艾塞那肽联合门冬胰岛素控制血糖疗效分析
2016-05-14杨智勇
杨智勇
[摘要] 目的 分析肥胖症合并2型糖尿病的确诊患者通过应用艾塞那肽注射液联合门冬胰岛素注射液控制血糖和体重指数的临床综合效果,及缩短血糖达标所需的时间。 方法 对我院2014年6月~2015年6月确诊为2型糖尿病且体重指数(BMI)>28 kg/m2的住院患者100例,随机分为对照组和观察组;两组基础治疗的方法均为门冬胰岛素注射液连续皮下注射日基础量,对照组在基础治疗的同时给予阿卡波糖片50 mg,每日3次,餐时嚼服;观察组在基础治疗的基础上应用艾塞那肽注射液5 μg皮下注射每日2次。两组均给予监测指端血糖每日8次。患者住院治疗30 d后观察血糖总体控制情况,综合评估疗效。 结果 观察组的治疗后血糖控制总体优于对照组,差异有统计学意义(P<0.05);观察组糖化血红蛋白与体重指数治疗后均优于对照组,差异有高度统计学意义(P<0.01),两组均未发生严重低血糖病例,观察组的血糖达标时间较对照组显著缩短,差异有高度统计学意义(P<0.01),观察组的胰岛素用量小于对照组,差异有高度统计学意义(P<0.01)。 结论 肥胖症合并2型糖尿病患者采用艾塞那肽注射液联合门冬胰岛素注射液控制血糖可使血糖达标率增高,体重指数稳中有降,血糖达标时间缩短,让患者最大限度受益,血糖达标的同时避免了体重增加的弊端,减少和防止了糖尿病并发症的发生,较口服阿卡波糖片联合门冬胰岛素注射液疗效显著,安全可靠,值得临床推广。
[关键词] 艾塞那肽注射液;门冬胰岛素;阿卡波糖片;2型糖尿病;胰岛素泵
[中图分类号] R587.1 [文献标识码] B [文章编号] 1673-9701(2016)06-0029-05
Effect analysis on exenatide combined with insulin aspart in blood glucose control
YANG Zhiyong
Department of Internal Medicine, Datong Huayang Hospital, Xining 810000, China
[Abstract] Objectives To analyze the clinical effect of exenatide injection combined with insulin aspart injection in controlling of blood glucose and weight indices and the reaching-standard time of blood glucose among 50 patients with obesity and type Ⅱ diabetes. Methods A total of 100 patients diagnosed as type Ⅱ diabetes with BMI >28 kg/m2 treated in our hospital from June 2014 to June 2015 were selected and randomly divided into the control group and the observation group. Patients in both groups were treated by daily insulin aspart injection continuous and subcutaneous basal therapy. Patients in the control group were additionally given acarbose tablet 50 mg, tid, chewing at meals; while patients in the observation group were additionally given exenatide subcutaneous injection, 5 μg, bid. Blood glucose of patients in both groups were monitored through finger tips for eight times per day. The overall control of blood glucose and curative effect were assessed after 30 days of hospitalization. Results The blood glucose control in general of observation group after treatment was better than the control group, the difference was statistically significant(P<0.05); glycosylated hemoglobin and body mass index (BMI) of observation group were better than control group after treatment, the difference was statistically significant(P<0.01), two groups were not cases of severe hypoglycemia, the time of blood glucose control of observation group was significantly shortened, the difference was statistically significant(P<0.01), the insulin dosage of observation group was less than the control group, the difference was statistically significant(P<0.01). Conclusion Exenatide subcutaneous injection combined with insulin aspart injection can increase the rate of reaching the standard of blood glucose among patients with obesity and type Ⅱ diabetes, and can keep the weight of patients stable with a slight decline, shorten the reaching-standard time, and benefit the patients to the maximum limit that effectively controlling blood glucose and avoiding gaining weight, as well as reducing the incidence of diabetic complications. Compared with oral administration of acarbose tablet combination of insulin aspart injection has more significant effectiveness and accredited safety, which is worthy to be promoted.
[Key words] Exenatide injection; Insulin aspart; Acarbose tablets; Type Ⅱ diabetes; Insulin pump
2型糖尿病是常见的代谢性疾病,我国糖尿病患者数目居世界首位,糖尿病给我国造成的的经济负担也逐年上升[1]。其主要发病原因为遗传与环境因素,胰岛素抵抗和β细胞功能缺陷、胰岛素α细胞功能异常和胰高血糖样肽-1(GLP-1)分泌缺陷。依据2型糖尿病的发病病因,补充外源胰岛素和GLP-1受体激动剂是有效的。为了更好的控制2型糖尿病患者的血糖,减少2型糖尿病患者的并发症的发生、发展在临床治疗过程中十分重要,本研究通过应用艾塞那肽注射液5 μg,每日2次,皮下注射,联合应用门冬胰岛素注射液连续皮下持续泵入基础量治疗控制血糖和体重指数的临床综合效果,现报道如下。
1 资料与方法
1.1 一般资料
选取2014年6月~2015年6月我院确诊为2型糖尿病并且体重指数>28 kg/m2的患者100例,在患者了解了实验全过程并且在知情同意的情况下实施,符合1999年WHO诊断及分型标准,FPG>11.1 mmol/L,年龄20~65岁,男性腰围≥85 cm,女性腰围≥80 cm;糖化血红蛋白(HbA1c)为8%~12%,排除标准:(1)1型糖尿病,妊娠糖尿病,糖尿病急性并发症。(2)严重肝、肾功能异常,既往有急慢性胰腺炎病史的患者。(3)有肿瘤病史。观察组男女比例为2.34∶1;平均年龄为(48.7±1.6)岁。对照组男女比例为2.31∶1;平均年龄为(48.5±1.8)岁。两组患者的一般情况无明显差异(P>0.05)。
1.2 治疗方法
两组患者均每日监测8次血糖(三餐前、三餐后2 h、睡前、凌晨3时)及HbA1c,两组均予以规范化、系统化糖尿病宣教,并严格控制饮食以及运动疗法,基础治疗均为基础量门冬胰岛素注射液(英文名:Insulin Aspart Injecion;国药准字:J20100123;进口药品注册标准:JS20100088;企业名称:Novo Nordisk A/S;生产地址:Novo Alle,DK-2880 Bagsvaerd,Denmark;规格:3 mL∶300单位(笔芯)持续皮下泵入,选用PH300胰岛素泵。观察组在基础治疗上加用艾塞那肽注射液(英文名称:Exenatide injection;生产厂家:Baxter Pharmaceutical Solution LLC.;执行标准:JX20080117;进口药品注册证号:5 μg(0.25 mg/mL,1.2 mL/支)的进口药品准字号:H20130434;规格:5 μg剂量刻度注射笔:0.25 mg/mL,1.2 mL/支,单次注射药量5 μg,内含60次注射的药量)5 μg/次,每日2次,对照组为在基础治疗上口服阿卡波糖50 mg,每日3次。两组治疗30 d为1个疗程,设为观察点,观察临床症状改变、空腹血糖、餐后2 h血糖、血糖达标时间、糖化血红蛋白、胰岛素用量、BMI值。
1.3 观察指标
使用华益精点血糖仪(型号ZE-808)检测末梢指间血糖8次/d,三餐前血糖、三餐后2 h血糖、睡前血糖、凌晨3时血糖及HbA1c、胰岛素用量、BMI值治疗前后的变化。
1.4 评定标准
本次实验中正常血糖的判断标准为:血糖控制目标以2011年美国糖尿病协会(ADA)指南:空腹血糖控制于(3.9~7.2)mmol/L,餐后血糖控制在10 mmol/L以下[2]。低血糖的判定标准为:出现低血糖的症状,血糖测定值为<3.9 mmo1/L,依据治疗后血糖波动范围,评估治疗2型糖尿病的疗效。
1.5 统计学方法
采用SPSS 19.0统计学软件处理,计量资料采用t检验,计数资料组间对比采用χ2检验,P<0.05为差异有统计学意义。
2 结果
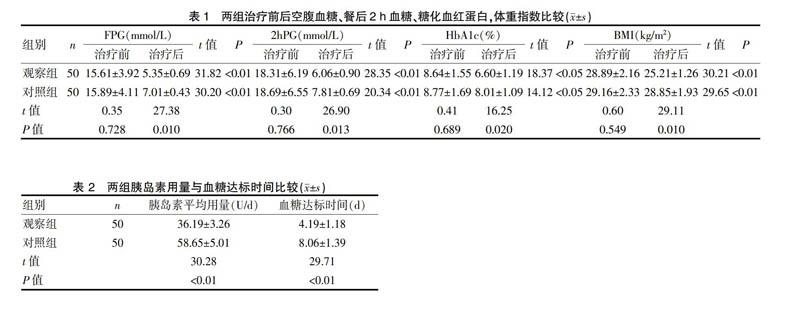
2.1 两组治疗前后空腹血糖、餐后2 h血糖、糖化血红蛋白,体重指数比较
分析治疗前后三餐血糖、HbA1c、胰岛素用量、BMI的变化,结果显示两组治疗前FPG、2 hPG及HbA1c、体重指数,差异均无统计学意义(P>0.05),观察组治疗后血糖控制总体优于对照组,差异有统计学意义(P<0.05);观察组糖化血红蛋白与体重指数治疗后均优于对照组,差异有统计学意义(P<0.05),说明艾塞那肽注射液联合门冬胰岛素注射液控制血糖的同时可以很好的控制体重。见表1。
2.2 两组胰岛素用量与血糖达标时间比较
两组均未发生严重低血糖病例,观察组的血糖达标时间较对照组显著缩短,差异有高度统计学意义(P<0.01),观察组的胰岛素用量小于对照组,差异有高度统计学意义(P<0.01),提示观察组胰岛素用量低,血糖控制达标时间短,速度快。见表2。
3 讨论
糖尿病以血糖持续升高为特征,患病率逐年上升。随着经济的发展及社会老龄化,糖尿病是继心脑血管疾病、癌症后的又一严重危害人民健康的慢性非传染性疾病[3]。2型糖尿病是非胰岛素依赖性糖尿病,与遗传因素、肥胖、饮食、生活 环境、胰岛素分泌障碍等因素有关[4]。同时也与胰岛素拮抗激素分泌失调、胰岛素抵抗、神经内分泌等多种因素有关。2型糖尿病的发病机制是:遗传因素;肥胖和不健康的生活方式;原发性胰岛素分泌不足及胰岛素拮抗激素分泌失调;胰 岛素抵抗。多种因素导致血糖升高和胰岛素的分泌量相对不足,形成恶性循环[5], 最终导致心血管系统、神经系统、泌尿生殖系统等多个系统出现并发症,严重者可导至死亡[6]。肥胖是2型糖尿病的独立危险因素,大多数的2型糖尿病患者伴有超重或肥胖[7]。随着生活方式的改变,目前2型糖尿病合并肥胖的患者逐年增加,这类患者血糖控制难度较大,在口服降糖药降糖效果不佳时,会用胰岛素控制血糖,同时可能使得体质量增加,因此,2型糖尿病合并肥胖症的血糖控制是糖尿病治疗中的难题[8-10]。
艾塞那肽注射液可显著降低血糖,并且能持续降低体重[11,12]。胰高糖素样肽 1(glucagon-ike peptide-1,GLP-1)是小肠黏膜 L 细胞在食物刺激下释放的激素,可促进葡萄糖依赖的胰岛素分泌[13,14]。艾塞那肽作用机制有:促进葡萄糖依赖的胰岛素分泌;抑制高血糖时胰升糖素的过度分泌;延缓胃排空;降低食欲,减少食物摄入;增加外周组织细胞,骨骼肌细胞对胰岛素敏感性;降低胰岛素抵抗,增加肝糖原储存[15]。在骨骼肌和脂肪细胞中,艾塞那肽通过激活磷脂酞肌醇-3一激酶途径增加葡萄糖摄取,改善外周组织对胰岛素的敏感性,促进胰岛β细胞增生、刺激β细胞新生和抑制β细胞凋亡,从而增加胰岛β细胞数量[16,17]。艾塞那肽可诱导胰腺导管上皮细胞、腺细胞和Nestin阳性导管细胞(潜在的多能胰岛干细胞)向胰岛素分泌细胞分化,也能促进胰腺十二指肠同源框基因(pancre-aticduodenal homeobox-1,PDX-1)的表达,PDX-1是胰腺内分泌细胞功能发育的必需因子,从而促进β细胞的新生和增殖,增加胰岛素分泌[18]。肝脏有艾塞那肽的同源性受体,艾塞那肽可通过抑制磷酸肌醇依赖性激酶1、蛋白激酶C等的磷酸化,降低肝脏的脂肪变性,保持肝脏正常功能。GLP-1在进食脂肪和碳水化合物后由空肠、回肠、结肠的L细胞分泌的一种肠降糖素,经由内分泌、神经 及底物刺激等途径作用于各种胰岛细胞,是具有包括调节血糖在内的多功能肽 类激素[19],因此,GLP-1类似物艾塞那肽在对2型糖尿病患者进行多靶点的治疗中就显得至关重要。艾塞那肽和诺和锐30均可在餐后迅速释放,但艾塞那肽可在有效控制高血糖的同时预防低血糖发生,这主要是由于艾塞那肽促胰岛素分泌呈完全血糖依赖性,此为艾塞那肽的独特优势[20],于此同时艾塞那肽还可抑制胰高血糖素的分泌,多方位控制血糖,同时延缓胃排空,减少食物摄入,降低体重,降低了2型糖尿病并发症的发病危险程度[21-26]。而艾塞那肽还可恢复一相和二相胰岛素分泌,促进β细胞增生,降糖的同时使用艾塞那肽治疗还可以降低心血管风险因子,调节血脂、降低血压等多方面益处[27]。已有许多体外研究显示,GLP-1具有诱导β细胞增殖和分化的直接作用。对胰岛细胞系 INS-1,GLP-1具有生长因子样作用,其可以剂量依赖性地增加 DNA的合成,表达并增加 DNA的结合活性[28]。GLP-1还可以诱导2种胰腺导管细胞系分化为能合成并分泌胰岛素的细胞。有研究者发现,GLP-1可以使肥胖的高血糖大鼠出现胰岛的生长,β 细胞的增殖[29]。研究者认为,在调控胰岛β细胞增殖的调节网络中,GLP-1发出β 细胞增殖的启动信号,从而通过增加β 细胞数量来满足机体对胰岛素的需求。研究发现[30],糖尿病大鼠加用GLP-1 治疗后,其β 细胞的凋亡数量显著减少,作为凋亡标志的凋亡小体等也显著减少,说明GLP-1 除了具有促进细胞增殖的作用外,抑制凋亡也是其增加胰岛细胞数量的重要机制[31]。阿卡波糖是一种α葡萄糖苷酶抑制剂,研究显示,碳水化合物在进入胃肠道后,经由 α 糖苷酶裂解生成一系列单糖,从而被小肠迅速吸收,并引起机体血糖水平的上升。α-葡萄糖苷酶抑制剂通过竞争性抑制作用对小肠上皮细胞中的α 葡萄糖苷酶的活性进行抑制,减缓机体对于碳水化合物的消化和吸收,从而促进餐后血糖水平的降低[32]。α-糖苷酶抑制剂的主要机制是在小肠上段通过可逆性地抑制肠系,膜刷状缘的α-糖苷酶,延缓α-糖苷酶将多糖(如淀粉、寡糖等)分解为单糖(主要为葡萄糖),使葡萄糖的吸收减缓。这种抑制作用是可逆的,葡萄糖的吸收并未被阻断而是推迟,能够使餐后的高血糖下降,下餐前也不易出现低血糖,相当于“分餐”的作用。α-糖苷酶抑制剂也能使2型糖尿病患者餐后过高的胰岛素水平下降,甚至在一定程度上改善胰岛素敏感性。此外,α-糖苷酶抑制剂能推进大量未消化的碳水化合物抵达低位小肠,该部位富含产生胰高糖素样肽-1(glucagon-like peptide 1,GLP-1)的L细胞,因而能够刺激GLP-1分泌持续增加,刺激胰岛素释放,进而降低血糖浓度。双糖、低糖以及多糖的葡萄糖吸收,对 FBG、HbA1c具有一定程度的降低作用,而且可以较好地降低餐后血糖。阿卡波糖还具有多重获益,可改善脂质代谢,显著升高高密度脂蛋白胆固醇和降低甘油三酯[33,34]。
本结果表明,观察组的治疗后血糖控制总体优于对照组,观察组糖化血红蛋白与体重指数治疗后均优于对照组,两组均未发生严重低血糖病例,观察组的血糖达标时间较对照组显著缩短,观察组的胰岛素用量小于对照组,说明艾塞那肽注射液联合门冬胰岛素基础量泵入治疗可有效控制血糖达标,缩短血糖达标时间,不使体重增加,减轻胰岛素抵抗,避免了高胰岛素血症的发生,改善了血脂代谢,解除了糖毒性和脂毒性,防止了并发症的发生和发展。
综上所述,艾塞那肽注射液特有的葡萄糖依赖性促进胰岛素分泌可以避免低血糖的发生,综合疗效优于阿卡波糖片联合门冬胰岛素泵入治疗,是2型糖尿病合并有肥胖症患者的较好的治疗方法,值得临床应用及推广。
[参考文献]
[1] 高菡璐,兰莉,乔冬菊,等. 1998~2010年哈尔滨市市区慢性病流行趋势分析[J]. 中华疾病控制杂志,2012,16(5):396.
[2] American Diabetes Association. Standards of medical care indiabetes-2011[J]. Diabetes Care,2011,34(Suppl 1):S11-S61.
[3] Hauber A,E A Gale. Themarketin diabetes[J]. Diabetologia,2006,49(2):247-252.
[4] Corbett JA. Aminoguanidine,anovelin hibit or of nitricoxide formation,prevents diabetic vasculardys function[J]. Diabetes,1992,41(4):552-556.
[5] Lerario AC. Algorithm for the treatment of type 2 diabetes:A positionstatement of Brazilian Diabetes Society[J]. Diabetol Metab Syndr,2010,2(1):35.
[6] Elgzyri T. Basic Management of diabetes mellitus:Practicalguidelines[J]. Libyan J Med,2006,1(2):176-184.
[7] Yang W,Liu J,Weng J,et al. Prevalence of diabetes among men and women in China[J]. N Engl J Med,2010,362(12):1090-1101.
[8] Kahn SE,Haffner SM,Heise MA,et al. Glycemic durability of rosiglitazone, metformin or glyburide monotherapy[J]. N Engl J Med,2006,355(23):2427-2443.
[9] UK Prospective Diabetes Study (UKPDS) Group. Intensi-tive blood-glycemia control with sulphonylureas or insulincompared with conventional treatment and risk of complica-tions in patients with type 2 diabetes[J]. Lancet,1998,352(9131):837-853.
[10] Nathan DM,Buse JB,Davidson MB,et al. Management of hyper-glycemia in type 2 diabetes:A consensus algrithm for the initiation and adjustment of therapy. A consensus statement from the American Diabetes Association and the Europe Association for the study of diabetes[J]. Diabetes Care,2008,31(1):173-175.
[11] Davies MJ,Donneiiy R,Barnett AH,et al. Exenatide com-pared with long-acting insulin to achieve glycaemic control with minimal weight gain in patients with type 2 diabetes:results of the helping evaluate exenatide in patients with diabetes compared with long-acting insulin(HEELA)study[J]. Diabetes Obes Metab,2009,11(12):1153-1162.
[12] Sudhakaran C,Fathima M,Anjana RM,et al. Effectiveness of exenatide in Asian Indians in a clinical care setting[J]. Diabetes Technol Ther,2010,12(8):613-618.
[13] Giugliano D,Standl E,Vilsboll T,et al. Is the current therapeutic armamentarium in diabetes enough to control the epidemic and its consequences what are the currentshortcomings[J]. Acta Diabetol,2009,46(3):173-181.
[14] Nauck MA. Unraveling the science of incretin biology[J].Eur J Intern Med,2009,20(Suppl 2):S303-S308.
[15] Knauf C,Cani PD,Perrin C,et al. Brain glucagon-like peptide-1 increases insulin secretionand insulinresistance to favor hepatic glycogenstorage[J]. J Clin Invest,2005,115(12):3554-3563.
[16] 刘敏,荆丹青,白桦,等. 艾塞那肽替代胰岛素治疗2型糖尿病患者的临床分析[J]. 山西医科大学学报,2012, 43(1):47-51.
[17] Nielsen LL,Young AA,Parkes DG. Pharmacology of exenatide(synthetic exendin-4):A potential therapeutic for improved glycemic control of type 2 diabetes[J]. Regul Pept,2004,117(2):77-88.
[18] Feanny,MA Facansp,Ballian N,et al. PDX-1 expression is assoriated with islet proliferation in vitro and vi[J]. Jbiol Chem,2003,278(1):471-478.
[19] Schmidt WE,EG Siegel,W Creutzfeldt,et al. Glucagon-likepeptide-1b utnot glucagon-like peptide-2 stimulates insulinrelease fromisolatedratpancreatic islets[J]. Diabetologia,1985,28(9):704-707.
[20] Noyan-Ashraf MH. GLP-1R agonist liraglutideactivates cytoprotective pathways and improves outcomes after experimental myocardial in farction in mice[J]. Diabetes,2009, 58(4):975-983.
[21] Nauck MA. Unravelingth escience of incretin biology[J]. Am J Med,2009,122(suppl):S3-S10.
[22] Nystrom T. Effects of glucagon-like peptide-1onendo the lial function in type 2 diabetes patients with stable coronaryartery disease[J]. A m J Physiol Endocrinol Metab,2004,287(6):E1209-E1215.
[23] Webb DM,Wintle M,MaloneJ K,et al. Exenatide effects on glucose meta bolism and meta bolicdis or derscommon to over weight and obese patients with type 2 diabetes[J]. Drug Devel Res,2006,67:666-676.
[24] Nauck MA,Duran S,Kim D,et al. A comparison of twice dailyexenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfony lrea and met formin:An oninferiority study[J].Diabetologia,2007,50:259-267.
[25] Zinman B,Hoogwerf BJ,Garcia SD,et al. The effect of adding exenatide to a thiazolidine dionein suboptimally control led type 2 diabetes[J]. Ann Intern Med,2007,146:477-485 .
[26] Mack CM,Laugero KD,Liu Q,et al. The rapeutic applic ationso fincretin mimetics for metabolic diseases:Preclinical studies[J]. Drug Dev Res,2006,67:553-558 .
[27] Blonde L,Klein EJ,Han J,et al. Interim analysis of the effect sofexenatide treatment on A1C,weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes[J]. Diabetes Obes Metab,2006, 8:436-447.
[28] Stoffers DA,Kieffer TJ,Hussain MA,et al. Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeodomain protein IDX-1 and increas islet size in mouse pancreas[J]. Diabetes,2000,49(5):741-748.
[29] Vahl TP,Paty BW,Fuller BD,et al. Effects of GLP-1-(7-36)NH2,GLP-1-(7-37),and GLP-1-(9-36)NH2,on intravenous glucose tolerance and glucose-induced Insulin secretion in healthyhumans[J]. J ClinEndocrinol Metab,2003,88(4):1772-1779.
[30] Farilla L,Bulotta A,Hirshberg B,et al. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsivenese of freshly isolated human islets[J]. Endocrinology,2003,144(12):5149-5158.
[31] Wang Q,Li L,Xu E,et al. Glucagon-like peptide-1 regulates proliferation and apoptosis via activation of protein kinase B in pancreatic INS-1 beta cells[J]. Diabetologia,2004,47(3):478-487.
[32] 张银波. 阿卡波糖联合二甲双胍对2型糖尿病进行治疗的临床疗效观察[J]. 中国现代药物应用,2015,9(13):145.
[33] Gao HW,Xie C,Wang HN,et al. Beneficial metabolic effects of nateglinide versus acarbose in patients with newly-diagnosed type 2 diabetes[J]. Acta Pharmacol Sin,2007,28(4):534-539.
[34] 仇维芝. 拜糖平治疗2型糖尿病疗效观察[J]. 现代中西医结合杂志,2013,22(26):2877-2879.
(收稿日期:2015-09-21)
