/g,25.9J/g,由于添加了1%-MWCNTs,导致实际吸热复合相变储能材料的比例减小,所以潜热值减小;A,B复合相变储能材料相变潜热值分别为111.9 J/g,169.5 J/g,B较A材料潜热值大,同时添加1%的120-MWCNTs的A,B复合相变储能材料相变潜热值分别为97.42 J/g,143.6 J/g,后者潜热值较大。
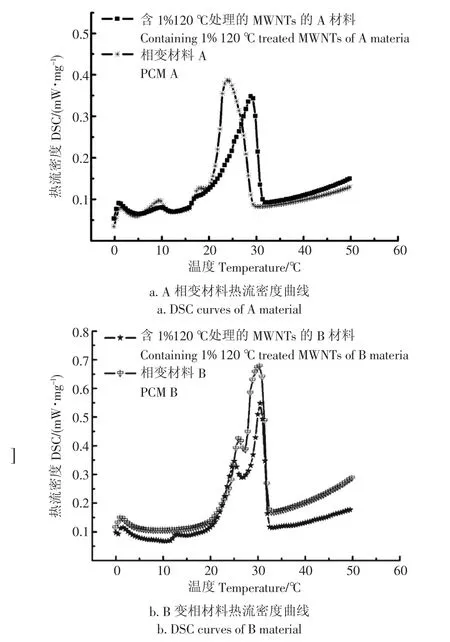
图7 复合相变储能材料的DSC图Fig.7 Different scanning calorimetry(DSC)photographs of composite phase change material
3 结论
1)酸化处理后的MWCNTs上形成羧基,90℃酸化处理的MWCNTs变化不明显,140℃酸化处理的MWCNTs剪短及团聚现象严重,120℃酸化处理的MWCNTs管长仍较长,有略微的团聚现象。
2)添加1%的120-MWCNTs的A,B类复合相变储能材料的过冷度降低最大;同时添加酸化处理的MWCNTs的A,B相变复合储能材料的相容性较好,无明显相分层现象。
3)相变温度点附近的含1%的120-MWCNTs的A、B复合相变储能材料的比热和导热系数最大,分别为5.095 mm2/s、0.932 5 w/mk、4.235 6 mm2/s、0.941 3 w/mk,比热的突变表明材料发生吸热现象,导热系数在相变点附近的左右两边都随温度的升高而增大。
4)含1%的120-MWCNTs复合相变储能材料B较A材料的潜热值大,分别为143.6 J/g,97.42 J/g,更适用于温室大棚。
[1]Abhat A.Low temperature latent heat thermal energy storage: heat storage materials[J].Solar Energy,1983,30:313-332.
[2]Sharma A,Tyagi V V,Chen C R,et al.Review on thermal energy storage with phase change materials and applications[J].Renew Sustain Energy Rev,2009,13:318-345.
[3]Khudhair A M,Farid M M.A review on energy conservation in building applications with thermal storage by latent heat using phase change materials[J].Energy Conversio nand Management, 2004,45:263-275.
[4]Mesalhy O,Lafdi K,Elgafy A.Carbon foammarices saturated with PCM for thermal protection purposes[J].Carbon,2006,44: 2080-2088.
[5]Lafdi K,Mesalhy O,Elgafy A.Graphite foams in filtrated with phase change materials as alter native materials for space and terrestrial thermal energy storage applications[J].Carbon,2008, 46(1):159-168.
[6]Lafdi K,Mesalhy O,Shaikh S.Experimental study on the influence of foam porosity and pore size on the melting of phase change materials[J].Journal of Applied Physics,2007,102(8): 083549-083549-6.
[7]Zalba B,Marin J,Cabeza L F,etal.Review on thermal energy storage with phase change materials heat transfer analysis and applications[J].AppliedThermalEngineering,2003,23:251-283.
[8] 盛强,邢玉明,王泽.八水氢氧化钡相变材料的强化传热实验[J].航空动力学报,2013,28(9):1927-1932.Sheng Qiang,Xing Yuming,Wang Ze.Experiment on heat transfer enhancement of barium hydroxide octahydrate phase change material[J].Journal of Aerospace Power,2013,28(9): 1927-1932.(in Chinese with English abstract)
[9]张雪梅,蔡路茵,章迪.相变储能化合物CH3COONa.3H2O的热分解行为[J].无机化学学报,2010,26(1):67-71.Zhang Xuemei,Cai Luyin,Zhang Di.Thermal behavior of CH3COONa.3H2O as phase change material[J].Chinese Journal of Inorganic Chemistry,2010,26(1):67-71.(in Chinese with English abstract)
[10]王宏丽,邹志荣,陈红武,等.温室中应用相变储热技术研究进展[J].农业工程学报,2008,24(6):304-307.Wang Hongli,Zhou Zhirong,Chen Hongwu,et al.Research advances in technologies of phase-change heat storage and its application in hothousess[J].Transactions of Chinese Society of Agricultural Engineering(Transactions of the CSAE),2008,24 (6):304-307.(in Chinese with English abstract)
[11]王宏丽,李晓野,邹志荣.相变蓄热砌块墙体在日光温室中的应用效果[J].农业工程学报,2011,27(5):253-257.Wang Hongli,Li Xiaoye,Zou Zhirong.Application of brick wall with phase change rice husk in solar hothousess[J].Transactions of Chinese Society of Agricultural Engineering(Transactions of the CSAE),2011,7(5):253-257.(in Chinese with English abstract)
[12]韩丽蓉.相变蓄热材料十水硫酸钠的改性及应用研究[D].杨陵:西北农林科技大学,2014.Han Lirong.Modification and Application Research of Phase ChangeHeat Storage Material Glauber Salt[D].Yangling: NorthwestA&FUniversity,2014.(inChinesewithEnglishabstract)
[13]蒋自鹏,铁生年.物理法制备芒硝基复合相变材料及其性能研究[J].人工晶体学报,2015,44(12):3120-3127. Jiang Zipeng,Tie Shengnian.Preparation and properties of Glauber’s salt-based composites phase change materials by physical method[J].Journal of Synthetic Crystals,2015,44(11): 3074-3080.(in Chinese with English abstract)
[14]Chen Ying,Jia Lisi,Mo Songping.Experimental investigation of crystallization process of nanofluids by DSC[J].Journal of Southeast University,2010,26(2):359-363.
[15]He Qingbo,Wang Shuangfeng,Tong Mingwei.Experimental study on thermophysical properties of nanofluids as phasechange material(PCM)in low temperature cool storage[J].Energy Conversion Management,2012,64:199-205.
[16]杨波,王蛟,刘军.碳纳米流体强化传热研究[J].强激光与粒子束,2014,26:051003.Yang Bo,Wang Jiao,Liu Jun.Heat transfer enhancement of carbon nanofluids[J].High Power Laser and Particle Beams,2014,26:051003.(in Chinese with English abstract)
[17]王姣,杨波,刘军,等.碳纳米流体相变特性实验研究[J].强激光与粒子束,2015,27:074102.Wang Jiao,Yang Bo,Liu Jun,et al.Experimental studies on phase change characterittics of carbon nanofluids[J].High Power Laser and Particle Beams,2015,27:074102.(in Chinese with English abstract)
[18]商红岩,刘晨光,徐永强.碳纳米管的表面修饰对Co-Mo催化剂HDS性能影响的研究[J].新型炭材料,2004,19(2):129-136.Shang Hongyan,Liu Chenguang,Xu Yongqiang.Effect of the surface modification of multi-walled carbon nanotubes (MWCNTs)on hydrodesulfurization activity of Co-Mo/MWCNTs catalysts[J].New Carbon Materials,2004,19(2):129-136.(in Chinese with English abstract)
[19]王继芬,谢华清,辛忠,等.酸化碳纳米管棕搁酸复合相变储能材料的研究[J].工程热物理学报,2010,31(8):1389-1391.Wang Jifen,Xie Huaqing,Xin Zhong,et al.Experimental study on palmitic acid composites containing carbon nanotubes by acidtreatment[J].Journal of Engineering Thermophysics,2010, 31(8):1389-1391.(in Chinese with English abstract)
[20]柳馨,铁健,铁生年.纳米粉体对Na2SO4.10H2O过冷及相分层现象的影响[J].人工晶体学报,2015,44(11):3074-3080.Liu Xin,Tie Jian,Tie Shengnian.Effect of nano powder addition on the subcooling and phase stratification of sodium sulfate decahydrate[J].Journal of Synthetic Crystals,2015,44(11): 3074-3080.(in Chinese with English abstract)
[21]石海荣,哈斯朝鲁,王全晶,等.Mn2-xFexP0.51Si0.49化合物的比热容与相变[J].稀有金属材料与工程,2012,41(2):600-602.Shi Hairong,Ha Sizhaolu,Wang Jinjing,et al.Specific heat and phase transition in Mn2-xFexP0.51Si0.49compounds[J].Rare Metal Materials and Engineering,2012,41(2):600-602.(in Chinese with English abstract)
[22]彭小飞,俞小莉,余凤芹.低浓度纳米流体比热容试验研究[J].材料科学与工程学报,2007,25(5):719-722.Peng Xiaofei,Yu Xiaoli,Yu Fengqin.Experimental study on the specific heat of nanofluids[J].Journal of Materials Science &Engineering,2007,25(5):719-722.(in Chinese with English abstract)
[23]Lee S,Choi U S.Measuring thermal conductivity of fluids containing oxide nanoparticles[J].Transaction of the ASME, 1999,121(5):280-284.
[24]Xuan Y M,Li Q.Heat transfer enhancement of nanofluids[J]. Int.J.Heat and Fluid Flow,2000,21:58-64.
Energy storage properties of mans nitro phase transition materials of multi-walled carbon nano-tubes of greenhouse
Liu Xin,Tie Jian,Tie Shengnian※
(New Energy(photovoltaic)Industry Research Center,Qinghai University,Xining 810016,China)
It is significant to study low temperature phase change materials(PCM)widely used in hothouses.The performance of low temperature sodium sulfate decahydrate based PCM composite affects hothouse temperature directly. Sodium sulfate decahydrate based PCM composite has suitable phase change temperature,but presents supercooling and phase stratification phenomenon.These problems restrict its practical application.The result of related study indicated that the heat conductive material had a certain influence on supercooling and phase stratification of PCM.We studied that the effect of nano-powders of sodium sulfate decahydrate on subcooling and phase stratification,of which the nano-carbon had the better effects on sodium sulfate decahydrate′s supercooling and phase stratification.This paper explored the effect of multi-walled carbon nano-tubes(MWCNTs)with high thermal conductivity on 2 kinds of sodium sulfate decahydrate based PCM composites,named PCM A and PCM B.PCM A consisted of 90wt%Na2SO4.10H2O and 10wt%KCl.PCM B consisted of 86.4wt%Na2SO4.10H2O,9.6wt%Na2CO3.10H2O,and 4wt%NaCl.Firstly,MWCNTs was modified on surface by acid treatment at 90,120 and 140℃respectively,in order to obtain good compatibility with PCM A and B.Fourier transform infrared spectroscopy was used to detect the acid-treated MWCNTs and the surface functional groups on the MWCNTs were analyzed.Low vacuum scanning electron microscope was used to observe the surface morphology of acid-treatment MWCNTs.The acid-treated MWCNTs were added into PCM A and B by physical dispersion method.The time dependence of the temperature of PCM A and B containing acid-treated MWCNTs or not was recorded by date acquisition instrument to explore the effects of acid treatment temperature and mass fraction of MWCNTs on the degree of supercooling of PCM.The phase stratification phenomenon was photographed and investigated.The specific heat and thermal conductivity were measured by thermal constant analyzer.And the thermal conductivity and specific heat of thermally conductive material added into the PCM A and B were analyzed.The latent heat of PCM was analyzed by different scanning calorimetry measurements.Latent heat value directly affects the size of the heat storage capacity of the material.The results indicated that carboxyl group was formed in MWCNTs after acid treatment,which could improve the compatibility between MWCNTs and PCM A or B.By the scanning electron microscope,the surface topography change of the MWCNTs without oxidization and the MWCNTs with the surface modified by acid treatment at 90℃was not obvious.And the MWCNTs with the surface modified by acid treatment at 120℃had slight agglomeration.The MWCNTs with the surface modified by acid treatment at 140℃had serious agglomeration and shear break.When 1wt%acid-treated MWCNTs at 120℃were added,both of PCM A and B showed low degree of supercooling.Their specific heat and thermal conductivity reached the maximum,which were 5.095 mm2/s,0.932 5 w/mk and 4.235 6 mm2/s,0.941 3 w/mk respectively in the vicinity of the phase transition temperature.Abrupt change of specific heat of the phase change material at this point showed that mutations occurred for latent heat material itself.The increase of thermal conductivity showed that the heat transfer effect of phase change energy storage material was enhanced in the process of heat absorption and heat transfer.Besides,PCM B containing 1wt% MWCNTs acid-treated at 120℃had higher latent heat than PCM A.They were 143.6 and 97.42 J/g respectively.These experiment results indicate that the PCM B containing 1wt%MWCNTs is more suitable for hothouse application.
greenhouse;phase change materials;sodium sulfate decahydrate;thermal storage performance
10.11975/j.issn.1002-6819.2016.06.031
S214.3;S625.5+1;O792;TB34
A
1002-6819(2016)-06-0226-06
2015-10-20
2016-01-13
青海省国际合作资助项目(2014-HZ-820);青海省重点实验室发展专项资金(2014-Z-Y31,2015-Z-Y02)
柳 馨,女(满),辽宁本溪人,主要从事相变储能技术研究。西宁 青海大学新能源光伏产业研究中心,810016。Email:qhuliuxin@163.com※通信作者:铁生年,男,青海西宁人,教授,主要从事新能源储能材料研究。西宁 青海大学新能源光伏产业研究中心,810016。
Email:tieshengnian@163.com
