水稻小穗颖壳发育的研究进展
2016-04-29徐乾坤任德勇李自壮曾大力郭龙彪钱前
徐乾坤 任德勇 李自壮 曾大力 郭龙彪 钱前
(中国水稻研究所水稻 生物学国家重点实验室,杭州 310006; #共同第一作者;*通讯联系人, E-mail: qianqian188@hotmail.com)
水稻小穗颖壳发育的研究进展
徐乾坤#任德勇#李自壮 曾大力 郭龙彪 钱前*
(中国水稻研究所水稻 生物学国家重点实验室,杭州 310006;#共同第一作者;*通讯联系人, E-mail: qianqian188@hotmail.com)
徐乾坤, 任德勇, 李自壮, 等. 水稻小穗颖壳发育的研究进展. 中国水稻科学, 2016, 30(1): 99-105.
摘要:水稻(Oryza sativa L.)是世界上重要的粮食作物,也是单子叶植物的模式植物。开花时间、花序和花器官的形态结构对其产量和品质均有重要影响。对花器官形态结构及发育机理的研究有助于提高水稻产量并改良其品质。花器官的形成和发育是水稻从营养生长转向生殖生长的重要过程,其发育模式和分子机理,一直是生物学研究的热点和焦点。水稻小穗的颖壳是禾本科特有的器官,主要包括内外稃、护颖和副护颖,关于其起源和形成的分子机制还知之甚少。近些年对颖壳的研究不断深入,不仅有助于深入认识水稻小穗或花器官的发育,而且能系统地了解水稻小穗或花器官发育的整个调控网络。本文主要介绍了水稻小穗颖壳发育的相关进展及植物花器官发育的ABCDE模型。
关键词:水稻; 内外稃; 护颖; 副护颖; ABCDE模型
水稻是单子叶的模式植物,具有与双子叶植物不同的花序结构,其小穗是花序的结构单元。一个典型的水稻小穗由一对副护颖、一对护颖、一个外稃、一个内稃、两个浆片、六个雄蕊和一个雌蕊构成[1-2]。与双子叶植物拟南芥相比,水稻小穗或小花在器官的轮次和形态结构上具有相似的雄蕊和雌蕊等生殖器官,但是不具有明显的花萼和花瓣结构,相反在对应的花器官轮次上形成了形态结构上存在较大差异的内外稃和浆片[3]。尽管有人推测水稻内外稃与双子叶植物花萼同源,但它们是否为同源器官且水稻内外稃是否同轮次器官或同一器官仍存在较大争议。另外,水稻的小穗还具有两对特有的颖壳:一对护颖和一对副护颖。关于护颖的特征与起源一直备受争议,其副护颖的特征与分子调控机制鲜有报道,通常被认为是极度退化的苞片[3-6]。为了深入了解水稻乃至禾本科小穗或小花中这些特有器官的特征、起源及分子遗传机制,需要发掘更多相关突变体并对其基因的功能进行研究。
1ABCDE模型
双子叶植物的小花通常由四轮花器官组成,由外到内依次是花萼、花瓣、雄蕊、雌蕊。在过去的二十多年里,大量的遗传和分子生物学的研究已经建立了经典的“ABCDE”模型,用于解释一些双子叶植物花发育的分子机制[7-11]。在拟南芥中,A、B、C、D和E五类基因通过独立或协同作用来调控双子叶植物花器官的形成和发育。目前,水稻中越来越多的花同源异型基因被克隆,基因功能研究表明该模型也部分适用于水稻[6,12]。在水稻的“ABCDE”模型中,B功能基因OsMADS2和OsMADS4与拟南芥的PI基因同源,OsMADS16/SUPERWOMEN1与拟南芥的AP3基因同源,主要决定浆片和雄蕊的特征[13-15]。C功能基因OsMADS3和OsMADS58与拟南芥的AG基因同源,主要决定雄蕊的特征和心皮的形态建成[16]。OsMADS13属于D功能基因,与矮牵牛的FBP7/FBP11及玉米的ZMM1/ZAG2同源,主要参与胚珠的形成[17]。E功能基因包括OsMADS1、OsMADS5、OsMADS7、OsMADS8和OsMADS34。其中,对OsMADS1和OsMADS34有较深入的研究,其有特化四轮花器官的特征和决定小穗分生组织的确定性[18-19]。
2水稻内外稃的发育
内稃和外稃是禾本科植物特有的外轮花器官且有着不同的特征和起源。通常认为内稃是小花轴上形成的先出叶(叶腋分生组织上形成的第一片叶),而外稃是小穗轴上形成的苞叶[20-22]。形态结构分析表明,内稃和外稃具有相同的细胞层数和外表皮细胞:如突起和毛状体结构;但是两者之间也存在较大的差异。与外稃的5条维管束相比,内稃仅仅3条维管束,同时内稃具有独特的膜状边缘结构且表面光滑[22]。AGAMOUS-LIKE6(AGL6)亚家族MADS-box基因MOSAICFLORALORGANS1(MFO1/OsMADS6)在内稃边缘组织中特异表达,决定内稃特征。mfo1突变体具有内稃内卷,维管束增多,边缘组织缺失,主体结构过度生长,内稃获得部分外稃的特征。同时,外稃特异表达的DROOPINGLEAF(DL)基因在mfo1突变体的内稃中异位表达[21]。在玉米中,与水稻MFO1基因同源的bearded-ear(bde)基因,同样只在内稃中表达,不在外稃中表达,表明AGL6亚家族基因在禾本科内稃中的表达较为保守[23]。水稻chimericfloralorgans1(cfo1/osmads32)突变体也表现出与mfo1突变体相似的内稃缺陷。在cfo1突变体中,内稃边缘区域扩大且外表面硅化,DL基因在内稃中异位表达。不同的是,cfo1突变体的内稃没有外稃状内卷且维管束数目不变。系统进化和测序结果表明MFO1基因和CFO1基因参与了禾本科小穗乃至禾本科内稃的调控和起源,但两者的功能出现了分化[24]。DEPRESSEDPALEA1/PALEALESS1(DP1/PAL1)编码一个AT-hook DNA结合蛋白,在内稃原基中强烈表达,影响内稃形成和花器官数量。DP1基因突变导致内稃主体结构完全丢失,仅保留了两个膜状器官[25]。DP1基因与玉米STALKFASTIGIATE1(BAF1)基因同源且功能保守[26]。RETARDEDPALEA1(REP1)位于DP1基因的下游,编码TCP结构域转录调节因子,与拟南芥CYCLOIDEA(CYC)基因同源,主要在内稃原基中表达,参与内稃特征和发育调控。在rep1突变体中,内稃主体部分显著退化,内稃变小,而内稃边缘区域则变宽。超表达REP1基因导致内稃主体部分变宽,边缘区域变窄[27]。MULTI-FLORETSPIKELET1(MFS1)基因编码ERF结构域转录因子,属于AP2/ERF结构域基因家族,主要参与内稃和护颖的特征调控。MFS1功能缺失导致内稃的主体部分退化,严重的仅剩下内稃的边缘部分,功能上部分类似于DP1基因[22,28]。CURVEDCHIMERICPALEA1/DEFORMEDFLORALORGAN1(CCP1/DFO1)基因与拟南芥EMBRYONICFLOWER1(EMF1)同源,主要调控花器官的特征,抑制了基因OsMADS58在内稃中异位表达[29]。ccp1/dfo1突变体内稃卷曲皱缩,内稃雌蕊化,外稃特征未发生任何改变。这些证据都支持了内稃可能是由内稃的主体部分和内稃的边缘部融合而成的假说[21-22](表1)。
尽管如此,也报道了很多同时控制水稻内外稃形成和发育的基因,如LEAFYHULLSTERILE1/OsMADS1(LHS1/OsMADS1),DEGENERATIVEPALEA/OsMADS15(DEP/OsMAD-S15),STAMENLESS1(SL1),OPENBEAK(OPB),DEGENERATEDHULL1(DH1)和TRIANGULARHULL1(TH1)/ABNORMALFLOWERANDDWARF1(AFD1),不仅参与内稃特征决定和发育过程的调控,也参与了外稃特征决表1内外稃发育相关基因定和发育过程的调控。LHS1/OsMADS1和DEP/OsMADS15主要通过控制内外稃特定细胞的分化来调控内外稃的发育和形成。LHS1突变后, 内外稃和浆片都伸长,同时向叶状器官转化, 内稃的维管束增多,类似于外稃[30]。DEP突变导致内外稃都伸长,但内部花器官未被影响[31]。SL1和OPB都编码C2H2锌指结构域转录因子。当这两个基因突变后,内外稃横向生长都受到抑制,变小且开裂不能闭合,外稃向内弯曲,雄蕊向雌蕊转变,暗示了SL1和OPB这两个基因在调控花器官特征和发育上功能部分相似[32-33]。DH1编码了一个LOB结构域的蛋白。在dh1突变体中,内外稃严重退化,仅剩下透明的膜状结构,有些甚至变成丝状器官[34]。有研究表明,H3K9DEMETHYLASE(JMJ706)的H3K9去甲基化是DH1基因正常表达所必需的,JMJ706 基因突变导致了内外稃数目的改变[35]。TH1/AFD1基因编码一个DUF640结构域转录因子,突变后内外稃横向和纵向生长均被影响,内外稃变小、增厚[36-37]。
Table 1. Relative genes of lemma and palea.
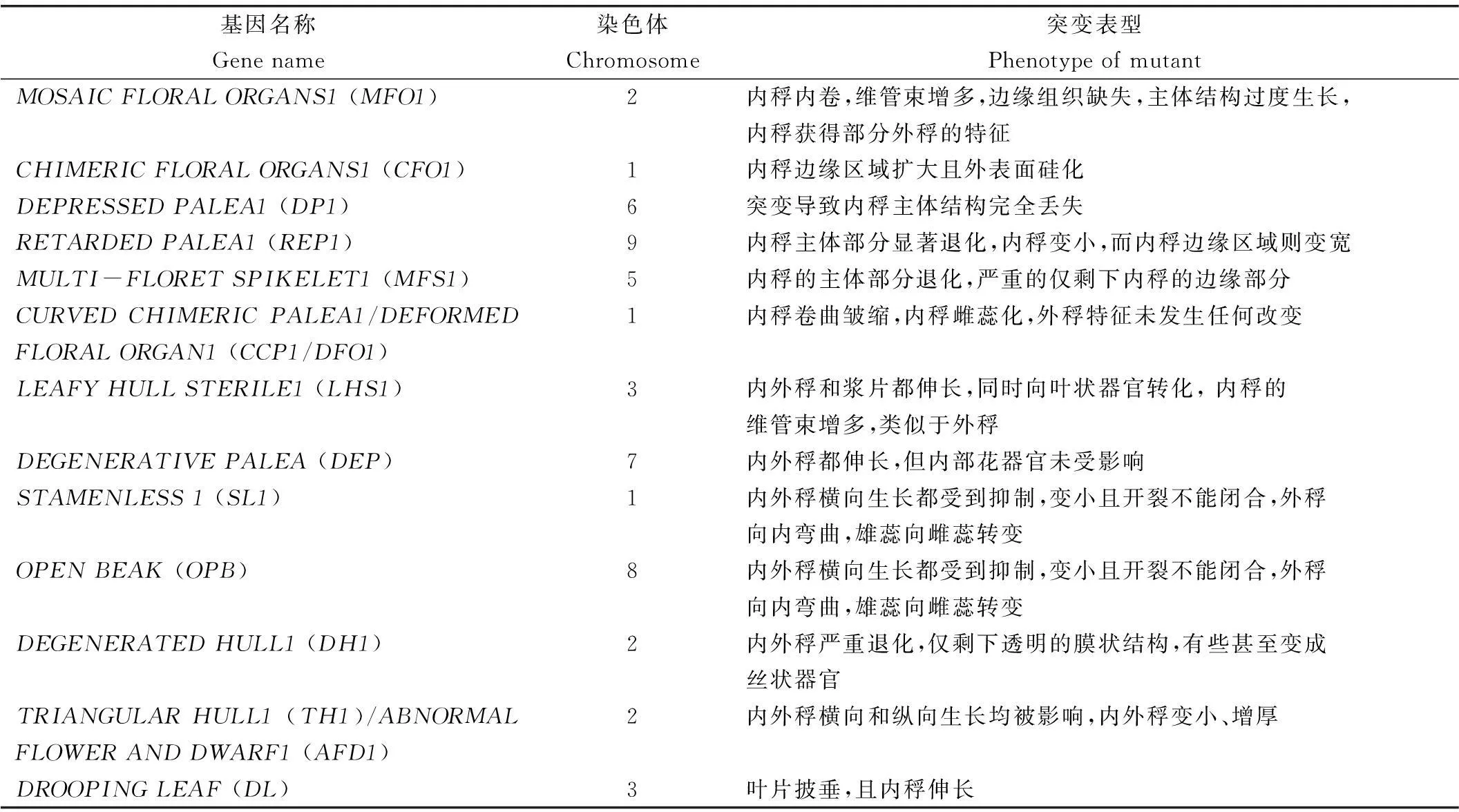
基因名称Genename染色体Chromosome突变表型PhenotypeofmutantMOSAICFLORALORGANS1(MFO1)2内稃内卷,维管束增多,边缘组织缺失,主体结构过度生长,内稃获得部分外稃的特征CHIMERICFLORALORGANS1(CFO1)1内稃边缘区域扩大且外表面硅化DEPRESSEDPALEA1(DP1)6突变导致内稃主体结构完全丢失RETARDEDPALEA1(REP1)9内稃主体部分显著退化,内稃变小,而内稃边缘区域则变宽MULTI-FLORETSPIKELET1(MFS1)5内稃的主体部分退化,严重的仅剩下内稃的边缘部分CURVEDCHIMERICPALEA1/DEFORMEDFLORALORGAN1(CCP1/DFO1)1内稃卷曲皱缩,内稃雌蕊化,外稃特征未发生任何改变LEAFYHULLSTERILE1(LHS1)3内外稃和浆片都伸长,同时向叶状器官转化,内稃的维管束增多,类似于外稃DEGENERATIVEPALEA(DEP)7内外稃都伸长,但内部花器官未受影响STAMENLESS1(SL1)1内外稃横向生长都受到抑制,变小且开裂不能闭合,外稃向内弯曲,雄蕊向雌蕊转变OPENBEAK(OPB)8内外稃横向生长都受到抑制,变小且开裂不能闭合,外稃向内弯曲,雄蕊向雌蕊转变DEGENERATEDHULL1(DH1)2内外稃严重退化,仅剩下透明的膜状结构,有些甚至变成丝状器官TRIANGULARHULL1(TH1)/ABNORMALFLOWERANDDWARF1(AFD1)2内外稃横向和纵向生长均被影响,内外稃变小、增厚DROOPINGLEAF(DL)3叶片披垂,且内稃伸长
目前,很少发现只影响水稻外稃发育的基因,但DL基因比较特别。DL基因属于YABBY家族,在外稃中脉中特异表达而在内稃中不表达,当DL基因失活后,外稃的形态和特征似乎未发生任何改变。同时,DL基因也在芒中强烈表达,暗示了DL基因可能参与了芒的形成且芒可能属于水稻外稃的一部分[38-39]。尽管如此,DL基因如何调控外稃的发育还有待进一步的研究。
3水稻护颖的发育
目前,关于护颖的起源存在两种观点。一种观点认为水稻祖先小穗包含一个顶生小花和两个侧生小花,在进化过程中侧生小花退化,只剩下外稃,最终退化成护颖[4,40-42]。另一种观点则认为,水稻小穗只有一朵小花,护颖是严重退化了的苞片状器官[5,6,40,43]。护颖是禾本科小穗特有的器官结构,其本质一直以来都是大家关注的焦点。在现存的稻族物种中,护颖的形态(尤其是大小)变化很大,比如野生稻(Oryzagrandiglumis)具有长而大的外稃状护颖[19,44];水稻的护颖比外稃小很多,可能是由外稃退化而来;然而在李氏禾(LeersiahexandraSwartz)中,护颖则完全退化[4,22]。LONGSTERILELEMMA/ELONGATEDEMPTYGLUME1(G1/ELE)编码DUF640结构域蛋白,属于植物特异的基因家族,该基因特异在护颖中强烈表达。在g1/ele1突变体中,护颖伸长,形态和结构上与外稃相似,包含四种细胞层和4~5条维管束[5,41]。
表2护颖发育相关基因
Table 2. Relative genes of sterile lemma.

基因名称Genename染色体Chromosome突变表型PhenotypeofmutantLONGSTERILELEMMA/ELONGATEDEMPTYGLUME1(G1/ELE)7护颖伸长,形态和结构上与外稃相似,包含四种细胞层和4~5条维管束OsMADS343护颖伸长,细胞层次和外表面结构都与外稃类似,具有5条维管束SUPERAPICALDORMANT(SAD1)8护颖不同程度伸长,其外表面具有毛状体和突起,形态结构上与外稃部分类似ABERRANTSPIKELETANDPANICLE1(ASP1)8护颖不同程度伸长,其外表面具有毛状体和突起,形态结构上与外稃部分类似
表3副护颖发育相关基因
Table 3. Relative genes of rudimentary glume.

基因名称Genename染色体Chromosome突变表型PhenotypeofmutantFRIZZYPANICLE(FZP)7没有正常的护颖,在对应的位置出现数目不确定的副护颖SUPERNUMERARYBRACT(SNB)7没有正常的护颖,在对应的位置出现数目不确定的副护颖MFS15护颖退化,形态结构类似副护颖OsINDETERMINATESPIKELET1(OsIDS1)3护颖退化,形态结构类似副护颖OsMADS343副护颖不同程度伸长,外表面结构在一定程度上具有护颖和外稃的特征ASP18副护颖不同程度伸长,外表面结构在一定程度上具有护颖和外稃的特征
OsMADS34基因编码MADS-box结构域转录因子,属于E功能基因,其突变后护颖伸长,细胞层次和外表面结构都与外稃类似,具有5条维管束[19,45-46]。另外,外稃特征基因DL也在osmads34突变体的护颖中被检测到,暗示了其护颖向外稃转变且获得了外稃的特征[45]。SUPERAPICALDORMANT(SAD1)和ABERRANTSPIKELETANDPANICLE1(ASP1)基因分别编码一个RNA聚合酶亚基和TOPLESS转录抑制因子。在sad1和asp1突变体中,护颖不同程度伸长,其外表面具有毛状体和突起,形态结构上与外稃部分类似[47-48]。以上研究表明G1/ELE、OsMADS34、SAD1和ASP1基因抑制护颖向外稃转化,暗示了护颖和外稃可能是同源器官,支持了护颖可能是退化的外稃,甚至是退化的侧生小花(表2)。
4水稻副护颖的发育
迄今为止,关于副护颖相关研究很少报道。通常情况下,副护颖被认为是严重退化的苞片[5-6,43]。FRIZZYPANICLE(FZP)和SUPERNUMERARYBRACT(SNB)都编码AP2/ERF结构域蛋白,均在副护颖中强烈表达。在fzp和snb突变体中,没有发现正常的护颖,在对应的位置出现数目不确定的副护颖[49-53]。MFS1和OsINDETERMINATESPIKELET1(OsIDS1)也编码AP2/ERF结构域转录因子,其突变后护颖退化,形态结构类似副护颖,暗示了护颖可能会同源异型转变成副护颖[22,49]。这些结果表明了该AP2/ERF基因家族可能在一定程度上承担着抑制护颖向副护颖转变的功能,并支持护颖和副护颖同是苞片结构的假说。值得一提的是,当OsMADS34和ASP1功能缺失后,副护颖不同程度伸长,外表面结构在一定程度上具有护颖和外稃的特征[46],暗示了OsMADS34和ASP1抑制了副护颖向护颖或者外稃转变。实际上,在大多数禾本科物种中,小穗由苞片状颖壳(副护颖的对应器官)和小花组成,缺乏护颖的对应器官,而苞片状颖壳并不像水稻副护颖一样严重退化,在形态结构上与其外稃相似,比如玉米和小麦等[1,5,20,22,40-41,54]。以上这些研究结果一起暗示了水稻副护颖、护颖和外稃可能是同源器官(表3)。
5问题及展望
水稻属于单子叶植物,与拟南芥等双子叶植物相比,水稻颖花发育的相关研究还相对滞后。这与单子叶植物花器官结构相对复杂、形态多变、缺乏相关突变体有关。虽然目前已经分离了一些与内外稃发育相关的基因,然而关于内外稃是否是花萼的同源物,是否是同一种器官的不同部分,或就是同一种器官,尚无定论。目前与护颖及副护颖发育相关的基因分离相对较少,还无法定论护颖及副护颖的起源及其进化,副护颖、护颖和外稃是否是同源器官还有待进一步考证。相信随着更多内外稃、护颖及副护颖相关突变体的分离与鉴定,基因的功能和基因之间互作研究的深入, 能更加深入地了解单子叶模式植物水稻与双子叶植物的花器官及水稻小穗本身器官间的同源关系,并更加清楚地认识水稻颖壳的发育机制,也必将推动水稻等禾本科植物小穗或者花发育研究的飞速发展,同时,整个遗传调控网络必将更清楚地呈现在人们的面前。另外,对水稻小穗或小花突变体进一步的深入研究,对改良稻米产量和品质性状具有重要意义。GS5和GW8主要通过影响颖壳横向细胞数目的数量来控制粒宽[55-57];PGL1和SRS5主要通过影响颖壳细胞的长度来控制谷粒的大小[58-59];SRS3和GL7同时控制颖壳细胞的大小和数目,从而影响产量[60-61]。实际生产中,水稻种子的颖壳也经常开裂和畸变,影响种子的产量和质量,也影响其发芽。同时在贮藏过程中,裂颖或畸变的种子易染菌、霉变,影响种子的生活力及发芽率,进而增加贮藏成本[62-63]。因此,利用已克隆的颖壳发育相关基因,通过分子标记辅助选择或转基因手段,可实现稻米产量和相关品质的遗传改良。
参考文献:
[1]Bommert P, Satoh-Nagasawa N, Jackson D, et al. Genetics and evolution of inflorescence and flower development in grasses.PlantCellPhysiol, 2005, 46: 69-78.
[2]Itoh J, Nonomura K, Ikeda K, et al. Rice plant development: From zygote to spikelet.PlantCellPhysiol, 2005, 46: 23-47.
[3]Ambrose B A, Lerner D R, Ciceri P, et al. Molecular and genetic analyses of thesilky1 gene reveal conservation in floral organ specification between eudicots and monocots.MolCell, 2000, 5: 569-597.
[4]Kellogg E A. The evolutionary history ofEhrhartoideae,Oryzeae, andOryza.Rice, 2009, 2: 1-14.
[5]Hong L, Qian Q, Zhu K, et al. ELE restrains empty glumes from developing into lemmas.JGenetGenom,2010, 37: 101-105.
[6]Schmidt R J, Ambrose B A. The blooming of grass flower development.CurrOpinPlantBiol, 1998, 1: 60-67.
[7]Coen E S, Meyerowitz E M. The war of the whorls: Genetic interactions controlling flower development.Nature,1991,53: 31-37.
[8]Weigel D, Meyerowitz E M. The ABCs of floral homeotic genes.Cell,1994,78: 203-209.
[9]Theissen G, Saedler H. Plant biology. Floral quartets.Nature, 2001, 409: 469-471.
[10]Soltis D E, Chanderbali A S, Kim S, et al. The ABC model and its applicability to basal angiosperms.AnnBot, 2007,100: 155-163.
[11]Litt A, Kramer E M. The ABC model and the diversification of floral organ identity.SeminCellDevBiol, 2010,21: 129-137.
[12]Kyozuka J, Kobayashi T, Morita M, et al. Spatially andtemporally regulated expression of rice MADS box genes with similarity toArabidopsisclass A, B and C genes.PlantCellPhysiol, 2004,1: 710-718.
[13] Shri R Y, Kalika P, Usha V. Divergent regulatoryOsMADS2 functions control size, shape and differentiation of the highly derived rice floret second-whorl organ.Genetics, 2007, 176: 283-294.
[14]Rita A, Pinky A, Swatismita R, et al. MADS-box gene family in rice: Genome-wide identification, organization and expression profiling during reproductive development and stress.BMCGenom, 2007, 8: 242.
[15]Xiao H, Wang Y, Liu D F, et al. Functional analysis of the rice AP3 homologueOsMADS16 by RNA interference.PlantMolBiol, 2003, 52: 957-966.
[16]Yun D P, Liang W Q, Dreni L, et al. OsMADS16 genetically Interacts with OsMADS3 and OsMADS58 in specifying floral patterning in rice.MolPlant, 2013, 6: 743-756.
[17]Li H F, Liang W P, Yin C S, et al. Genetic interaction of OsMADS3, DROOPING LEAF, and OsMADS13 in specifying rice floral organ identities and meristem determinacy.PlantPhysiol,2011, 156: 263-274.
[18]Hu Y, Liang W P, Yin C S, et al. Interactions of OsMADS1 with floral homeotic genes in rice flower development.MolPlant, 2015, 8:1366-1384.
[19]Lin X L, Wu F, Du X Q, et al. The pleiotropic SEPALLATA-like gene OsMADS34 reveals that the ‘empty glumes’ of rice (Oryzasativa) spikelets are in fact rudimentary lemmas.NewPhytiol, 2013, 202: 689-702.
[20]Kellogg E A. Evolutionary history of the grasses.PlantPhysiol, 2001, 125: 1198-1205.
[21]Ohmori S, Kimizu M, Sugita M, et al. MOSAIC FLORAL ORGANS1, an AGL6-like MADS box gene, regulates floral organ identity and meristem fate in rice.PlantCell, 2009, 21: 3008-3025.
[22]Ren D Y, Li Y F, Zhao F M, et al. MULTI-FLORET SPIKELET1, which encodes an AP2/ERF protein, determines spikelet meristem fate and sterile lemma identity in rice.PlantPhysiol, 2013, 162: 872-884.
[23]Thompson B E, Bartling L, Whipple C, et al. Bearded-ear encodes a MADS box transcription factor critical for maize floral development.PlantCell, 2009, 21:2578-2590.
[24]Sang X, Li Y, Luo Z, et al. CHIMERIC FLORAL ORGANS1, encoding a monocot-specific MADS box protein, regulates floral organ identity in rice.PlantPhysiol, 2012, 160: 788-807.
[25]Jin Y, Luo Q, Tong H, et al. An AT-hook gene is required for palea formation and floral organ number control in rice.DevBiol, 2011, 359: 277-288.
[26]Gallavotti A, Malcomber S, Gaines C, et al. BARREN STALK FASTIGIATE1 is an AT-hook protein required for the formation of maize ears.PlantCell, 2011, 23: 1756-1771.
[27]Yuan Z, Gao S, Xue D W, et al. RETARDED PALEA1 controls palea development and floral zygomorphy in rice.PlantPhysiol, 2009, 149: 253-244.
[28]Ren D Y, Li Y F, Wang Z, et al. Identification and gene mapping of a multi-floret spikelet 1 (mfs1) mutant associated with spikelet development in rice.JIntegerAgr, 2012, 11: 1574-1579.
[29]Yan D W, Zhang X M, Zhang L, et al. CURVED CHIMERIC PALEA 1 encoding an EMF1-like protein maintains epigenetic repression of OsMADS58 in rice palea development.PlantJ, 2015, 82: 12-24.
[30]Prasad K, Parameswaran S, Vijayraghavan U. OsMADS1, a rice MADS-box factor, controls differentiation of specific cell types in the lemma and palea and is an early-acting regulator of inner floral organs.PlantJ, 2005, 43: 915-928.
[31]Wang K, Tang D, Hong L, et al. DEP and AFO regulate reproductive habit in rice.PLoSGenet, 2010, 1: 1-9.
[32]Duan Y L, Diao Z J, Liu H Q, et al. Molecular cloning and functional characterization ofOsJAGgene based on a complete-deletion mutant in rice (Oryza sativa L.).PlantMolBiol, 2010, 74:605-615.
[33]Xiao H, Tang J F, Li Y F, et al. STAMENLESS 1, encoding a single C2H2zinc finger protein, regulates floral organ identity in rice.PlantJ, 2009, 59:789-801.
[34]Li A, Zhang Y, Wu X, et al. A LOB domain-like protein required for glume formation in rice.PlantMolBiol, 2008, 66:491-502.
[35]Sun Q W, Zhou D X. Rice jmjC domain-containing gene JMJ706 encodes H3K9 demethylase required for floral organ development.ProcNatlAcadSciUSA, 2008, 105:13679-13684.
[36]Li X J, Sun L J, Tan L B, et al.TH1, a DUF640 domain-like gene controls lemma and palea development in rice.PlantMolBiol, 2012, 78: 351-359.
[37]Ren D, Rao Y, Wu L, et al. The pleiotropic ABNORMAL FLOWER AND DWARF1 affects plant height, floral development and grain yield in rice.JIntegrPlantBiol, 2015, doi: 10.1111/jipb.12441.
[38]Toriba T, Hirano H Y. The DROOPING LEAF and OsETTIN2 genes promote awn development in rice.PlantJ, 2014, 77: 616-626.
[39]Li H, Liang W, Hu Y, et al. Rice MADS6 interacts with the floral homeotic genes SUPERWOMAN1, MADS3, MADS58, MADS13, and DROOPING LEAF in specifying floral organ identities and meristem fate.PlantCell, 2011,23: 2536-2552.
[40]Takeoka Y, Shimizu M, Wada T, et al. Science of the Rice Plant. Vol I.Nobunkyo,Tokyo, 1993, 295-326.
[41]Yoshida A, Suzaki T, Tanaka W, et al. The homeotic gene long sterile lemma (G1) specifies sterile lemma identity in the rice spikelet.ProcNatlAcadSciUSA, 2009, 106:20103-20108.
[42]Kobayashi K, Maekawa M, Miyao A, et al. PANICLE PHYTOMER2 (PAP2), encoding a SEPALLATA subfamily MADS-box protein, positively controls spikelet meristem identity in rice.PlantCellPhysiol, 2010, 51: 47-57.
[43]Terrell E E, Peterson P M, Wergin W P. Epidermal features and spikelet micromorphology inOryzaand related genera (Poaceae:Oryzeae).SmithsonianContrBot, 2001, 91:1-50.
[44]Zamora A, Barboza C, Lobo J, et al. Diversity of native rice (Oryzapoaceae) species of Costa Rica.GenetResCropEvol, 2003, 50: 855-870.
[45]Gao X C, Liang W Q, Yin C S, et al. The SEPALLATA-like gene OsMADS34 is required for rice inflorescence and spikelet development.PlantPhysiol, 2010, 153:728-740.
[46]Kaoru K, Masahiko M, Akio M, et al.PANICLEPHYTOMER2 (PAP2), encoding a SEPALLATA subfamily MADS-box protein, positively controls spikelet meristem identity in rice.PlantCellPhysiol, 2010, 51: 47-57.
[47]Li W Q, Akiko Y, Megumu T, et al. SAD1, an RNA polymerase I subunit A34.5 of rice, interacts with Mediator and controls various aspects of plant development.PlantJ, 2015, 81: 282-291.
[48]Akiko Y, Yoshihiro O, Hidemi K, et al. ABERRANT SPIKELET AND PANICLE1, encoding a TOPLESS-related transcriptional co-repressor, is involved in the regulation of meristem fate in rice.PlantJ, 2012, 70: 327-339.
[49]Lee D Y, An G. Two AP2 family genes, Supernumerary bract (SNB) and Osindeterminate spikelet 1 (OsIDS1), synergistically control inflorescence architecture and floral meristem establishment in rice.PlantJ, 2012, 69: 445-461.
[50]Lee D Y, Lee J, Moon S, et al. The rice heterochronic gene SUPERNUMERARY BRACT regulates the transition from spikelet meristem to floral meristem.PlantJ, 2007, 49: 64-78.
[51]Tsuneo K, Akira H. A novel frameshift mutant allele,fzp-10, affecting the panicle architecture of rice.Euphytica, 2012, 184: 65-72.
[52]Yi G, Choi J H, Jeong E G, et al. Morphological and molecular characterization of a new frizzy panicle mutant, "fzp-9(t)", in rice (OryzasativaL.).Hereditas, 2005, 142: 92-97.
[53]Mai K, Atsushi C, Yasuo N, et al. FRIZZY PANICLE is required to prevent the formation of axillary meristems and to establish floral meristem identity in rice spikelets.Development, 2003, 130: 3841-3850.
[54]Li M, Xiong G, Li R, et al. Rice cellulose synthase-like D4 is essential for normal cell-wall biosynthesis and plant growth.PlantJ, 2009, 60: 1055-1069.
[55]Li Y B, Fan C C, Xing Y Z, et al. Natural variation in GS5 plays an important role in regulating grain size and yield in rice.NatGenet, 2011, 12: 1266-1269.
[56]Xu C J, Liu Y, Li Y B, et al. Differential expression of GS5 regulates grain size in rice.JExpBot, 2015, 9: 2611-2623.
[57]Wang S K, Wu K, Yuan Q B, et al. Control of grain size, shape and quality by OsSPL16 in rice.NatGenet, 2012, 8: 950-954.
[58]Heang D, Sassa H. Antagonistic actions of HLH/bHLH proteins are involved in grain length and weight in rice.PLoSOne, 2012, 2: e31325.
[59]Shuhei S, Izumi K, Tsuyu A, et al. Small and round seed 5 gene encodes alpha-tubulin regulating seed cell elongation in rice.Rice, 2012, 5: 4.
[60]Kanako K, Shigeru K, Katsuyuki O, et al. A novel kinesin 13 protein regulating rice seed length.PlantCellPhysiol, 2010, 8: 1315-1329.
[61]Wang Y X, Xiong G S, Hu J, et al. Copy number variation at the GL7 locus contributes to grain size diversity in rice.NatGenet, 2015, 8: 944-948.
[62]Zhu W, Tong J P, Wu Y J. Preliminary study and selection of rice germplasm with glume gaping resistance.JPlantGenetResour, 2004, 5: 52-55.
[63]Wei X G, Zhang X W, Shao G N, et al. Fine mapping of BH1, a gene controlling lemma and palea development in rice.PlantCellRep, 2013, 9: 1455-1463.
Research Progresses in Rice Spikelet Glume Development
XUQian-kun#, REN De-yong#, LI Zi-zhuang, ZENG Da-li, GUO Long-biao, QIAN Qian*
(StateKeyLaboratoryofRiceBiology,ChinaNationalRiceResearchInstitute,Hangzhou310006,China;#These authors contributed equally to this work; *Corresponding author, E-mail: qianqian188@hotmail.com)
XU Qiankun, REN Deyong, LI Zizhuang, et al. Research progresses in rice spikelet glume development. Chin J Rice Sci, 2016, 30(1): 99-105.
Abstract:Rice (Oryza sativa L.), a monocot model plant, is an important cereal crop in the world. The flowering time, inflorescence and floral organ morphological structure have significant influence on rice yield and quality. The research on the structure and development of floral organs is helpful to improve the grain yield and rice quality. The development and morphogenesis of floral organ is a vital process from vegetative growth to reproductive growth in rice. More and more biological researches focus on its developmental pattern and molecular mechanism. The glumes of rice spikelets are unique organs and consisted of lemmas, paleae, sterile lemmas and rudimentary glumes. The molecular mechanisms of the formation and origin of glumes keep poor understanding. Recently, further study of the glumes help to not only understand the rice spikelet and floral organ development, but also facilitate understanding of the regulatory network involved in rice spikelet and floral organ development. In this paper, we focus on the rice glume development and review the ABCDE model of floral organ specialization.
Key words:rice (Oryza sativa L.); palea and lemma; sterile lemma; rudimentary glume; ABCDE model
文章编号:1001-7216(2016)01-0099-06
中图分类号:Q944.46; S511.01
文献标识码:A
基金项目:国家自然科学基金资助项目(31401464);浙江省“重中之重”学科“生物学”开放基金资助项目(KFJJ2014006)。
收稿日期:2015-10-19; 修改稿收到日期: 2015-10-30。
