Synthesis and Properties of 5-(3-Amino-1,2,5-oxadiazol-4-yl)tetrazol-1-ol and Its Ammonium and Hydroxylammonium Salts
2016-05-08ZHAILianjieFANXuezhongWANGBozhouHUOHuanLIYananBIFuqiangZHANGJunlin
ZHAI Lian-jie, FAN Xue-zhong, WANG Bo-zhou, HUO Huan, LI Ya-nan, BI Fu-qiang, ZHANG Jun-lin
(1. Xi′an Modern Chemistry Research Institute, Xi′an 710065, China; 2. State Key Laboratory of Fluorine & Nitrogen Chemicals, Xi′an 710065, China)
1 Introduction
Nitrogen-rich azole-based energetic salts are promising compounds that fulfill many requirements in the pursuit of HEDMs with higher performance and/or decreased sensitivity.Not only does the high nitrogen content of triazoles and especially tetrazoles bring the environmental benefit of releasing mainly N2after decomposition, it also increases the heat of formation due to inherently energetic C—N and N—N bonds contained in the molecule[1-4]. On the other hand, salt-based energetic materials often possess advantages of lower vapor pressure, higher density, and better thermal stability over nonionic molecules[5-7]. Additional, a further way of azole functionalization is the oxidation of the heterocycles to its corresponding N-oxides. For example, the tetrazoleN-oxides have been shown to usually result in an increased density, oxygen balance and performance of the energetic material in comparison with the corresponding nonoxidized tetrazoles, but at the same time also lower its sensitivity to mechanical friction (i.e., shock, impact)[8-9]. This type is strikingly represented by dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate. It has a rather high density of 1.87 g·cm-3, a detonation pressure of 42.4 GPa, a detonation velocity of 9698 m·s-1, and an impact sensitivity of 20 J, all of which are close to or superior to those of HMX[10].
In our continuing efforts to seek more powerful, less sensitive, eco-friendly and low-cost energetic materials, we were interested in some furazan-functionalized azolate-based (triazole, tetrazole and its N-oxides) salts that contain a high percentage of both oxygen and nitrogen. The promising properties of energetic salts of the 5-(3-amino-1,2,5-oxadiazol-4-yl)tetrazolate anion[11], attracted our attention to the 5-(3-amino-1,2,5-oxadiazol-4-yl)tetrazol-1-ol (4), which was reported by Tselinskii[12]. Compound 4 and its salts are expected to higher density and oxygen balance due to introduction of N-oxide group. Herein, we now report our results on the synthesis, full analytical and spectroscopic characterization, energetic properties and X-ray structures of nitrogen-rich salts of the 5-(3-amino-1,2,5-oxadiazol-4-yl)tetrazol-1-ol; the salts display significant physical properties relative to their precursor,compond 4, as shown in Scheme 1.
2 Experimental
2.1 Materials and Measurements
1H NMR,13C NMR, and15C NMR spectra were obtained in DMSO-d6with a Bruker AV500 NMR spectrometer. IR spectra were obtained from KBr pellets with a Nicolet NEXUS870 Infrared spectrometer in the range of 4000-400 cm-1. Elemental analyses were performed with a VARI-El-3 elementary analysis instrument. Differential scanning calorimetry (DSC) test was carried out in a platinum sample container using a Q-200 at a heating rate of 10 ℃·min-1. The impact sensitivity was determined by drop hammer apparatus, applying a standard method using a 2 kg drop weight. The friction sensitivity was determined using a Julius Peters apparatus.
All chemicals were obtained from commercial sources and used without further purification.
2.2 Synthetic Routes
Compounds 2-6 were prepared by the above-mentioned synthetic route (Scheme 1), in which compounds 2 and 3 were prepared with a high yield according to the literature procedure[13]

Scheme 1
2.3 Synthesis of 5-(3-Amino-1,2,5-oxadiazol-4-yl)tetrazol-1-ol(4)
4-Amino-1,2,5-oxadiazole-3-carbohydroximoyl chloride (3.25 g, 20 mmol) is dissolved in 20 mL of dimethylformamide at room temperature. The solution is cooled to 0 ℃ and NaN3(1.69 g, 26 mmol) is added in batches. After stirred for 30 min at 0 ℃, diethyl ether (150 mL) and water (80 mL) were added to the mixture, respectively. Then the diethyl ether solution was separated and cooled to 0 ℃ in a salt-ice bath. Gaseous HCl was bubbled through the diethyl ether reaction mixture at 0-5 ℃ while stirring for 2 hours and then the flask was sealed and stirred at room temperature over night. The precipitate was collected by filtration, washed with ice water and dried to yield compound 4 as white solid. After recrystallization from MeOH/H2O (V/V=1∶1), 3.03 g (89.8%) colorless crystals of compound 4 were obtained. DSC(5 ℃·min-1): 189 ℃(m.p.), 191 ℃ (dec.); IR(KBr,ν/cm-1): 3456, 3336, 1639, 1610, 1540, 1469, 1433, 1392, 1262, 1176, 1107, 1048, 988, 906, 860, 713, 612, 446;1H NMR (DMSO-d6, 500 MHz)δ: 7.36 (s, 2H, NH2), 7.06 (s, 1H, OH);13C NMR (DMSO-d6, 125 MHz)δ: 156.1, 138.0, 134.7;15N NMR (DMSO-d6, 50.6 MHz)δ: 30.9, -4.0, -18.3, -18.9, -57.4, -115.4, -337.8; Aual.Calcd. for C3H3N7O2(%) : C 21.31, H 1.79, N 57.98; Found, C 21.49, H 1.78, N 57.62.
2.4 Synthesis of Ammonium 5-(3-Amino-1,2,5-oxadiazol-4-yl)tetrazol-1-olate (5)
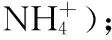
2.5 Synthesis of Hydroxylammonium 5-(3-Amino-1,2,5-oxadiazol-4-yl)tetrazol-1-olate (6)
A solution of compound 4 (0.85 g, 5 mmol) in a minimum amount of methanol was stirred at room temperature while adding hydroxylamine (50% in water, 0.26 mL, 5.2 mmol). After 1 h the precipitate was filtered and air-dried to yield compound 6 (0.94 g, 93%) as a colorless solid. DSC (5 ℃·min-1): 200 ℃ (dec.); IR (KBr,ν/cm-1): 3455, 3361, 3146, 2960, 1635, 1604, 1532, 1505, 1451, 1395, 1300, 1239, 1186, 1153, 985, 885, 769, 582;1H NMR (DMSO-d6, 500 MHz)δ: 10.12 (s, 4H, NH3OH+);13C NMR (DMSO-d6, 125 MHz)δ: 156.3, 137.8, 135.1; Aual.Calcd. for C3H6N8O3(%): C 17.83, H 2.99, N 55.44; Found, C 17.50, H 2.78, N 55.18.
2.6 Crystal Structure Determination
X-ray single-crystal diffraction (XRD) data for compounds 4, 5, and 6 were collected on a Bruker SMART Apex II CCD X-ray diffractometer equipped with a graphite-monochromatized MoKα radiation (λ= 0.71073 Å). The structures were solved either with SHELXS-97[14], refined with SHELXL-97[15]. The full-matrix least-squares refinement onF2included atomic coordinates and anisotropic thermal parameters for all non-H atoms. The H atoms were found and refined. CCDC-1444340 (for 4), -1444341 (for 5), -1444342 (for 6) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/ data_request/cif.
3 Results and Discussion
3.1 Multinuclear NMR Spectra
The structures of compounds 4, 5 and 6 its salts were investigated using1H and13C NMR spectra. Additionally,15N NMR spectra of compound 4 and 5 were recorded, and chemical shifts are given in Fig.1. The1H NMR spectra of compound 4 exhibits two sharp single peaks at 7.36 and 7.06 for the amine andN-hydroxy groups, respectively. For compound 5, all N-connected protons are visible as only one singlet, which appears around 7.1. In contrast to compound 5, an exceptionally high value ofδ= 10.12 is observed for compound 6. In the13C NMR spectrum, compound 4 shows three resonances at 156.1 for the furazan carbon bonded to NH2, 134.7 for the other furazan carbon and at 138.0 for 1-hydroxytetrazole. For the anionic species in 5-6, three single resonances atδ=134.5-135.8 are observed, which does not significantly differ from the signal of the neutral species 4. It should be mentioned that the13C NMR resonance of the tetrazole-oxide carbon is at a higher field than that of the 5-(3-amino-1,2,5-oxadiazol-4-yl)tetrazolate salts[11], which suggests the higher electron-donating ability of tetrazole oxide than that of tetrazolate.
Selected15N NMR spectra of compound 4, and 5 are shown in Fig. 1. The assignments are based on comparison with similar molecules in the literature. In the neutral compound 4, the tetrazole-N-oxide ring shows four well resolved resonances; the signals can be observed at shifts of -115.4 (N1), -18.9 (N2), -4.0 (N3), and -57.4 (N4), comparable with the signals of the similar 5,5′-bistetrazole-1,1′-diol[10]. The signals of the furazan nitrogen atoms at 30.9 (N5), and -18.3 (N6) can be found in both cases in the expected range similar to the recently published 3,4-bis(1-hydroxytetrazolyl)furazan[16]. In addition, the signals of the amino groups bonded to furazans can be assigned to the resonance peaks at highest field (δ≈-337) as a triplet due to NH coupling. Similar nitrogen shifts were observed in the ammonium salt. In the spectrum of compound 5, additional signals atδ=-360.5 for ammonium are visible, respectively.
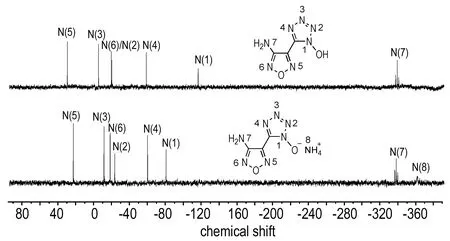
Fig.115N NMR spectra of compounds 4 and 5 in DMSO-d6
3.2 Crystal Structure
Compound 4·H2O could only be obtained in the crystalline form with inclusion of one crystal water molecule. It crystallizes in the monoclinic space groupP21/nwith four formula units in the unit cell and a density of 1.697 g·cm-3at 293 K. As shown in Fig.2, the 1-hydroxytetrazole ring and the furoxan ring are nearly planar with a dihedral angle between them of 2.4°. The C—N and N—N single and double bonds within the azole rings are all in the range of formal C—N and N—N single and double bonds (C—N: 1.47 Å, 1.22 Å; N—N: 1.48 Å, 1.20 Å)[17]. The N—O bond length [O(2)—N(7): 1.352(18) Å] of theN-hydroxy group is silghtly shorter than the N—O distances in the furazan ring [O(1)—N(3): 1.367 Å, O(1)—N(2): 1.404 Å]. These findings support the presence of a delocalizedπ-system in the five-membered ring of compound 4. Since the amino andN-hydroxy groups in compound 4 are excellent hydrogen-bonding donors, the discrete compound 4 are linked into a 2D double layer by the hydrogen-bonding interactions between different independent molecules [N(1)—H(1A)…N(2i): 3.109 Å; N(1)—H(1B)…O(2ii): 3.082 Å. Symmetry codes: i: -x-1, -y+1, -z+1; ii: -x+1/2, y+1/2, -z+3/2]. The 3D network of the structure is formed further by the hydrogen-bonding interactions from water molecules and adjacent layers [O(3)—H(3B)…N(5): 2.882 Å; O(2)—H(2)…O(3iii): 2.590 Å; O(3)—H(3B)…O(1iv): 3.151 Å. Symmetry codes: iii: x-1/2, -y+1/2, z-1/2; iv: x+3/2, -y+1/2, z+1/2] (Fig.3). It should be noted that one strong hydrogen bond is observed in the structure of compound 4 and its anion between the NH2group of the furazan ring and the nitrogen atom of theN-hydroxy tetrazole ring.
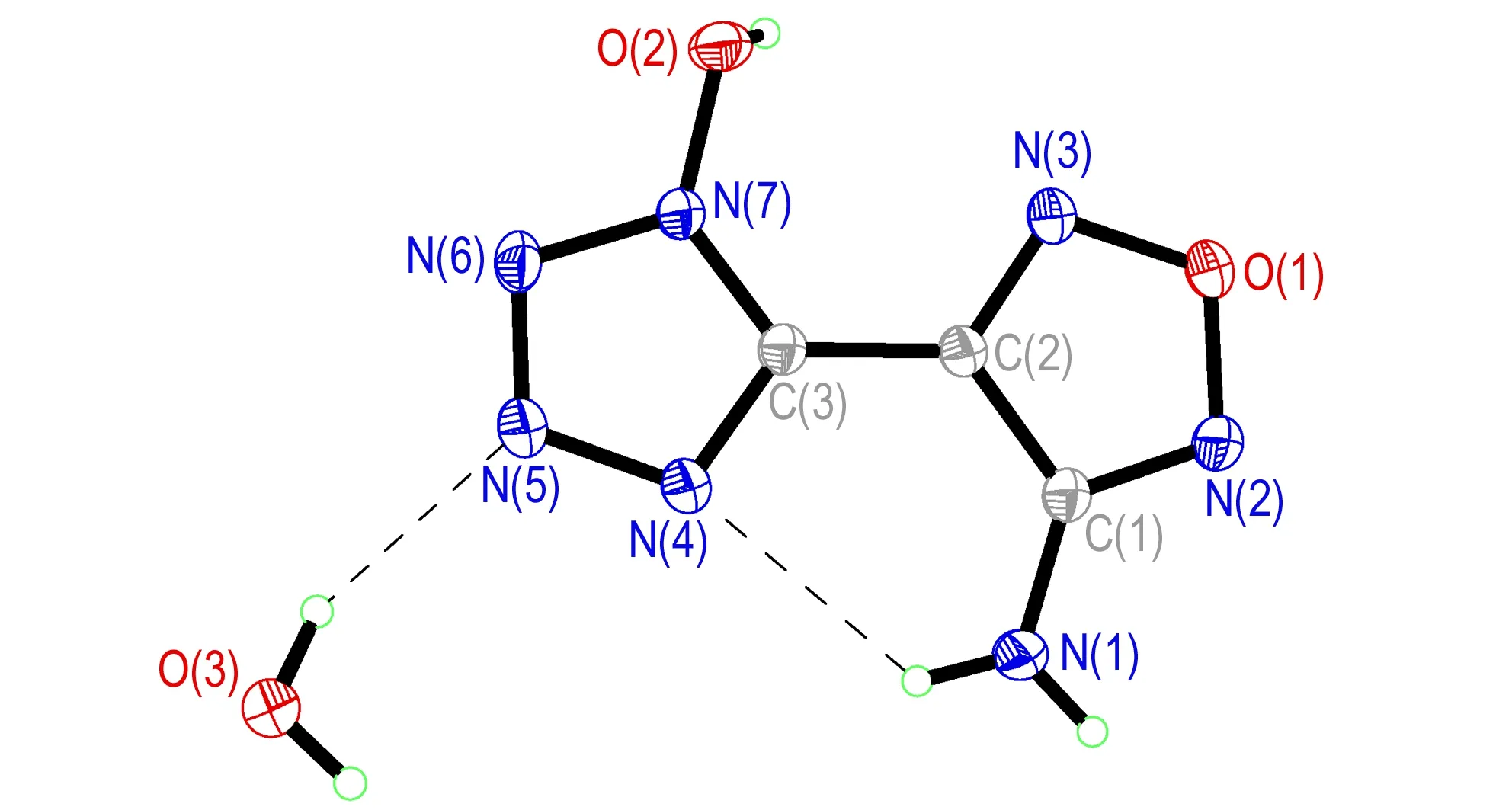
Fig.2 Crystal structure of compound 4·H2O with thermal ellipsoids at 30% probability
Ammonium 5-(3-amino-1,2,5-oxadiazol-4-yl)tetrazol-1-olate 5 crystallizes in the same space group as compound 4 with two formula units in the unit cell and a density of 1.643 g·cm-3. The transfer of the proton from the N-hydroxy tetrazole to amine was confirmed by the crystal data, as shown in Fig.4. The deprotonation of compound 4 yields significant changes in the bond lengths of the anion. As expected the N(1)—O(1) bond length is shortened by 0.03 Å, whereas the N(2)—N(3) distance is slightly elongated by 0.02 Å. The C(1)—N(1)—N(2) angle of theN-oxide anion has an value of 109.14(18)° as compared to 110.11(14)° for the neutral compound 4. The structure is strongly influenced by the formation of numerous hydrogen bonds and all hydrogens of compound 5 participate in hydrogen bonds (Fig.5). Each ammonium cation in compound 5 forms four significant hydrogen bonds with three N-oxide oxygen atoms and one nitrogen atom of the surrounding tetrazole-oxide rings. The donor-acceptor distances in the hydrogen bonds are all under 3.0 Å (2.768-2.899 Å) and are directed with D—H…A angles in the ragne of 161.1°-166.1°. They are therefore again more of a directed rather than only of an electrostatic nature.

Fig.3 View along thebaxis showing the two different 2D layers of the molecules (layer distanced=3.14 Å) that are connected by hydrogen bonds from water molecules
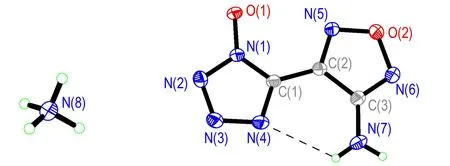
Fig.4 Crystal structure of compound 5 with thermal ellipsoids at 30% probability
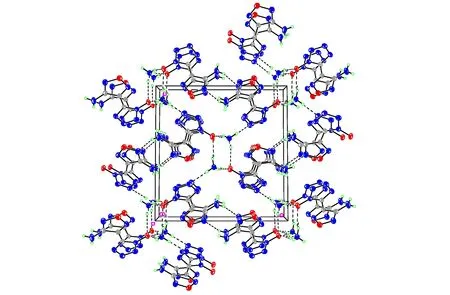
Fig.5 View of the unit cell of compound 5 along theaaxis showing the hydrogen bonding in the structure
Compound 6 crystallizes in the monoclinic space groupP21/cwith four formula units in the unit cell. The calculated density at 296 K is 1.786 g·cm-3, which is notably higher than the corresponding ammonium salt of compound 4 (1.643 g·cm-3). Interestingly the tetrazole-oxide ring and the furazan ring in compound 4 anions are distinctly twisted with the dihedral angle of 17.36° (Fig.6), while other anion in the salts are nearly in one plane, including theN-oxide oxygen atom and the NH2nitrogen atom. As shown in Fig.6, all hydrogens of compound 6 participate in hydrogen bond formation. The anions and hydroxylammonium cations are arranged in wave-like layers by seven hydrogen bonds along theb-axes. Five of the hydrogen-bond lengths lie well within the sum of the van der Waals radii [rw(N)+rw(N)=3.20 Å,rw(O)+rw(N)=3.07 Å] with a D…A length between 2.783 Å and 3.057 Å, but they are not strongly directed, with D—H…A angles between 112° and 151°. Finally, these wave-like 2D layers are linked to form the 3D network structure via two hydrogen bonds [N(8)—H(8A)…O(1): 2.740 Å; N(8)—H(8B)…N(4i): 3.157 Å. Symmetry codes: i: x-1, y, z-1] (Fig.7). The numerous intramolecular and intermolecular hydrogen bonds in compound 6, especially the hydrogen bond N(8)—H(8A)…O(1i) (2.740 Å) strongly directed with a small D—H…A angle of 175°, could explain the remarkably high density.
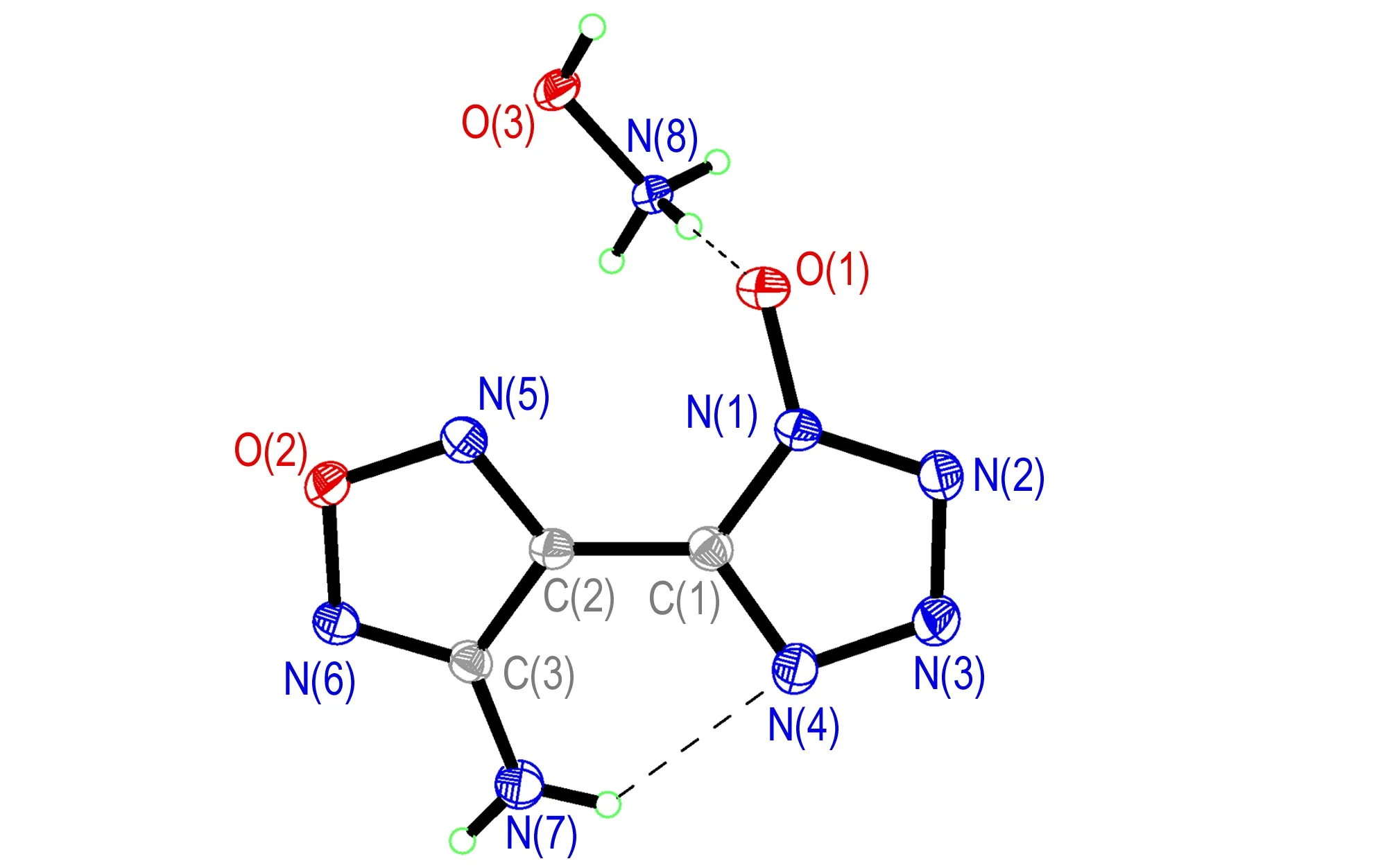
Fig.6 Crystal structure of compound 6 with thermal ellipsoids at 30% probability

Fig.7 Molecular packing diagram of compound 6
3.3 Thermal Behavior
The thermal behavior of compounds 4-6 is determined by DSC at a heating rate of 10 ℃·min-1and depicted in Fig.8. The neutral compound 4 has a high melting point (Tm=189 ℃) and low thermal decomposition temperature (Td=191 ℃). Compositions 5 and 6 exhibit excellent thermal stabilities with thermal decomposition temperatures (Td) in 257 ℃ and 200 ℃ respectively, which are comparable to that of 1,3,5-trinitro-1,3,5-triazinane (RDX) (Td=210 ℃). Compound 5 is the most thermally stable (Td=257 ℃), which most likely arises from four strong hydrogen bonds with distances below 2.9 Å between the cations and anions. For the neutral compound 4 its DSC curves show a small endothermic peak at 67 ℃ respectively, which corresponding to the loss of crystal water.
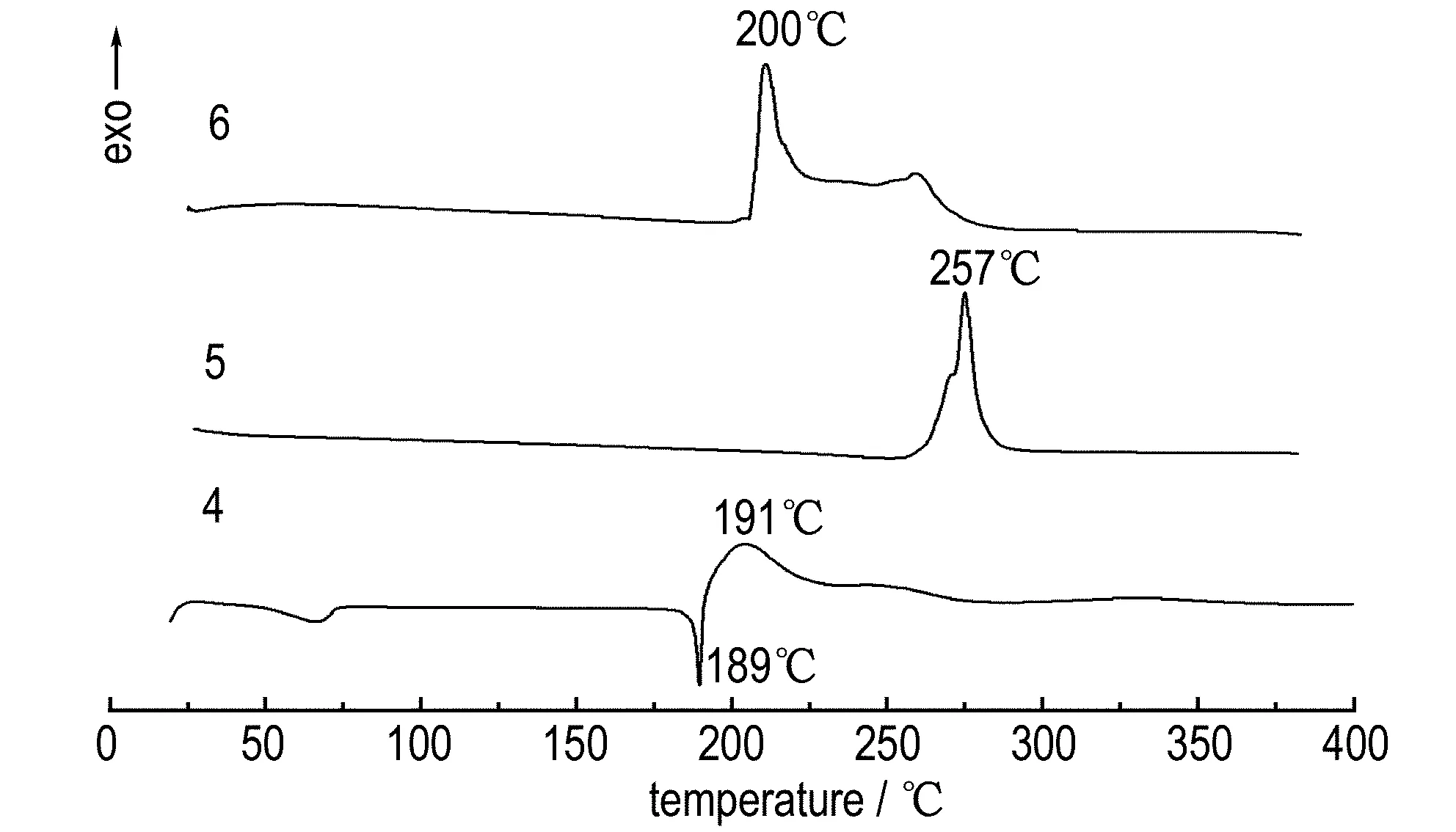
Fig.8 DSC curves of compounds 4-6 at a heating rate of 10 ℃·min-1
3.4 Sensitivities
The impact sensitivities of compounds 5 and 6 are more than 24 J, and both of them are less sensitive to impact than the parent molecule 4 (19 J), possibly due to the extensive hydrogen-bonding interactions between the cations and anions after salt formation. Friction sensitivities of compounds 4-6 are greater than 360 N. These facts show that compounds 5 and 6 are quite insensitive explosives.
4 Conclusion
5-(3-Amino-1,2,5-oxadiazol-4-yl)tetrazol-1-ol and its ammonium salt, hydroxylammonium salt were synthesized and fully characterized. Their structures were confirmed by XRD. In these salts there are extensive hydrogen-bonding interactions between the cations and anions. The intramolecular and intermolecular hydrogen bonds play a pivotal role on their molecular density, thermal stability, and sensitivities. Compared to its parent compound 4, compounds 5 and 6 have low impact sensitivity (>24 J) and good thermal stabilities (200-257 ℃). The most promising compound is compound 6, which possesses low sensitivities, excellent thermal stability and satisfactory density. Because the parent compound 4 and its salts are easily prepared including only four cheap and facile steps and are chemically and thermally stable compounds with relatively good energetic properties, compounds 4-6 can be used as environmentally friendly and new-generation insensitive energetic materials.
[1] GAO Hai-xiang, Shreeve J M. Azole-based energetic salts[J].ChemicalReviews, 2011, 111(11): 7377-7436.
[2] Fischer D, Klapötke T M, Stierstorfer J. Potassium 1,1′-dinitramino-5,5′ -bistetrazolate: a primary explosive with fast detonation and high initiation power[J].AngewandteChemieInternationalEdition, 2014, 53(31): 8172-8175.
[3] HUANG Hai-feng, ZHOU Zhi-ming, LIANG Li-xue, et al. Nitrogen-rich energetic monoanionic salts of 3, 4-bis(1H-5- tetrazolyl)furoxan[J].Chemistry-AnAsianJournal, 2012, 7(4): 707-714.
[4] LIU Xiang-yu, GAO Wen-juan, SUN Pan-pan, et al. Environmentally-friendly high-energy MOFs: crystal structure, thermostability, insensitivity and remarkable detonation performance[J].GreenChemistry, 2015, 17(2): 831-836.
[5] LIN Qiu-han, LI Yu-chuan, QI Cai, et al. Nitrogen-rich salts based on 5-hydrazino-1H-tetrazole: a new family of high-density energetic materials[J].JournalofMaterialsChemistry, 2013, 1(23): 6776-6785.
[6] LIANG Li-xuan, HUANG Hai-feng, WANG Kai, et al. Oxy-bridged bis(1H-tetrazol-5-yl)furazan and its energetic salts paired with nitrogen-rich cations: highly thermally stable energetic materials with low sensitivity[J].JournalofMaterialsChemistry, 2012, 22(41): 21954-21964.
[7] Klapötke T M, Mayr N, Stierstorfer J, et al. Maximum compaction of ionic organic explosives: bis(hydroxylammonium) 5,5′-dinitromethyl-3,3′-bis(1,2,4-oxadiazolate) and its derivatives[J].Chemistry-AEuropeanJournal, 2014, 20(5): 1410-1417.
[8] Göbel M, Karaghiosoff K, Klapötke T M, et al. Nitrotetrazolate-2N-oxides and the strategy of N-oxide introduction[J].JournaloftheAmericanChemicalSociety, 2010, 132(8): 17216-17226.
[9] Dippold A A and Klapötke T M. A study of dinitro-bis-1,2,4-triazole-1,1′-diol and derivatives: design of high-performance insensitive energetic materials by the introduction of N-oxides[J].JournaloftheAmericanChemicalSociety, 2013, 135(26): 9931-9938.
[10] Fischer N, Fischer D,Klapötke T M, et al. Pushing the limits of energetic materials-the synthesis and characterization of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate[J].JournalofMaterialsChemistry, 2012, 22(38): 20418-20422.
[11] WANG Rui-hu, GUO Yong, ZENG Zhou, et al. Furazan-functionalized tetrazolate-based salts: a new family of insensitive energetic materials[J].Chemistry-AEuropeanJournal, 2009, 15(11): 2625-2634.
[12] Tselinskii I V, Mel′nikova S F, and Romanova T V. Synthesis and reactivity of carbohydroximoyl azides: II 4-substituted 1,2,5-oxadiazole-3-carbohydroximoyl azides and 1-hydroxy-5-(4-R-1,2,5-oxadiazol-3-yl)tetrazoles[J].RussianJournalofOrganicChemistry, 2001, 37(11): 1638-1642.
[13] Martinez H, Zheng Zhao-Yun, Dolbier Jr. R. D. Energetic materials containing fluorine. Design, synthesis and testing of furazan-containing energetic materials bearing a pentafluorosulfanyl group[J].JournalofFluorineChemistry, 2012, 143(10): 112-122.
[14] Sheldrick G M. SHELXS-97, Program for crystal structure solution[CP], University of Göttingen, Germany 1997.
[15] Sheldrick G M. SHELXL-97, Program for crystal structure refinement[CP], University of Göttingen, Germany 1997.
[16] Fischer D, Klapötke T M, Reymann M, et al. Energetic alliance of tetrazole-1-oxides and 1,2,5-oxadiazoles[J].NewJournalofChemistry, 2014, 8(3): 1619-1627.
[17] Allen F H, Kennard O, Watson D G, et al. Tables of bond lengths determined by X-Ray and neutron diffraction. Part I. bond lengths in organic compounds.JournalofChemicalSociety,PerkinTransactionsII, 1987, 12, 1-19.
杂志排行
含能材料的其它文章
- Crystal Structure and Thermal Behavior of Potassium Dinitromethane
- Coatings of Activated Metal Hydride and Application in the Fuel-rich Propellant
- An Insensitive Energetic Compound 5,7-Diamino-4,6-dinitrobenzotriazol-3-ium-1-oxide: Synthesis, Characterization and Performances
- The Tensile Properties and Creep Performance of a Long-term Thermally Aged Plastic Bonded Explosive
- Energies of Combustion and Specific Heat Capacities of Diaminofurazan, Dinitrofurazan and Diaminoazofurazan
- Thermal Behaviors of 1-Amino-2-nitroguanidine
