Mismatch repair enzyme expression in primary and castrate resistant prostate cancer
2016-04-27BelinNghiemXiotunZhngHungMingLmLwreneTrueIlsColemnCelestiHignoPeterNelsonColinPrithrColmMorrissey
Belin Nghiem,Xiotun Zhng,Hung-Ming Lm,b, Lwrene D.True,Ils Colemn,Celesti S.Higno,e, Peter S.Nelson,e,Colin C.Prithr,Colm Morrissey,*
aDepartment of Urology,University of Washington,Seattle,WA,USA
bState Key Laboratory of Quality Research in Chinese Medicine,Macau Institute for Applied Research in Medicine and Health,Macau University of Science and Technology,Macau(SAR),China
cDepartment of Pathology,University of Washington,Seattle,WA,USA
dDivison of Human Biology,Fred Hutchinson Cancer Research Center,Seattle,WA,USA
eDepartment of Medicine,University of Washington,Seattle,WA,USA
fDepartment of Laboratory Medicine,University of Washington,Seattle,WA,USA
Original Article
Mismatch repair enzyme expression in primary and castrate resistant prostate cancer
Belinda Nghiema,Xiaotun Zhanga,Hung-Ming Lama,b, Lawrence D.Truec,Ilsa Colemand,Celestia S.Higanoa,e, Peter S.Nelsond,e,Colin C.Pritcharde,f,Colm Morrisseya,*
aDepartment of Urology,University of Washington,Seattle,WA,USA
bState Key Laboratory of Quality Research in Chinese Medicine,Macau Institute for Applied Research in Medicine and Health,Macau University of Science and Technology,Macau(SAR),China
cDepartment of Pathology,University of Washington,Seattle,WA,USA
dDivison of Human Biology,Fred Hutchinson Cancer Research Center,Seattle,WA,USA
eDepartment of Medicine,University of Washington,Seattle,WA,USA
fDepartment of Laboratory Medicine,University of Washington,Seattle,WA,USA
Mismatch repair;
Castration resistant
prostate cancer;
MLH1;
MSH2;
MSH6;
PMS2
Objective:Although the utility of immunohistochemistry(IHC)for assessing mismatch repair(MMR)protein expression has been demonstrated in solid tumors including primary prostate cancer(PCa),its utility has not been assessed in castration-resistant PCa (CRPC).
Methods:Tissue microarrays were constructed from 127 radical prostatectomies and 155 CRPC metastases from 50 patients.MMR(MLH1,MSH2,MSH6,and PMS2)expression was assessed by IHC and gene expression arrays.Associations between MMR protein expression in PCa and CRPC and biochemicalrecurrence(BCR)ortime from diagnosisto death respectively were determined. Results:There was no correlation between levels of MMR protein and BCR.Absence of MSH2 and MSH6 wasthe mostpronounced at15%and 22%in PCa and 17.8%and 16%in CRPCpatients,respectively.MSH2 and MSH6 protein were absent in 9.4%and 8%of PCa and CRPCrespectively.Absence of individual MMR proteins did not correlate with BCR or time from diagnosis to death.However absent MSH2/MSH6 in CRPC was associated with shortertime to death(p=0.0006).Loss of MSH2 was verified at the gene expression level.This finding correlated with microsatellite instability previously reported in this CRPC cohort.
Conclusion:The absence of MLH1,MSH2,MSH6,and PMS2 protein and combinations thereof are frequent in PCa.Loss of MSH2/MSH6 protein may predict poor outcome in patients with CRPC.
©2016 Editorial Office of Asian Journal of Urology.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/ licenses/by-nc-nd/4.0/).
1.Introduction
MLH1,MSH2,MSH6,and PMS2 are all mismatch repair(MMR) enzymes that are associated with microsatellite instability in cancer[1].Microsatellite instability has been observed in prostate cancer(PCa)with Christians et al.[2]reporting one of 30 patients displaying instability and Suzuki et al.[3] reporting seven of 48 patients displaying stability,however, these differences may re flect differences in the patient populations studied.To repair mismatched nucleotides the MLH1,MSH2,MSH6,and PMS2 MMR enzymes form a complex through the initial heterodimerization of MSH2/MSH6 which first identi fies the mismatch and accommodates the MLH1/ PMS2 heterodimer to initiate repair of the mismatch defect [4,5].Our study focused on assessing the expression of MLH1,MSH2,MSH6,and PMS2 in PCa by immunohistochemistry(IHC).
Mismatch repair enzyme loss has previously been assessed by IHC in PCa.In a study of 81 PCa’s on a tissue microarray there was no complete loss of MLH1 protein[6]. Interestingly PMS2 expression has been shown to be elevated in PCa[7]and has been described as a predictor for biochemical recurrence after radical prostatectomy[8]. MSH2 IHC staining intensity has been shown to correlate with Gleason score,overall and disease-free survival in PCa [6,9,10].Additional IHC studies have focused on patient populations that are carriers for MMR enzyme deficiencies considered to be more at risk for the development of PCa [11-14].Loss of MLH1,PMS2 and MSH2 proteins has also been described in PCa cell lines(DU145,LNCaP,p69SV40T, M2182,and M12)[15-17].
Fifteen percent of colorectal cancers have a hypermutated phenotype and microsatellite instability[18].The hypermutated phenotype has also been described in PCa [19].Pritchard et al.[20]estimated that 12%of CRPCs are hypermutated,and that all of the hypermutated cancers had mismatch repair gene mutations and microsatellite instability.
We analyzed a primary PCa cohort and further analyzed the Pritchard cohort to further characterize MMR expression in CRPC by IHC.We observed that the absence of MSH2 and MSH6 expression by IHC are frequent events in both primary PCa and CRPC.Furthermore,we found no substantial decrease in MMR protein levels by IHC in CRPC versus primary PCa.Contrary to other IHC studies of MMR protein expression we did not observe any association between MMR protein expression and time to biochemical recurrence.However,in our cohort of CRPC patients the absence of MSH2/MSH6 expression by IHC was associated with rapid disease progression.
2.Materials and methods
2.1.Tissue acquisition and microarray construction
Human PCa specimens were obtained as part of the University of Washington Medical Center Prostate Cancer Donor Program,which is approved by the University of Washington Institutional Review Board[21].All specimens for IHC were formalin fixed(and,for bone specimens,decalcified in formic acid),paraffin embedded and examined histologically for presence of non-necrotic tumor.Tissue microarrays (TMA)were constructed with 1 mm-diameter duplicate cores from primary PCa(consisting of 127 radical prostatectomy specimens;clinical data are detailed in Supplemental Table 1),and CRPC(consisting of 155 CRPC metastases including 73 visceral metastases and 82 bone metastases from 50 patients within 8 h of death,up to 4 sites per patient).The clinical data have been previously reported[22].
2.2.IHC
Five micron thick sections of formalin-fixed paraffinembedded tissue were deparaffinized.Antigen retrieval was performed with heat-induced epitope retrieval. Endogenous peroxidase and biotin were blocked and sections were then blocked with 5%normal goat-horse-chicken serum,and incubated with the primary antibody.After washing with PBS,slides were incubated with biotinylated secondary antibody(Vector Laboratories Inc.,Burlingame, CA,USA),followed by ABC reagent(Vector Laboratories Inc.)and stable diaminobenzidine(Invitrogen Corp.,Waltham,MA,USA).All sections were lightly counterstained with hematoxylin and mounted with Cytoseal XYL(Richard Allan Scientific).Mouse or rabbit immunoglobulin-G was used at the same concentration as the primary antibody for negative controls.Antibodies and dilutions used for IHC are described in Supplemental Table 2.
2.3.IHC assessment
All assessments were performed on 1 mm-diameter cores from primary PCa and CRPC.
2.3.1.MMR enzyme expression levels
Immunostaining of nuclei was assessed using a quasicontinuous score system,created by multiplying each intensity level(“0”for no brown color,“1”for faint and fine brown chromogen deposition,and“2”for clear and coarse granular chromogen clumps)with the percentage of cells stained at each respective intensity.We then summed allvalues to a final score for each sample(scores ranged from 0 to 200)[23].
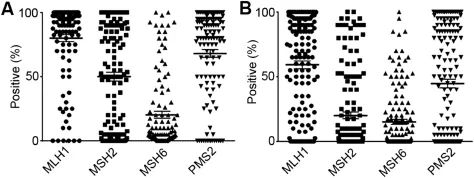
Figure 1Nuclear mismatch repair protein expression in primary prostate cancer and castration-resistant prostate cancer metastases.Immunohistochemistry analysis of MLH1,MSH2,MSH6,and PMS2 in primary PCa(A)and CRPC metastases(B).Median nuclear staining was reduced in CRPC metastases when compared to primary PCa.Of note,median MSH6 staining was lower than MLH1,MSH2,and PMS2.
2.3.2.Presence or absence of MMR enzyme expression
MMR protein positive patients were defined as those patients where nuclei of any tumor cell within the patient specimen stained positive.Negative patients were defined as those patients where there was an absence of nuclear staining in any tumor cell in the patient specimen.For metastasis specimens where multiple metastases were available for analysis from one patient all sites of metastasis needed to be negative for the patient to be defined as a negative patient.Samples with missing or damaged sections were excluded from analysis.
2.4.RNA isolation,amplification and microarray hybridization
Gene expression data have been published[24](GEO accession#GSE77930).Briefly,total RNA was isolated from laser capture micro dissected frozen CRPC metastases,using the Arcturus Pico Pure RNA Isolation Kit(Thermo Fisher,Waltham,MA,USA)and DNAse treated using the Qiagen RNase-Free DNase Set(Hildan,Germany).RNA was amplified for two rounds using the Ambion Message Ampa RNA Kit(Waltham,MA,USA).Agilent 44 K whole human genome expression oligonucleotide microarrays(Agilent Technologies,Inc.) were used to profile the CRPC metastases.
2.5.Statistical analysis
Significance of differences for the transcript analyses was calculated using a student’s t-test.P values≤0.05 indicated statistical significance.Biochemical recurrence and survival proportions were compared by Kaplan-Meier plot with log-rank test using GraphPad Prism version 6.02 (La Jolla,CA,USA).
3.Results
3.1.Mismatch repair enzyme levels in primary PCa
Nuclear staining was verified by IHC for all four MMR proteins(Supplemental Figs.1-4).Nuclear scores ranged from negative(0)to completely positive(200).To determine if the levels of MMR enzyme expression were associated with biochemical recurrence we compared the outcomes of high (≥100)vs.low(<100)expressing MLH1,MSH2,and PMS2 patients.Since the mean staining score of MSH6 was 20.3±29.5,we used a threshold value of high(≥50)vs.low (<50)(Fig.1A).There was no statistically significant association of protein expression level with biochemical recurrence for each of the MMR proteins(Supplemental Fig.5).
3.2.Mismatch repair enzyme levels in CRPC
To determine if levels of MMR proteins are lower in patients with advanced disease we compared the nuclear score in CRPC relative to primary PCa.Mean expression levels dropped from 80.1±29.4 to 59.2±37.3 for MLH1, 50.1±38.3 to 20.1±31.5 for MSH2,20.3±29.5 to 15.2±23.8 for MSH6,and 68.1±33.5 to 44.7±40.6 for PMS2 in primary PCa compared to CRPC(Fig.1).Thus we found moderate decreases in the expression of all of the MMR enzymes in CRPC relative to primary PCa.In addition, we found that different metastases within the same patient expressed each protein at different levels,however,no differences in MMR enzyme expression were observed between bone and visceral metastases.
3.3.Complete loss of mismatch repair enzyme levels in primary PCa
Since the complete loss of protein expression by IHC has been used to identify patients and specimens with MMR deficiency and microsatellite instability,we switched our focus to evaluating specimens with complete loss of MMR expression in both primary PCa and CRPC.In primary PCa we determined that 5.0%,15.0%,17.8%,and 11.2%of the patients specimens did not stain for MLH1,MSH2,MSH6,and PMS2,respectively(Table 1).Since MLH1 forms a functional heterodimer with PMS2 and MSH6 forms a functional heterodimer with MSH2,we also determined the absence of MLH1/PMS2(2.6%)and MSH2/MSH6(9.4%)in primary PCa. We observed an absence of staining for all four MMR proteins in 2.6%of the primary PCa specimens(Table 1; Supplemental Table 3).Absent staining was not associated with biochemical recurrence for any of the four MMR proteins or MSH2/MSH6 in primary PCa(Supplemental Fig.6).
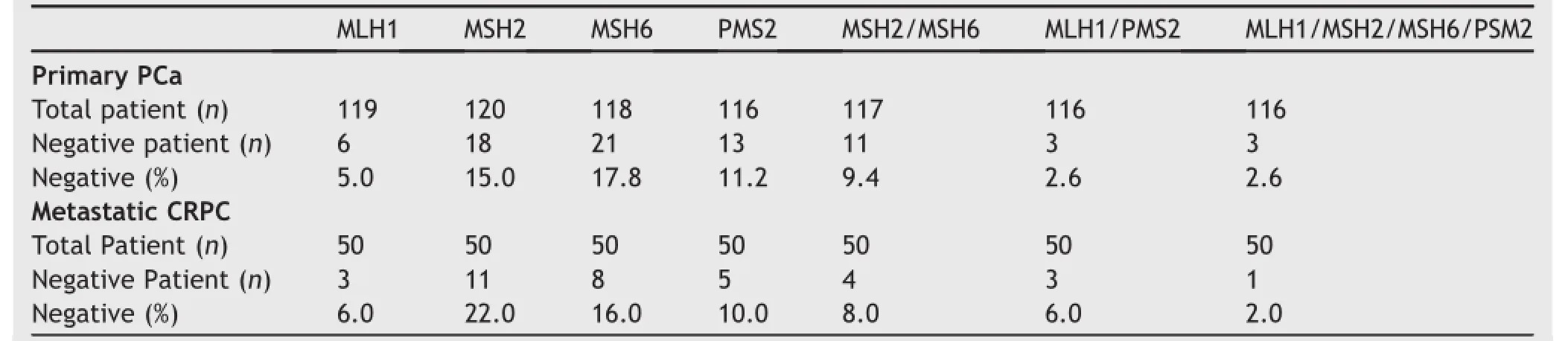
Table 1Absence of MMR protein expression by IHC in primary PCa and CRPC metastasis.The absence of MMR protein expression in primary PCa and CRPC metastases expressed as a%of total patients/cohort.Absence of MSH2 and MSH6 expression was pronounced in both primary PCa and CRPC patients.
3.4.Complete loss of mismatch repair enzyme levels in CRPC
In CRPC we determined that 6.0%,22.0%,16.0%,and 10.0% of the patients specimens did not stain for MLH1,MSH2, MSH6,and PMS2,respectively(Table 1).Six percent of the CRPC patients had no observable MLH1 and PMS2(MLH1/ PMS2)protein expression and 8%had no observable expression of MSH2 and MSH6(MSH2/MSH6).MMR protein staining for all four MMR proteins was absent in 2.0%of the CRPC patients(Table 1;Supplemental Table 4).We determined from previously published data that all four MSH2/MSH6-negative patients had microsatellite instability[20].The absence of staining was not associated with a shortened time from diagnosis to death for the four individual MMR proteins in CRPC patients(Fig.2A-D). However,the absence of MSH2/MSH6 protein expression was associated with a shortened time from diagnosis to death(p=0.0006)(Fig.2E).Loss of MSH2 and MSH6 protein staining usually indicates a germline MSH2 mutation[25]therefore we compared gene expression in CRPC specimens to determine if the loss of MSH6 transcript was associated with the loss of MSH2 transcript.The MSH2 transcript was significantly decreased in patients missing MSH2/MSH6 protein relative to all other specimens in the CRPC patients(p<0.0001;Supplemental Fig.7). The MSH6 transcript was also significantly decreased in patients missing MSH2/MSH6 protein relative to all other specimens in the CRPC patients(p=0.0018; Supplemental Fig.7).
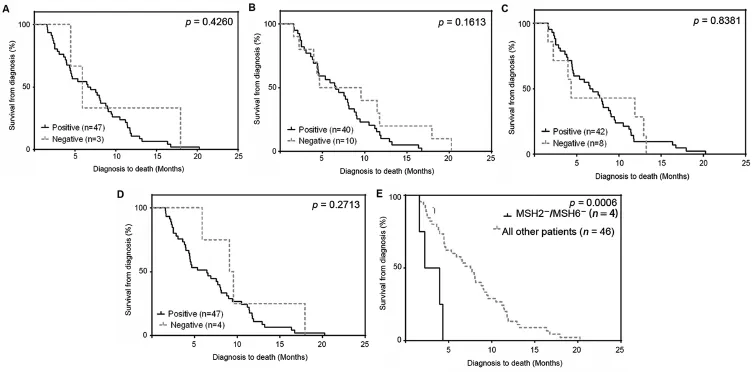
Figure 2Absence of MSH2/MSH6 in castration-resistant prostate cancer(CRPC)metastases is associated with shortened time from diagnosis to death.Kaplan-Meier analysis on the individual mismatch repair(MMR)enzymes determined the absence of staining was not associated with shortened time from diagnosis to death for each of the four MMR proteins(A is MLH1,B is MSH2,C is MSH6,and D is PMS2.)in CRPC patients.(E)Kaplan-Meier analysis of MSH2/MSH6(n=4)vs.all other patients(n=46).Absence of MSH2/MSH6 revealed a significant trend towards a shortened survival time(p=0.0006).
4.Discussion
There is precedence for using TMAs to assess MMR protein expression in cancer specimens.In a study of colon cancer Hendricks et al.[26]compared whole slides to a TMA from a cohort of 129 patients and assessed MLH1,MSH2,and MSH6 protein expression and MSI.The TMA,showed a concordance of 85%,95%,and 75%for MLH,MSH2,and MSH6, respectively.TMAs have also been used to determine MMR protein expression in ovarian cancer,endometrial cancer, and PCa[10,27,28].
Differential expression of MMR enzymes has been associated with disease recurrence in PCa.Velasco et al.[9] observed that decreased MSH2 expression in PCa specimens from 73 patients was associated with a decreased risk for time to PSA recurrence after radical prostatectomy (p=0.08).Prtilo et al.[10]in a cohort of 223 men also demonstrated that patients with low MSH2 expressing tumors had a significant survival advantage(p<0.0004). Additionally,in a cohort of 58 patients Burger et al.[6] observed a significant association between moderate/ strong MSH2 and tumor recurrence(p=0.039).Further, Norris et al.[8]in a cohort of 166 patients determined the mean level of PMS2 protein was higher in tumors of patients with high grade tumors who recurred,compared with nonrecurrent patients and that PMS2 was an independent predictor of time-to-recurrence(P<0.001).However,in our cohort of 127 patients we observed no association of MLH1,MSH2,MSH6,or PMS2 expression levels with recurrence.The discordance between our study and those of others may be due to differences in patient numbers, immunohistochemical methods,follow-up times or the cohorts studied.For example,while approaching significance Velasco et al.[9]did not observe a statistically significant association of MSH2 with biochemical recurrence,Burger et al.[6]examined the association of MSH2 with recurrence over 3 years and Norris et al.[8]focused on patients with aggressive disease.However,Prtilo et al.[10]assessed MSH2 in considerably more patient specimens(n=243) using similar methods and determined that MSH2 expression correlated with biochemical disease-free survival (P=0.018).
Our study focused more on the absence of staining rather than changes in MMR protein expression levels.We reasoned that since the loss of MMR function leads to MSI only the complete loss of any MMR protein would lead to genetic instability and a more aggressive tumor phenotype. Nevertheless,we did not observe any significant decrease in time to recurrence in patients where we observed an absence of any of the four MMR proteins(MLH1,MSH2, MSH6,or PMS2)or combinations thereof.When comparing the absence of the four MMR proteins in primary vs. castration resistant disease we determined there was little to no difference in the incidence of MMR protein negativity between the hormone naı¨ve and late stage CRPC disease. This result suggests that the absence of MMR protein expression does not necessarily lead to an increase in disseminated disease in hormone naı¨ve tumors.
Pritchard et al.[20]have shown that complex structural rearrangements in mismatch DNA repair genes MSH2 and MSH6 are a major mechanism that result in MSI.Our data comprise some of the individuals in the Pritchard data set. We determined the four CRPC patients that were negative for both MSH2 and MSH6 protein in CRPC patients from the Pritchard dataset had MSI[20]and a shorter time from diagnosis to death.Furthermore,we determined there was a significant decrease in MSH2 and MSH6 at the transcript level in the MSH2/MSH6 negative specimens(which was more pronounced for MSH2).
The loss of MSH6 protein expression usually follows the loss of MSH2 expression suggesting that the absence of both proteins in a patient specimen may clearly define a group of patients in PCa that have MSI.Whether MMR enzyme inactivity and subsequently MSI in advanced CRPC provides the tumor with more effective ways to evade androgen deprivation therapy and conventional treatment,remains to be seen.
5.Conclusion
The absence of MLH1,MSH2,MSH6,and PMS2 protein and combinations thereof are frequent in PCa.The number of patients whose cancers did not express any MMR protein by IHC was similar in primary PCa and CRPC.No significant difference in biochemical recurrence was observed between patients with tumor tissues expressing MMR proteins versus negative cancers.These data suggest that(i)the frequency of MSH2-/MSH6-tumors are similar in primary PCa and CRPC,and(ii)the absence of MSH2/MSH6 in metastases may impact survival in patients with CRPC.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
We thank the patients and their families who were willing to participate in the Prostate Cancer Donor Program.The investigators Drs.Robert Vessella,Bruce Montgomery,Evan Yu,Heather Cheng,Elahe Mostaghel,Paul Lange,and Martine Roudier for their contributions to the University of Washington Medical Center Prostate Cancer Donor Rapid Autopsy Program.This research was supported by funding by the Pacific Northwest Prostate Cancer SPORE(P50CA97186), R01CA165573,and the Richard M.LUCAS Foundation.Colm Morrissey is a recipient of a Career Development Award from Jim and Catherine Allchin.
Appendix A.Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ajur.2016.09.002.
[1]Shia J,Stadler Z,Weiser MR,Rentz M,Gonen M,Tang LH, et al.Immunohistochemical staining for DNA mismatch repair proteins in intestinal tract carcinoma:how reliable are biopsy samples?Am J Surg Pathol 2011;35:447-54.
[2]Christians F,Connolly D,Tsuchiya K,True L,Loeb L.Lack of microsatellite instability in human prostate-cancer.Int J Oncol 1995;6:1173-6.
[3]Suzuki H,Komiya A,Aida S,Akimoto S,Shiraishi T,Yatani R, et al.Microsatellite instability and other molecular abnormalities in human prostate cancer.Jpn J Cancer Res 1995;86: 956-61.
[4]Sharma M,Predeus AV,Kovacs N,Feig M.Differential mismatch recognition specificities of eukaryotic MutS homologs,MutSalpha and MutSbeta.Biophys J 2014;106:2483-92.
[5]Pal T,Permuth-Wey J,Sellers TA.A review of the clinical relevance of mismatch-repair deficiency in ovarian cancer. Cancer 2008;113:733-42.
[6]Burger M,Denzinger S,Hammerschmied CG,Tannapfel A, Obermann EC,Wieland WF,et al.Elevated microsatellite alterations at selected tetranucleotides(EMAST)and mismatch repair gene expression in prostate cancer.J Mol Med(Berl) 2006;84:833-41.
[7]Norris AM,Woodruff RD,D’Agostino Jr RB,Clodfelter JE, Scarpinato KD.Elevated levels of the mismatch repair protein PMS2 are associated with prostate cancer.Prostate 2007;67: 214-25.
[8]Norris AM,Gentry M,Peehl DM,D’Agostino Jr R, Scarpinato KD.The elevated expression of a mismatch repair protein is a predictor for biochemical recurrence after radical prostatectomy.Cancer Epidemiol Biomarkers Prev 2009;18: 57-64.
[9]Velasco A,Hewitt SM,Albert PS,Hossein M,Rosenberg H, Martinez C,et al.Differential expression of the mismatch repair gene hMSH2 in malignant prostate tissue is associated with cancer recurrence.Cancer 2002;94:690-9.
[10]Prtilo A,Leach FS,Markwalder R,Kappeler A,Burkhard FC, Cecchini MG,et al.Tissue microarray analysis of hMSH2 expression predicts outcome in men with prostate cancer.J Urol 2005;174:1814-8.
[11]Zeinalian M,Emami MH,Naimi A,Salehi R,Hashemzadeh-Chaleshtori M.Immunohistochemical analysis of mismatch repair proteins in Iranian colorectal cancer patients at risk for lynch syndrome.Iran J Cancer Prev 2015;8:11-7.
[12]Rosty C,Walsh MD,Lindor NM,Thibodeau SN,Mundt E, Gallinger S,et al.High prevalence of mismatch repair def iciency in prostate cancers diagnosed in mismatch repair gene mutation carriers from the colon cancer family registry.Fam Cancer 2014;13:573-82.
[13]Fredriksson H,Ikonen T,Autio V,Matikainen MP,Helin HJ, Tammela TL,et al.Identification of germline MLH1 alterations in familial prostate cancer.Eur J Cancer 2006;42: 2802-6.
[14]Soravia C,van der Klift H,Brundler MA,Blouin JL,Wijnen J, Hutter P,et al.Prostate cancer is part of the hereditary nonpolyposis colorectal cancer(HNPCC)tumor spectrum.Am J Med Genet A 2003;121A:159-62.
[15]Leach FS,Velasco A,Hsieh JT,Sagalowsky AI,McConnell JD. The mismatch repair gene hMSH2 is mutated in the prostate cancer cell line LNCaP.J Urol 2000;164:1830-3.
[16]Chen Y,Wang J,Fraig MM,Metcalf J,Turner WR,Bissada NK, et al.Defects of DNA mismatch repair in human prostate cancer.Cancer Res 2001;61:4112-21.
[17]Yeh CC,Lee C,Dahiya R.DNA mismatch repair enzyme activity and gene expression in prostate cancer.Biochem Biophys Res Commun 2001;285:409-13.
[18]Boland CR,Goel A.Microsatellite instability in colorectal cancer.Gastroenterology 2010;138:2073-87.
[19]Kumar A,White TA,MacKenzie AP,Clegg N,Lee C,Dumpit RF, et al.Exome sequencing identifies a spectrum of mutation frequencies in advanced and lethal prostate cancers.Proc Natl Acad Sci USA 2011;108:17087-92.
[20]Pritchard CC,Morrissey C,Kumar A,Zhang X,Smith C, Coleman I,et al.Complex MSH2 and MSH6 mutations in hypermutated microsatellite unstable advanced prostate cancer.Nat Commun 2014;5:4988.
[21]Roudier MP,True LD,Higano CS,Vesselle H,Ellis W,Lange P, et al.Phenotypic heterogeneity of end-stage prostate carcinoma metastatic to bone.Hum Pathol 2003;34:646-53.
[22]Zhang X,Coleman IM,Brown LG,True LD,Kollath L,Lucas JM, et al.SRRM4 expression and the loss of REST activity may promote the emergence of the neuroendocrine phenotype in castration-resistant prostate cancer.Clin Cancer Res 2015;21: 4698-708.
[23]Zhang X,Morrissey C,Sun S,Ketchandji M,Nelson PS,True LD, et al.Androgen receptor variants occur frequently in castration resistant prostate cancer metastases.PLoS One 2011;6. e27970.
[24]Kumar A,Coleman I,Morrissey C,Zhang X,True LD,Gulati R, et al.Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer.Nat Med 2016;22:369-78.
[25]Zhang L.Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome.Part II.The utility of microsatellite instability testing.J Mol Diagn 2008;10:301-7.
[26]Hendriks Y,Franken P,Dierssen JW,De LW,Wijnen J,Dreef E, et al.Conventional and tissue microarray immunohistochemical expression analysis of mismatch repair in hereditary colorectal tumors.Am J Pathol 2003;162:469-77.
[27]Coppola D,Nicosia SV,Doty A,Sellers TA,Lee JH,Fulp J,et al. Uncertainty in the utility of immunohistochemistry in mismatch repair protein expression in epithelial ovarian cancer.Anticancer Res 2012;32:4963-9.
[28]Resnick KE,Frankel WL,Morrison CD,Fowler JM,Copeland LJ, Stephens J,et al.Mismatch repair status and outcomes after adjuvant therapy in patients with surgically staged endometrial cancer.Gynecol Oncol 2010;117:234-8.
Received 29 June 2016;received in revised form 19 August 2016;accepted 31 August 2016
Available online 12 September 2016
*Corresponding author.Genitourinary Cancer Research Laboratory,Department of Urology,Box 356510,University of Washington, Seattle,WA,USA.
E-mail address:cmorriss@uw.edu(C.Morrissey).
Peer review under responsibility of Second Military Medical University.
http://dx.doi.org/10.1016/j.ajur.2016.09.002
2214-3882/©2016 Editorial Office of Asian Journal of Urology.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
杂志排行
Asian Journal of Urology的其它文章
- Metastasis in renal cell carcinoma:Biology and implications for therapy
- Developing immunotherapy strategies in the treatment of prostate cancer
- Novel immunotherapy approaches for metastatic urothelial and renal cell carcinoma
- Intrinsic subtypes and bladder cancer metastasis
- Cultured circulating tumor cells and their derived xenografts for personalized oncology
- Specific bone region localization of osteolytic versus osteoblastic lesions in a patient-derived xenograft model of bone metastatic prostate cancer
