An Insensitive Energetic Compound 5,7-Diamino-4,6-dinitrobenzotriazol-3-ium-1-oxide: Synthesis, Characterization and Performances
2016-05-08HUOHuanWANGBozhouZHAILianjieBIFuqiangDONGJunLIANPeng
HUO Huan, WANG Bo-zhou, ZHAI Lian-jie, BI Fu-qiang, DONG-Jun, LIAN Peng
(Xi′an Modern Chemistry Research Institute, Xi′an 710065)
1 Introduction
In the late decade, efforts to improve the safety and survivability of munitions led to the concept of insensitive munitions. Recently, 1,2,3-triazole becomes an interesting structural framework found in all kinds of energetic materials and medicament[1-4]. In addition,N-oxides have been widely studied in the field of energetic materials. The introduction of N-oxides to the energetic heterocyclic compounds has been paid much more attentions owing to their unique characteristics, such as high crystal density and low sensitivities[5-6]. For example, 5,7-diamino-4,6-dinitrobenzotriazol-3-ium-oxide was identified as a kind of the potential high-performance insensitive explosive and has some desirable traits, including a high density (1.76 g·cm-3), a low impact sensitivity (20 J), and a low friction sensitivity (>360 N). So it can be used as a main insensitive energetic component of explosives and propellants to substitute TATB, RDX or HMX[7].
In the literature [7] , using 1,1,1-trimethylhydrazinium iodide (THMI) as a vicarious nucleophilic substitution (VNS) reagent, 5,7-diamino-4,6-dinitrobenzotriazol-3-ium-1-oxide(2) was synthesized from 4,6-dinitrobenzotriazol-3-ium-1-oxide (1) with a yield of 64% in dimethylsulfoxide. Because THMI decomposes easily, it requires being prepared from two expensive reagents unsymmetric dimethylhydrazine and iodomethane. So that VNS reaction process was firstly improved in this paper. Using cheaper NH2OH·HCl as a substitute for THMI, the title compound 2 was synthesized with a similar yield (63.2%) in water, which brought on a lower reaction cost. In addition, the cyclization mechanism of the picryl chloride and hydrazine hydrate was discussed. The detonation performances, thermal behaviors, electrostatic potential (ESP), and molecular orbital of the compound 2 were studied.
2 Experimental
2.1 Materials and Instruments
Picryl chloride was prepared and purified by Xi'an Modern Chemistry Research Institute, and other reagents were purchased from the commercial sources.1H NMR and13C NMR were obtained in DMSO-d6on a Bruker AV500 NMR spectrometer. Infrared spectra were obtained from KBr pellets on a Nicolet NEXUS870 Infrared spectrometer in the range of 4000-400 cm-1. Elemental analyses (C, H and N) were performed on a VARI-El-3 elemental analyzer.
2.2 Synthesis and Characterization
According to the reference [7], using a cheaper VNS reagent NH2OH·HCl as a substitute for the expensive THMI, the title compound 2 was synthesized via cyclization and VNS with picryl chloride and hydrazine hydrate as the starting materials (Scheme 1). Total yield is 31.0%.
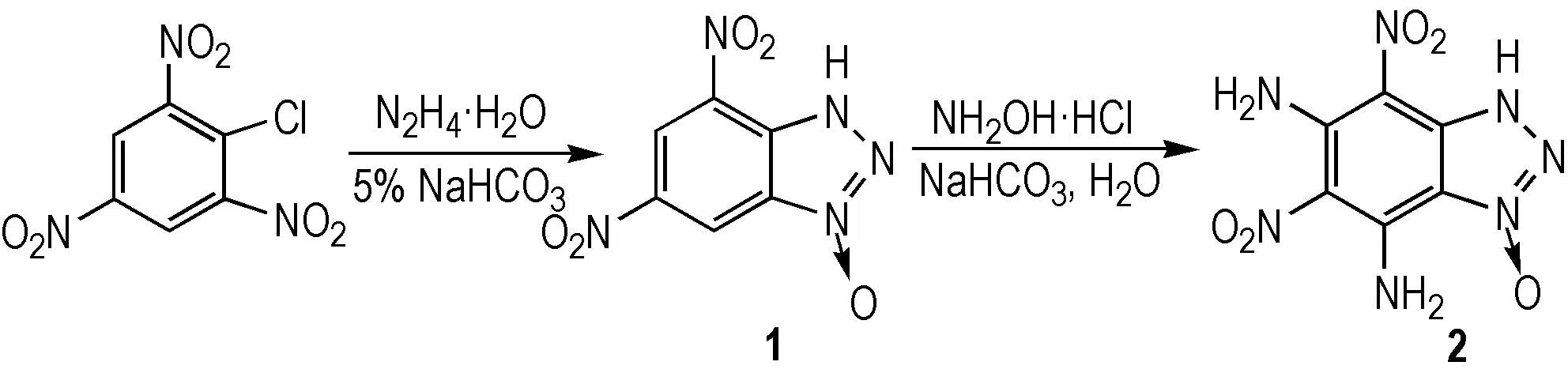
Scheme 1 Synthetic route for the title compound
2.2.1 Synthesis of Compound 1
Hydrazine hydrate (0.75 mL, 15.46 mmol) and 80 mL 5% aqueous sodium hydrogen carbonate solution were mixed in a three-necked round-bottomed flask with a stirrer at 0 ℃. To the reaction mixture, picryl chloride (0.738 g, 2.98 mmol) was added in one portion. The solution was stirred for 20 min at 0 ℃ and then for 2 h at ambient temperature. After heating for 1 h at 60 ℃, activated charcoal (1 g) was added to the hot solution. It was filtered hot, cooled to 0 ℃ and acidified to pH 2 with conc. hydrochloric acid. Then the solvent was removed in vacuo and the residue was adequately dissolved with acetic ester. The solvent was filtered and the filtrate was concentrated in vacuum. The 0.4 g orange precipitate was obtained with a yield of 55.3% and a purity of 98.7% (HPLC). DSC (10 ℃· min-1):Tdec=201.3 ℃. IR(KBr,ν/ cm-1):3105(NH), 2654,2296, 2230, 2142, 1783, 1703, 1641, 1434(triazole), 1557, 1368(NO2),1542,1506, 1434, 1383, 1337, 1278, 1245, 1189, 1184,1176, 1156, 1055, 982, 934, 916, 895, 884, 827, 805, 769, 752, 734, 723, 704.1H NMR (DMSO-d6, 500 MHz): 9.103 (d,J=2 Hz, 1H, CH), 8.885 (d,J=2 Hz,1H, CH).13C NMR (DMSO-d6, 125 MHz): 144.983, 137.457, 136.783, 130.207, 117.539(CH), 115.188 (CH). Anal.Calcd. for C6H3N5O5(%): C 32.01, H 1.34, N 31.11; Found: C 32.12, H 1.48, N 31.06.
2.2.2 Synthesis of compound 2
Sodium hydrogen carbonate (1.11 g, 13.3 mmmol), compound 1 (0.6 g,2.66 mmol) and 40 mL water were mixed in a three-necked round-bottomed flask with a stirrer. To the reaction mixture, hydrochloric hydroxylamine (0.74 g, 10.64 mmol) was added in one portion. The solution was stirred for 5 h at 20 ℃. Then cooled to 0 ℃ and 12 mL 4 N sodium hydroxide solution was added dropwise. After stirring for 2 h at 0 ℃, the solution was acidified to pH 2 by 2 N hydrochloric acid. The orange precipitate was filtered and 0.43 g solid was obtained with a yield of 63.2% and a purity of 98.7% (HPLC). DSC: (10 ℃·min-1):Tdec=248.5 ℃. IR (KBr,ν/cm-1): 3414, 3363 (NH2), 3245(NH), 1611, 1445(triazole), 1544, 1384(NO2), 1290, 1258, 1235, 1194, 1165, 1105, 974, 883, 817, 773, 728, 691, 669;1H NMR(DMSO-d6, 500 MHz):9.784-10.358(m, 4H, NH2);13C NMR(DMSO-d6, 125 MHz):149.73, 145.686, 144.32, 132.00, 115.28, 111.37; Anal.Calcd. for C6H5N7O5(%): C 28.24, H 1.98, N 38.43; Found C 28.33, H 2.00, N 38.04.
3 Results and Discussion
3.1 Mechanism of Triazol-3-ium-1-oxide Cyclization
One reasonable and possible mechanism is brought forward in Scheme 2. Firstly, the bond C—Cl on picryl chloride was activated by the adjacent nitro groups, and the C atom was attacked by hydrazine hydrate to form the intermediate Ⅰ. Then it is proposed that the intermediate Ⅱ was formed via base-promoted intramolecular amino-nitro nucleophilic addition with the elimination of a H2O molecular. This intermediate undergoes an electron shift to produce intermediate Ⅲ, followed by formation of the NN bond. Finally, the target compound 5,7-diamino-4,6-dinitrobenzotriazol-3-ium-oxide was obtained by acidified with the hydrochloric acid.
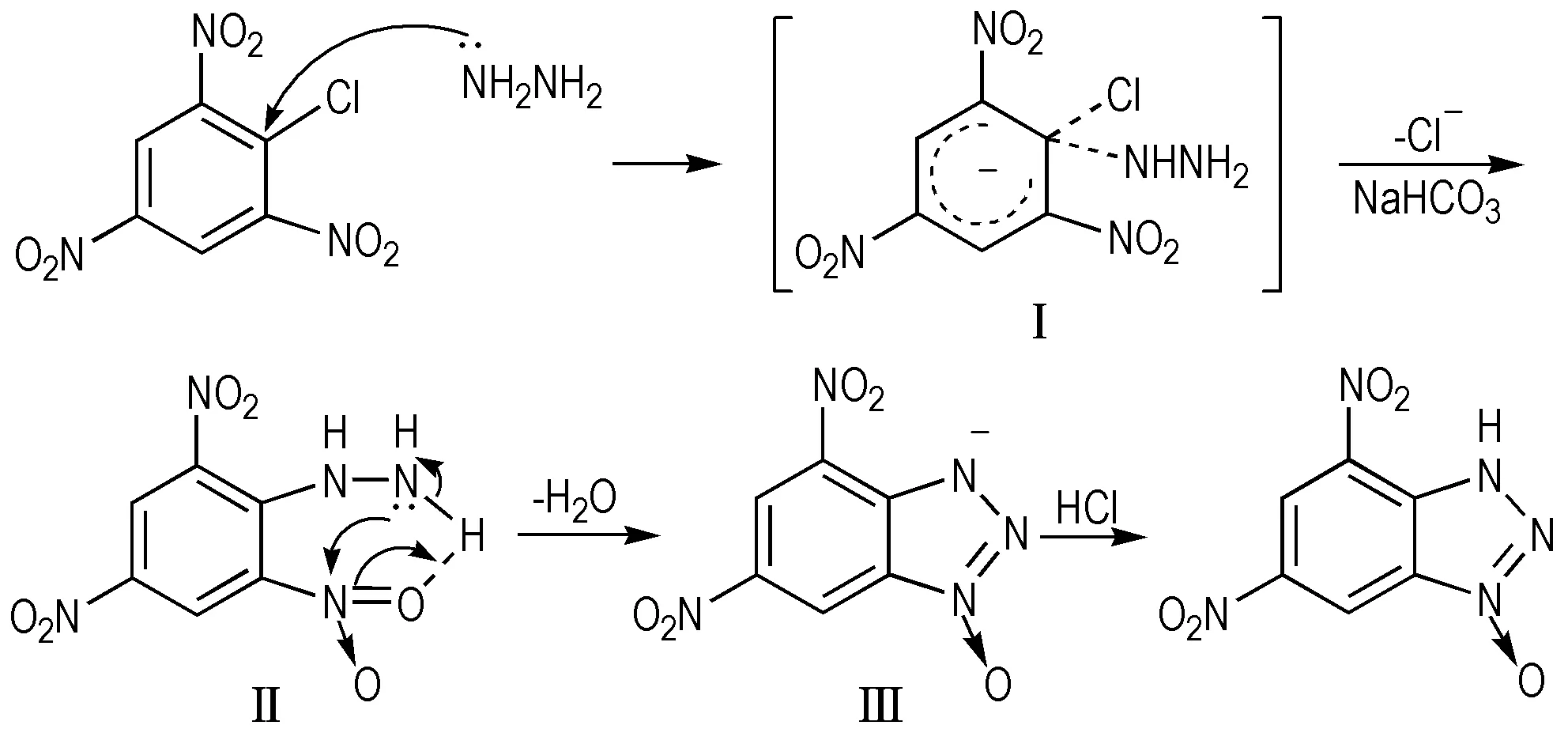
Scheme 2 Mechanism of triazol-3-ium-1-oxide
3.2 VNS Reaction
THMI is an expensive VNS reagent and decomposes easily, so we look forward to find another VNS reagent to substitute THMI. We discussed the effect on reaction of the different VNS reagents, such as NH2OH·HCl, THMI and 3-amino-1,2,4-triazole(ATA). The result shows that using NH2OH·HCl as VNS reagent, the yield was 63.2%, and consistent with that of using THMI (64%). NH2OH·HCl is cheaper than THMI very much, so that the cost of synthesis could be greatly decreased. Furthermore, the effect of reaction time on yield was discussed, and the best reaction time at room temperature was 5 h. The results are listed in Table 1.
Table 1 VNS reaction with different reagents
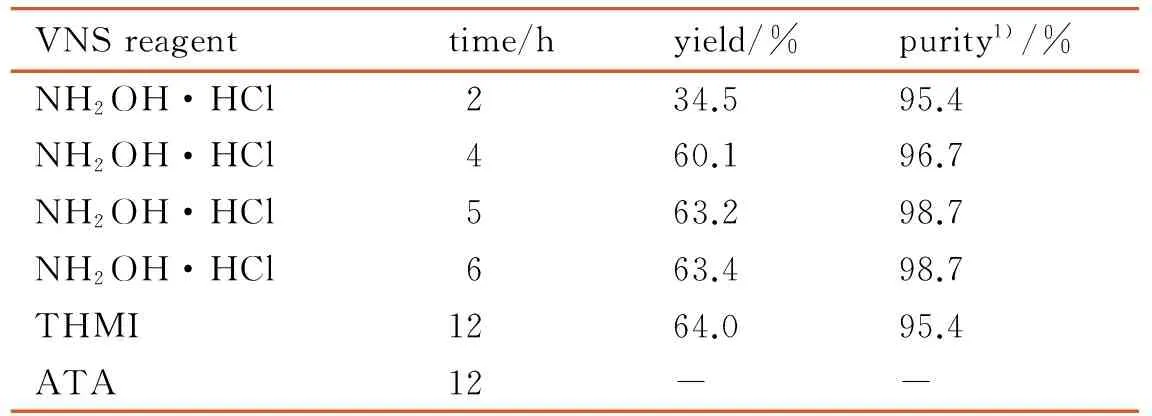
VNSreagenttime/hyield/%purity1)/%NH2OH·HCl234.595.4NH2OH·HCl460.196.7NH2OH·HCl563.298.7NH2OH·HCl663.498.7THMI1264.095.4ATA12--
Note: 1) the purity characterized by HPLC.
3.3 Properties of Compounds 1 and 2
The decomposition temperatures were obtained by the DSC, and the other performances were obtained by calculation, such as density and the enthalpy of formation were calculated by Gaussian 09 program[8], its detonation velocity and detonation pressure were calculated by VLW method[9]. It was found that the title compound 2 and its intermediate compound 1 had better performances, some main properties of the title compound 2 were obtained by calculation or test as follows: density is 1.76 g·cm-3, detonation velocity is 7396.7 m·s-1, enthalpy of formation is 4750 kJ·kg-1and its decomposition point is 248.5 ℃. Due to the presence of hydrogen bonds and the addition of two amino groups by VNS method, compared with compound 1, the title compound 2 exhibited a higher density and a better thermal stability, while the alternating amino and nitro groups should ensure stability and insensitivity. The physicochemical and detonation properties of compounds 1 and 2 were listed in Table 2.
Table 2 Properties of compounds 1 and 2
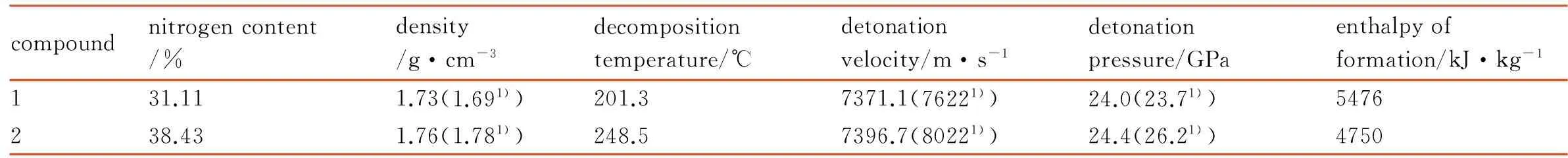
compoundnitrogencontent/%density/g·cm-3decompositiontemperature/℃detonationvelocity/m·s-1detonationpressure/GPaenthalpyofformation/kJ·kg-1131.111.73(1.691))201.37371.1(76221))24.0(23.71))5476238.431.76(1.781))248.57396.7(80221))24.4(26.21))4750
Note: 1) reference [7], detonation parameters were calculated using the EXPLO5 6.01 code.
3.4 Thermal Behaviors
The DSC curve of compound 1 in Fig.1 indicated a melting point,Tmax, at 86.7 ℃, and one thermal decomposition peak at 201.3 ℃. The TG-DTG curve of compound 1 in Fig.2 showed two main mass loss stages. The first stage amounts to 7.33% in the temperature range of 64.9-90.6 ℃, it is mainly attributed to the part of crystal water. The second stage begins at 184.1 ℃ and ends at 214.37 ℃, accompanied with 24.17% mass loss, corresponding to the mass of residual triazol-3-ium-oxide, and 34.71% residue at 491.23 ℃. The result indicates that there are a few remains at the end of the decomposition.
The DSC curve of compound 2 in Fig.3 exhibited one thermal decomposition peak at 248.5 ℃, according to the one obvious mass-loss stage in the TG-DTG curve in Fig.4, which can be confirmed by the mass-loss stage in the temperature range of 198.24-319.41 ℃ with mass-loss of 60.96%, and 15.28% residue at 447.71 ℃.The result indicates that there are a few remains at the end of the decomposition.
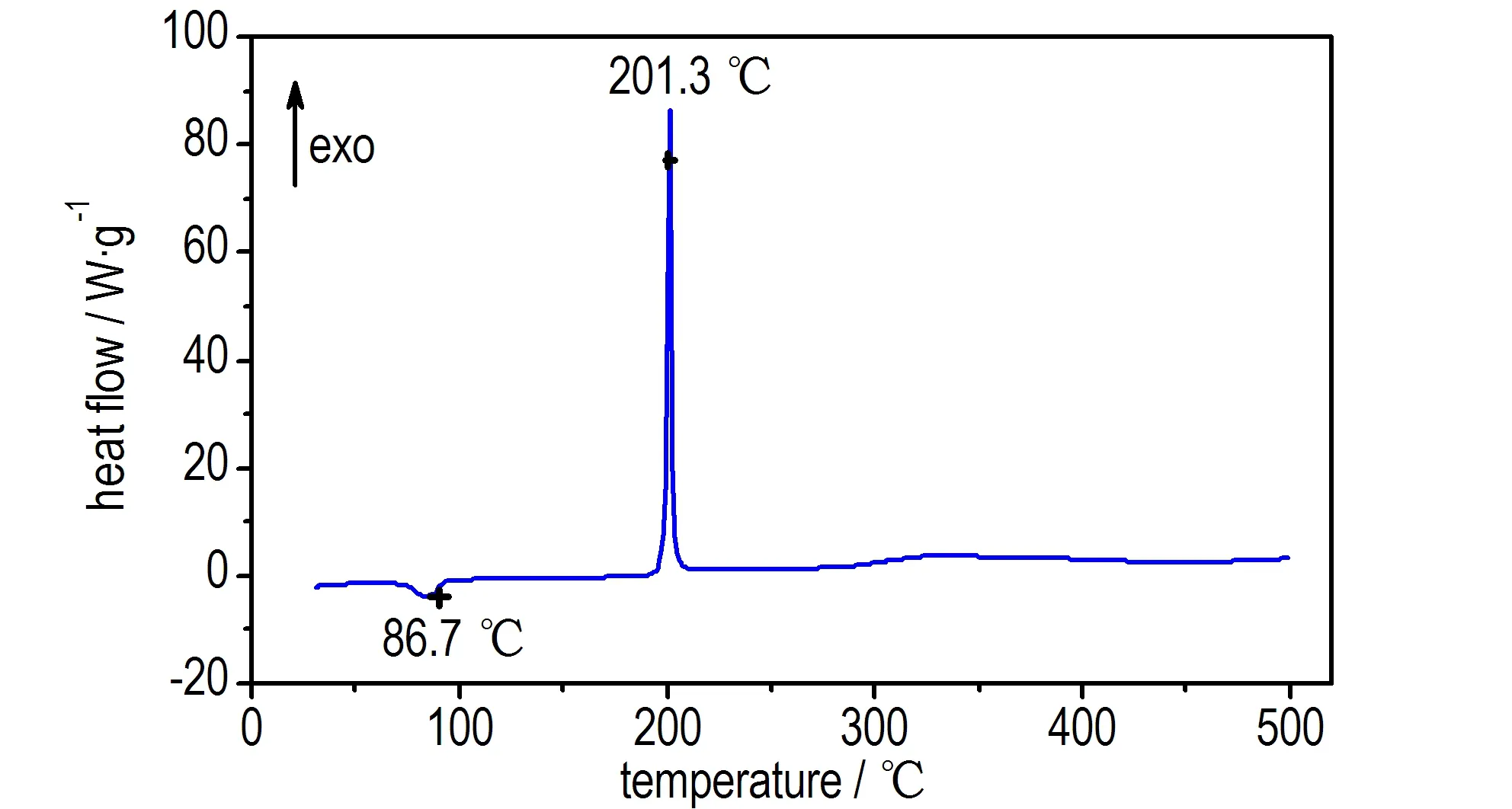
Fig.1 DSC curve of compound 1
Above-mentioned DSC results showed that the first decomposition temperatures of compounds 2 and 1 were 248.5 ℃ and 201.3 ℃, respectively. This fact that decomposition temperature of compound 2 was over 240 ℃, showed an excellent thermal stability and potential application for gas-generator or rocket propellants.
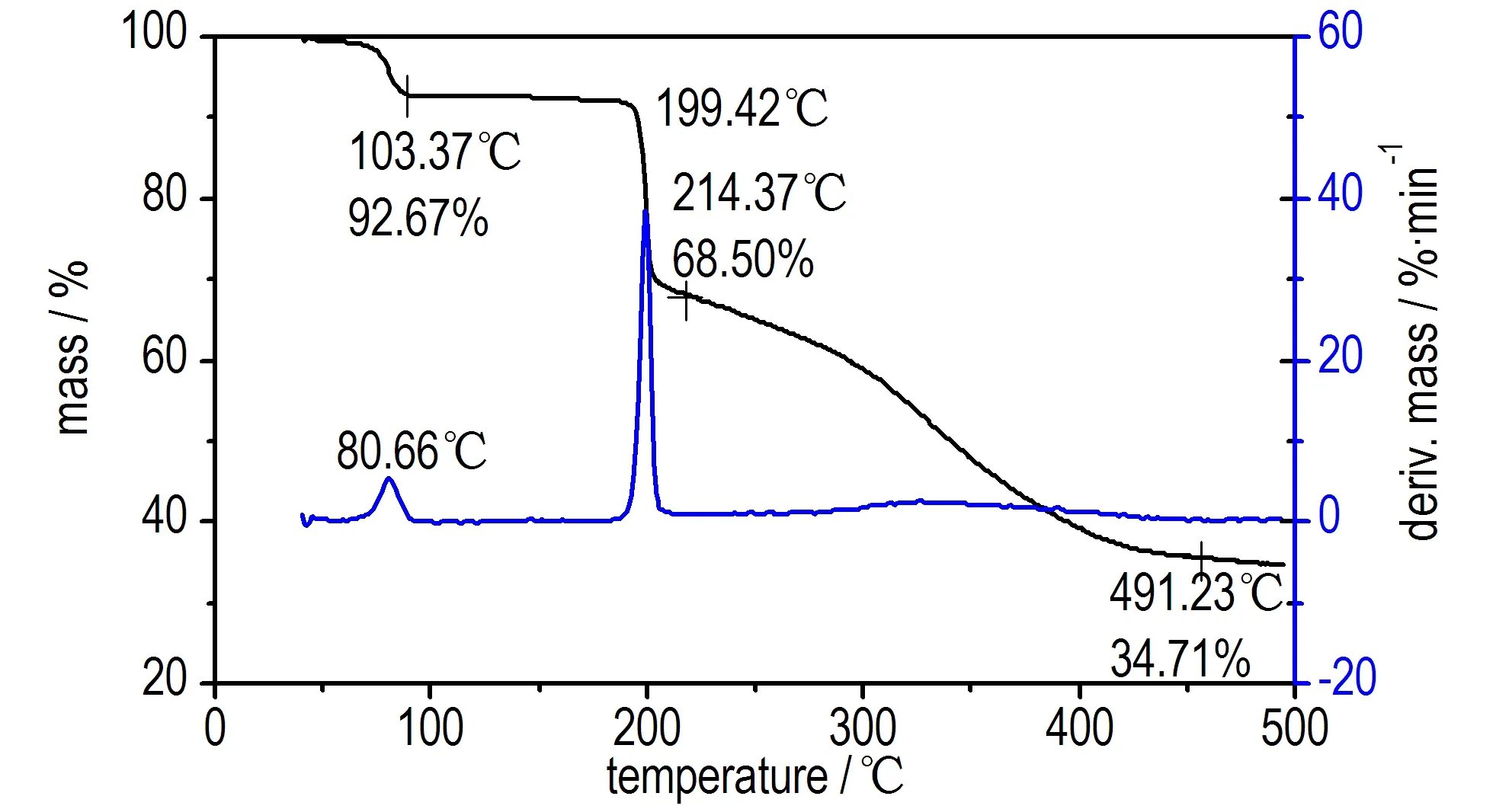
Fig.2 TG-DTG curve of compound 1
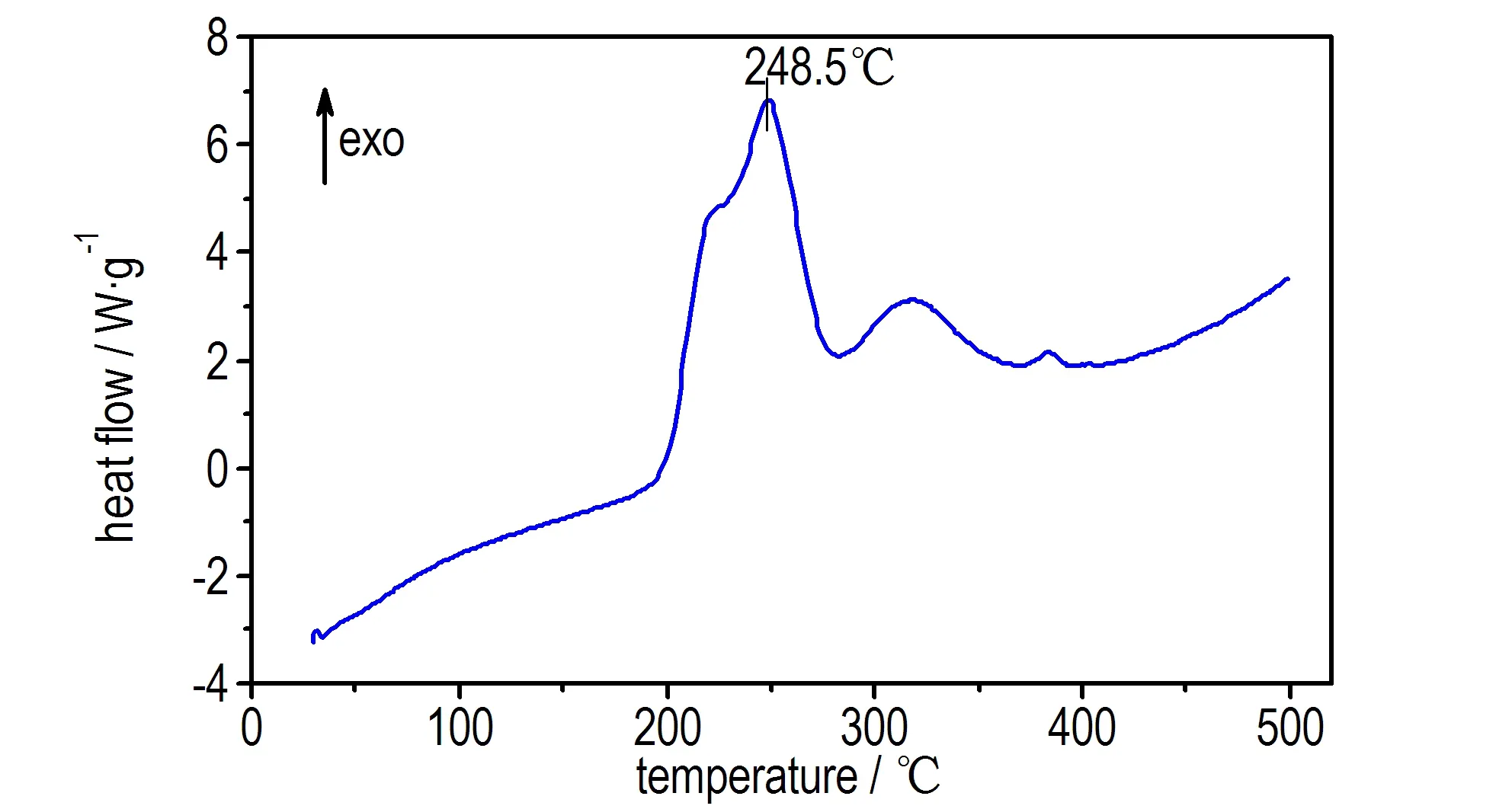
Fig.3 DSC curve of compound 2
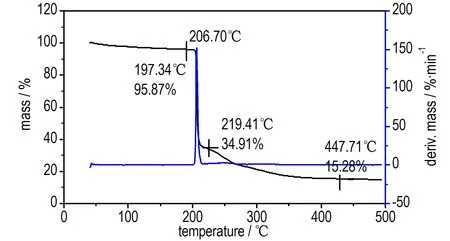
Fig.4 TG-DTG curve of compound 2
3.5 Electrostatic Potential (ESP) and Molecular Orbital
To obtainfurther understanding of the chemical and physical properties for target compound, electrostatic potential (ESP) and molecular orbital calculations were performed by the B3LYP/6-31++g (d, p) level of theory based on the optimized structure. Figure 5 shows the ESP for the 0.001 electron/bohr of the electron density evaluated at the B3LYP method. It has recently been found, and is extensively used, that the computed ESP is generally related to the impact sensitivity of the bulk energetic materials[10-13]. Basically a higher charge separation and larger and stronger positive potentials lead to especially higher sensitivity values. In Fig.5, it can be clearly seen that the positive ESP region of the title compound is smaller and also a lower charge separation; this is in good accord with the experimental result of compound 2 (IS=20 J, FS>360 N).
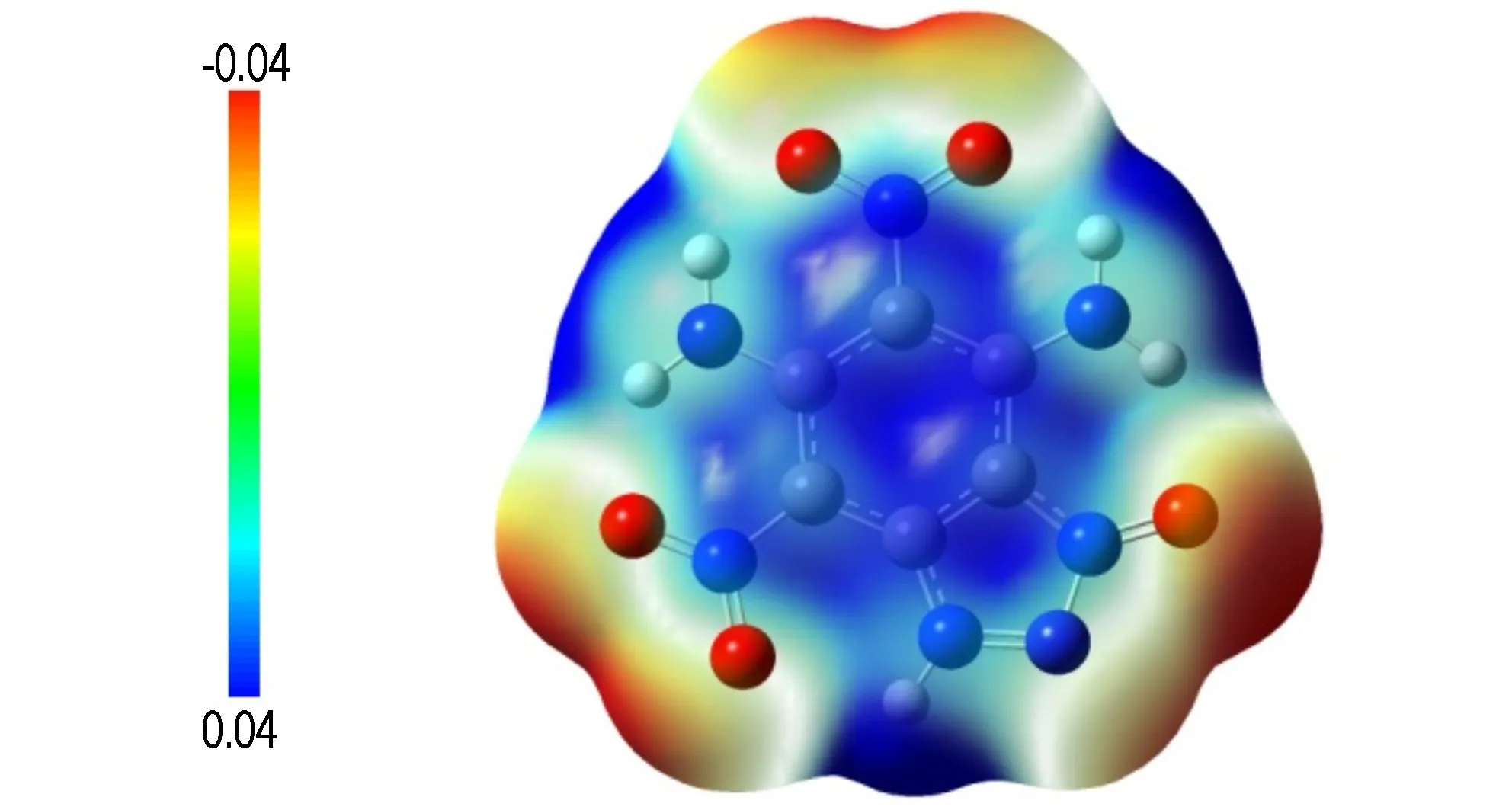
Fig.5 Calculated electrostatic potential of the title compound( The red regions represent electron rich regions, the blue regions is electron extremely deficient regions. The 3D isosurface of electron density is shown between -0.04 hartree and +0.04 hartree)
The highest occupied molecular orbitals (HOMOs) and the lowest unoccupied molecular orbitals (LUMOs) of compound 2 were shown in Fig.6. It can be seen that one animo group and the imino group mainly occupy the HOMO, whereas the nitro group and the other amino group occupy the LUMO. The results indicate that the group of —NH2make an important difference to some properties of the title compound. Since the gap energy (ΔE) of HOMO and LUMO is an important parameter to measure the stability of the energetic material, the highest occupied molecular orbital energy (EHOMO), the lowest unoccupied molecular orbital energy (ELOMO) and their gaps (ΔE=ELOMO-EHOMO) were obtained as -0.00044, 0.01423 and 0.01467 Hartree, respectively. The acceptable ΔElikely explains the reasonable thermal stabilities.
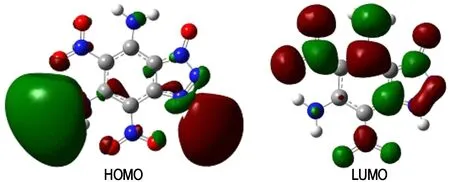
Fig.6 The highest occupied molecular orbital (HOMO, left) and the lowest unoccupied molecular orbital (LUMO, right) of the title compound
4 Conclusions
(1) Using cheap NH2OH·HCl as a substitute for expensive and instable THMI, 5,7-diamino-4,6-dinitrobenzotriazol-3-ium-1-oxide was synthesized via VNS reaction from 4,6-dinitrobenzotriazol-3-ium-1-oxide with a yield of 63.2%. Compared with the literature, the cost of VNS reaction was greatly decreased.
(2) A possible mechanism of triazol-3-ium-1-oxide cyclization is presented.
(3) The nitrogen content, density, decomposition temperature, detonation velocity, detonation pressure and enthalpy of formation of the title compound are 38.43%, 1.76 g·cm-3, 248.5 ℃, 7396.7 m·s-1, 24.4 GPa and 4750 kJ·kg-1, respectively.
(4) The highest occupied molecular orbital energy (EHOMO), the lowest unoccupied molecular orbital energy (ELOMO) and their gaps (ΔE=ELOMO-EHOMO) are -0.00044, 0.01423 and 0.01467 Hartree, respectively, revealing that 5,7-diamino-4,6-dinitrobenzotriazol-3-ium-1-oxide possess reasonable thermal stabilities.
[1] Yan Z Y, Zhao Y B, Fan M J, et al. General synthesis of (1-substituted-1H-1,2,3-triazole-4-ylmethyl)-dialkylamines via a copper(I)- catalyzed three-component reaction in water[J].Tetrahedron, 2005, 61: 9331-9335.
[2] SHl Hong-gang, Ll Sheng-hua, Ll Yu-chuan, et al.Synthesis of 1-amino-1,2,3-triazole[J].ChineseJournalofEnergeticMaterials(HannengCailiao), 2008, 16(6): 676-678.
[3] DONG Hai-shan, The development and countermeasure of high energy density materials[J].ChineseJournalofEnergeticMaterials(HannengCailiao), 2004(Suppl.): 1-12.
[4] HUO Huan, WANG Bo-zhou, ZHOU Cheng, et al. Synthesis and characterization of 4-amino-5-nitro-1,2,3-trazole[J].ChineseJournalofEnergeticMaterials(HannengCailiao),2008, 16(1): 49-52.
[6] DippoldA A, Klapötke T M. A Study of Dinitro-bis-1,2,4-triazole-1,1′-diol and derivatives: design of high-performance insensitive energetic materials by the introduction of N-oxides[J].JAmChemSoc, 2013, 135: 9931-9938.
[8] FrischM J, Trucks G W, Schlegel H B, et al. GAUSSIAN 09[CP], Gaussian, Inc, Wallingford C, 2009.
[9] WU Xiong, LONG Xin-ping, HE Bi, et al. The VLW equation of state for detonation products[J].ScienceinChina, 2008, 38(12):1129-1131.
[10] FischerD, Klapötke T M, Stierstorfer J. Synthesis and characterization of diaminobisfuroxane[J].EurJInorgChem, 2014, 34: 5808-5811.
[11] Zhang J H, Shreeve J M. 3,3′-Dinitroamino-4,4′-azoxyfurazan and its derivatives: an assembly of diverse N—O building blocks for high-performance energetic materials[J].JAmChemSoc, 2014, 136: 4437-4445.
[12] KlapötkeT M, Nordheider A, Stierstorfer J. Synthesis and reactivity of an unexpected highly sensitive 1-carboxymethyl-3-diazonio-5-nitrimino-1,2,4-triazole[J].NewJChem, 2012, 36: 1463-1468.
[13] Hammerl A, Klapötke T M, Nöth H, et al. Synthesis structure, molecular orbital and valence bond calculations for tetrazole azide, CHN7[J].Propellants,Explosives,Pyrotechnics, 2003, 28(4): 165-173.
杂志排行
含能材料的其它文章
- Crystal Structure and Thermal Behavior of Potassium Dinitromethane
- Coatings of Activated Metal Hydride and Application in the Fuel-rich Propellant
- Synthesis and Properties of 5-(3-Amino-1,2,5-oxadiazol-4-yl)tetrazol-1-ol and Its Ammonium and Hydroxylammonium Salts
- The Tensile Properties and Creep Performance of a Long-term Thermally Aged Plastic Bonded Explosive
- Energies of Combustion and Specific Heat Capacities of Diaminofurazan, Dinitrofurazan and Diaminoazofurazan
- Thermal Behaviors of 1-Amino-2-nitroguanidine
