Metastasis in renal cell carcinoma:Biology and implications for therapy
2016-04-27JunGongMnuelCitnoMiNzliDizmnAmeishGovindrjnSumntPl
Jun Gong,Mnuel Citno Mi,Nzli Dizmn, Ameish Govindrjn,Sumnt K.Pl,*
aDepartment of Medical Oncology&Experimental Therapeutics,City of Hope Comprehensive Cancer Center,Duarte,CA,USA
bDivision of Medical Oncology,Instituto Hemomed de Oncologia e Hematologia.Av.Arnolfo de Azevedo,121-Cerqueira Ce´sar-CEP 01248-040,Sao Paulo,Brazil
cDepartment of Internal Medicine,Istanbul Medeniyet University Goztepe Research and Training Hospital,Istanbul,Turkey
Metastasis in renal cell carcinoma:Biology and implications for therapy
Jun Gonga,1,Manuel Caitano Maiab,1,Nazli Dizmanc, Ameish Govindarajana,Sumanta K.Pala,*
aDepartment of Medical Oncology&Experimental Therapeutics,City of Hope Comprehensive Cancer Center,Duarte,CA,USA
bDivision of Medical Oncology,Instituto Hemomed de Oncologia e Hematologia.Av.Arnolfo de Azevedo,121-Cerqueira Ce´sar-CEP 01248-040,Sao Paulo,Brazil
cDepartment of Internal Medicine,Istanbul Medeniyet University Goztepe Research and Training Hospital,Istanbul,Turkey
Renal cell carcinoma;
Metastasis;
Vascular endothelial
growth factor;
Mammalian target of
rapamycin;
Hypoxia inducible
factor
Although multiple advances have been made in systemic therapy for renal cell carcinoma(RCC),metastatic RCC remains incurable.In the current review,we focus on the underlying biology of RCC and plausible mechanisms of metastasis.We further outline evolving strategies to combat metastasis through adjuvant therapy.Finally,we discuss clinical patterns of metastasis in RCC and how distinct systemic therapy approaches may be considered based on the anatomic location of metastasis.
©2016 Editorial Office of Asian Journal of Urology.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/ licenses/by-nc-nd/4.0/).
1.Introduction
Approximately one-third of patients with renal cell carcinoma(RCC)present with metastatic disease,and amongst those patients with localized disease,a substantial proportion will recur[1].For patients with metastatic renal cell carcinoma(mRCC),the landscape of therapy has evolved dramatically over the past decade.Prior to 2005, immunotherapy represented the mainstay of therapy with agents such as interleukin-2(IL-2)and interferon-α(IFN-α) [2,3].Estimates of overall survival(OS)in that era coalesced around 1 year.Since 2005,multiple targeted therapies have been approved,primarily directed at vascular endothelial growth factor(VEGF)or its cognate receptor (VEGF receptor,or VEGFR),or the mammalian target of rapamycin(mTOR)[4].In this generation of therapies,inhibitors of VEGF include axitinib,bevacizumab,pazopanib, sorafenib and sunitinib,while inhibitors of mTOR includeeverolimus and temsirolimus[5-10].These agents have collectively improved median survival estimates to approximately 2.5-3 years[11].Over the past year,3 additional FDA approvals have been granted for mRCC for a VEGFR/MET/AXL inhibitor(cabozantinib),a programmed death-1(PD-1)inhibitor(nivolumab)and a multikinase inhibitor(lenvatinib,approved with everolimus)[12-14].It remains to be seen how these agents will alter OS estimates for mRCC,although it will surely move the bar in a positive direction.
Despite these critical advances,the reality is that the vast majority of patients with mRCC have incurable disease [15].A goal of treatment is to maximize the yield of existing systemic therapies through personalized approaches.In the current review,we explore how clinical and biological properties of metastases may potentially alter paradigms for systemic therapy.
2.Biology of RCC
2.1.Differing biology by histology
It is critical to acknowledge that mRCC is comprised of multiple distinct histologies,each with unique biologic underpinnings.The most common histology is clear cell, comprising 75%-80%of cases.Approximately 70%of patients with clear cell RCC bear alterations in the Von-Hippel Lindau (VHL)gene[16].Wild type VHL protein functions as an ubiquitin ligase,participating in degradation of hypoxia inducible factor(HIF).In patients bearing VHL alteration,the resulting high levels of HIF result in upregulation of VEGF.VEGF activates VEGFR,triggering the phosphoinositol-3-kinase(PI3K)-Akt signaling cascade.Downstream,mTOR is activated and leadsto transcription ofa variety oftumor-promoting factors, resulting in increased cellular migration and angiogenesis. Although VEGFR has typically been implicated as the key driver of tumor progression in RCC,there is emerging evidence that other transmembrane receptors may potentially drive metastasis,including METand AXL[17].
Non-clear cell RCC histologies comprise roughly 20%-25% of patients overall.The most prevalent of these is papillary RCC,which represents 10%-15%of patients.Papillary RCC is frequently subdivided into type I and type II disease.Type I disease is characterized by alterations in the MET protooncogene,while type II is characterized by a variety of alterations.Recent data from The Cancer Genome Atlas (TCGA)investigators highlighted alterations in SETD2, CKDN2A and TFE3 fusions as frequent events in type II papillary RCC[18].Chromophobe type disease comprises approximately 5%of all RCC cases.TCGA data pertaining to chromophobe RCC suggest frequent changes in the TERT promoter region,and mitochondrial DNA analyses suggest changes in mitochondrialfunction[19].Beyond papillary and chromophobe RCC,otherhistologies of RCCrepresent<1%of all cases.Despite their rarity,there are efforts to characterize the genomic changes occurring in these entities.For instance,our group has identified frequent alterations in NF2 in patients with collecting duct RCC,an exquisitely rare diagnosis with a dismal prognosis[20].
Admixed with any histological subset of RCC may be sarcomatoid elements.Sarcomatoid RCC is thought to coexist with other histologies in about 25%of cases[21]. Sarcomatoid disease tends to be particularly aggressive, although(as discussed subsequently)the current treatment paradigm is not distinct from clear cell disease.Our group has identified frequent alterations in the aurora kinase pathway,and NF2 alterations have also been detected in this disease[22,23].
2.2.Tumor heterogeneity
Although histology is frequently used to offer prognostic data to patients,it is critical to acknowledge that the biology of tumors may differ across sites of metastasis.One of the first detailed studies to identify this intratumoral heterogeneity was from Gerlinger and colleagues[24].In an effort that included just 4 patients with mRCC,separate sites of metastasis were evaluated.Alterations in the mTOR pathway were variable across sites of metastasis,as were alterations in SETD2,PTEN and KDM5C.Subsequent sections will highlight potential therapeutic strategies for these alterations.With the evolution of novel immunotherapeutic strategies,there has also been substantial interest in characterizing PD-L1 expression in metastatic sites.A recent study from the Dana Farber Cancer Institute compared tissues derived from 53 primary RCC specimens and 73 corresponding metastases[25].PD-L1 expression appeared to be consistent,although PD-L1 expression was noted to be heterogeneous within lesions.
2.2.1.Biological mediators of metastasis
Little is agreed upon regarding the biological mechanisms that drive RCC metastasis.On a macromolecular level, Grange et al.[26]have proposed that tumor-derived microvesicles(which essentially break off from the primary site)may disperse tumors through hematogenous routes.These microvesicles appear to bear CD105-positive cells,which carry a cancer stem cell phenotype,and microRNAs which stimulate angiogenesis.The immune milieu may also play a critical role in the evolution of metastases.In preclinical models,neutrophilic infiltration in the lungs(accompanied by secretion of neutrophil chemokines)was accompanied by suppression of pulmonary metastases of RCC[27].In contrast,loss of neutrophil chemokines in the lung was accompanied by an increase in pulmonary metastases.Other immune cells with negative effects on antitumor immunity(e.g.,myeloid derived suppressor cells,or MDSCs)have been shown to have a proangiogenic effect and cause propagation of RCC in preclinical models[28].
Beyond these macromolecular events,several molecular mediators of RCC metastasis have been identified.In the setting of clear cell RCC bearing VHL alteration,it has been proposed that CUB-domain-containing protein(CDCP1)may drive metastasis[29].CDCP1 is regulated through HIF dependent pathways and drives activation of protein kinase C-δ(PKCδ),which in turn increases cellular migration. Expression of MUC1,a membrane-bound glycoprotein,is also HIF-dependent,and knockdown of MUC1 has been shown to markedly decrease cellular invasion and migration in in vitro RCC models[30].Various chemokine receptors, including CXCR4,also appear to be upregulated in thecontext of clear cell RCC.An increased expression of CXCR4 and its ligand CXCL12 in the setting of VHL alteration has been associated with increased metastatic spread in preclinical studies[31].
3.Prevention of tumor metastasis:role of adjuvant therapy
3.1.Localized disease
At present,there is no defined role for adjuvant therapy for localized RCC following partial or radical nephrectomy.The current standard of care involves serial imaging with computerized tomography,typically up to a span of 5 years. However,multiple trials have evaluated the strategy of using approved agents in the metastatic setting as adjuvant therapy[32].Both adjuvant VEGF-tyrosine kinase inhibitors (VEGF-TKIs)and mTOR inhibitors have been evaluated, including sunitinib,sorafenib,pazopanib,axitinib and everolimus.
The first phase III study to report out exploring adjuvant therapy is the phase III ASSURE trial[33].This study randomized 1943 patients to receive one of two VEGF-TKIs (sunitinib or sorafenib)or placebo.The study had to be modified on the basis of early toxicity events;in a modif ication to the original design,patients were started on a lower dose of sunitinib and sorafenib.Doses were escalated if no toxicities were incurred after a 2-month period.The primary endpoint of the study was disease-free survival, and the trial identified no significant difference across the three cohorts(5.8 years with sunitinib,6.1 years with sorafenib and 6.6 years with placebo).Aside from failing to meet the primary endpoint of the study,there were also concerns regarding treatment related toxicity.Toxicities encountered were typical for VEGF-directed therapies, with the most common adverse events being hypertension, hand-foot syndrome,rash and fatigue.A total of five deaths occurred within the first month of protocol-based therapy. Four of these patients had received sunitinib,with deaths attributable to neurologic dysfunction,gastrointestinal perforation,pulmonary embolus and disease progression, respectively.The remaining patient received sorafenib and developed infectious colitis during therapy.In the adjuvant setting,even if a modest benefit with adjuvant therapy was observed,it would need to be counterbalanced against the toxicity profile and the potential risk of treatment-related mortality.
The remaining trials of adjuvant targeted therapy in RCC have yet to report out.Each of these studies includes a slightly different patient population and treatment strategy,and thus,it is possible that the results may differ from the aforementioned ASSURE trial(Table 1).For instance, the phase III ATLAS study compares a total of 3 years of axitinib therapy to placebo,and the phase III SORCE study similarly examines 3 years of adjuvant sorafenib.The phase III EVEREST trial uniquely explores the mTOR inhibitor everolimus-it is possible that the increased tolerability of mTOR inhibition may make enhance adherence and dose intensity.Although ASSURE is the first study to report final results,data from the phase III S-TRAC trial are anticipated shortly.A recent press release suggested that this trial(a comparison of 1 year of adjuvant therapy with sunitinib to placebo)met its primary endpoint of improved disease-free survival[34].The study differed from ASSURE in that it included a higher risk population.Specifically,while ASSURE allowed enrollment of patients with pT1b and grade 3-4 disease,or pT2-4 disease and any grade,S-TRAC enrollment was limited to patients with pT3-4 disease with any grade.The study included just over 670 patients,a sample size considerably smaller than ASSURE.
It is curious that S-TRAC would yield a positive result.A subset analysis of the ASSURE study limited to pT3-4 patients still failed to show a benefit in disease-free survival, despite including a sample size comparable to the overall STRAC study population.Subtle differences such as radiographic interpretation may potentially account for the discordant results-S-TRAC employed central radiographic review,while local review was performed in the ASSURE trial.In any case,the implementation of results from STRAC will likely be contingent on a number of factors as yet unknown,including the magnitude of benefit with adjuvant sunitinib in this study and the degree of toxicity incurred.
Beyond VEGF-and mTOR-inhibitors,the favorable toxicity profile of PD-1 inhibitors makes these agents attractive for use in the adjuvant setting.Furthermore,as previously noted,studies have shown relatively balanced expression of PD-L1 in both primary tumor and metastases. The Eastern Cooperative Oncology Group(ECOG)has unveiled a plan for assessing both neoadjuvant and adjuvant therapy with nivolumab in a phase III clinical trial.Other agents such as atezolizumab,a PD-L1 inhibitor,may soon be explored in this setting.
3.1.1.Metastatic disease
Metastasectomy is the standard of care for patients who have limited sites of metastasis from RCC.There are limited adjuvant studies that incorporate patients who have had metastasectomy for stage IV RCC-ostensibly, these patients carry the highest potential risk of recurrence,with some retrospective series suggesting a median time to recurrence as low as 16 months[35].The standard of care in this setting is vigilant observation-although no guidelines exist,monitoring every 3 months with computerized tomography represents a reasonable practice.ECOG 2810 is a prospective,phase III study randomizing patients with fully resected clear cell mRCC to either pazopanib or placebo for 1 year.The study will accrue a total of 126 patients.Previous trials of adjuvant therapy,such as ASSURE,have been criticized for inclusion of low-risk patients,which thereby increases the necessary effect size for any intervention.The high risk of recurrence in the ECOG 2810 study population may poise the trial well for a positive outcome.
4.Patterns of metastases in RCC
One of the most widely cited studies of RCC metastasis distribution is derived from the National Inpatient Sample [36].In this study,11,157 patients with mRCC were identified from 1998 to 2007.The most common sites of metastases were lung(45%),following by bone(30%)and lymph node(22%).Liver metastases were noted in 20%ofpatients and adrenal metastases were noted in 9%of patients.Brain metastases occurred in approximately 9%of patients(Fig.1).
Several series have pointed towards distinct outcomes depending on patterns of metastasis.In an assessment of 2027 mRCC patients from the International mRCC Database Consortium(IMDC)experience,liver metastases were noted to occur in a larger proportion of patients with poorrisk by IMDC criteria[37].Furthermore,the hazard ratio for death(adjusted for IMDC risk factors)was 1.4 for patients with bone metastases(95%CI 1.22-1.62)and 1.42 for patients with liver metastases(95%CI 1.17-1.73).IMDC data have also been used to assess the outcome of patients with brain metastases[38].Approximately 106 patients were identified with brain metastases,of which only 12% fell into the favorable IMDC risk group.Most patients(90%) had cerebral metastases,while a smaller subset(17%)had cerebellar metastases.As one might anticipate,a higher number of brain metastases were associated with poorer outcome.
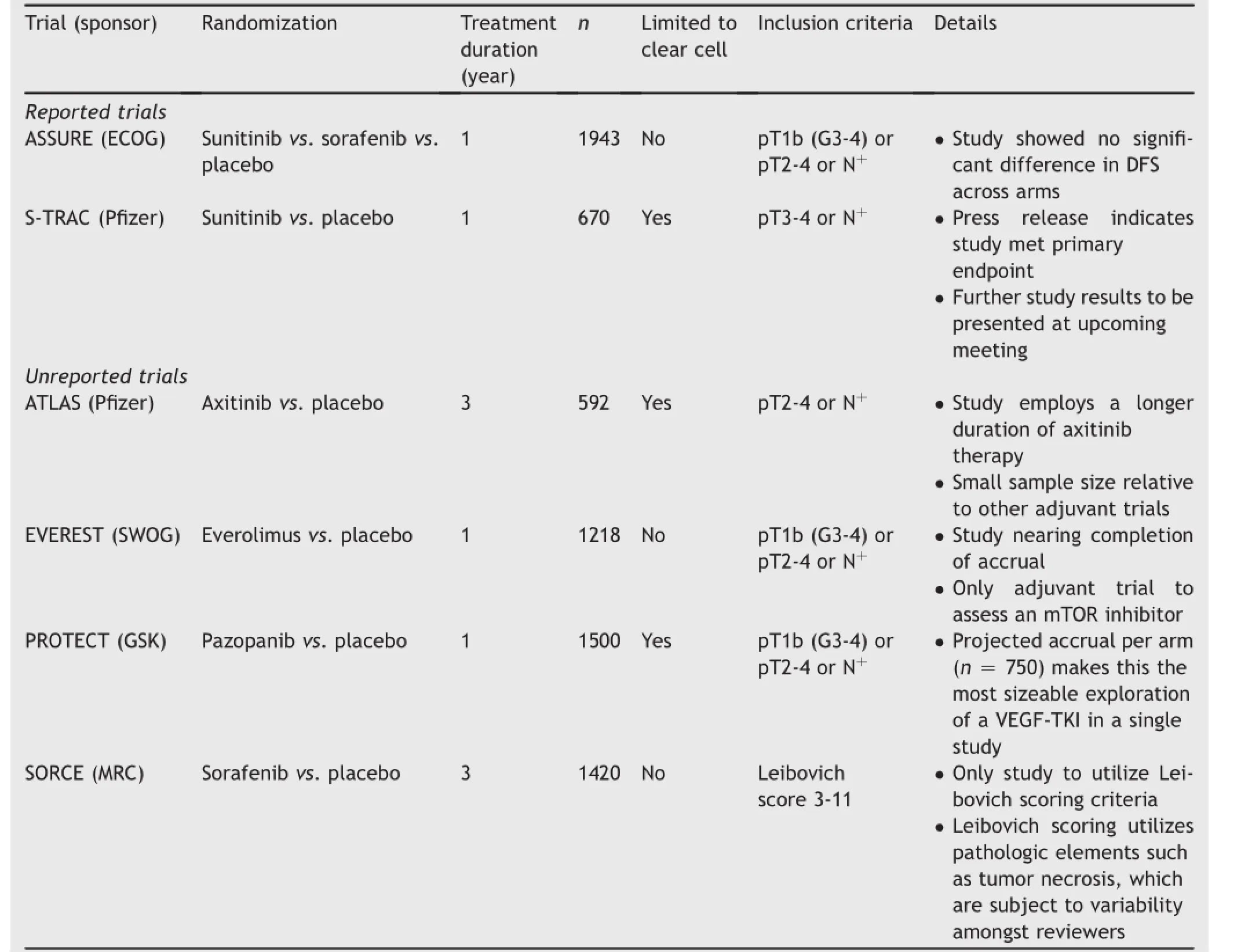
Table 1Trials of adjuvant targeted therapy in RCC.
There are several sites of metastases that are associated with more favorable prognoses.Multiple anecdotal reports exist documenting regression of lung metastases following either brief local or systemic therapy for mRCC,and broadly,patients with lung-only metastases are thought to carry a favorable prognosis[39-41].Interestingly,pancreatic metastases are also thought to confer a favorable prognosis.In a retrospective series from MD Anderson,228 patients with pancreatic metastases were characterized. Median OS was 39 months in patients with pancreatic metastases as compared to 26 months in patients without (p<0.010).
5.Treatment by anatomic site
5.1.Bone metastases
While a comprehensive discussion of RCCtreatmentoptionsis beyond the scope of this review,we discuss herein uniquesystemic therapy considerations that pertain to specific anatomic sites.In particular,bone and brain metastases representsignificant therapeutic dilemmas.Forpatientswith bone metastases,recent data have alluded to the potential utility of cabozantinib.Cabozantinib,a multikinase inhibitor with activity against VEGFR,MET and AXL,has previously been demonstrated to have impressive activity in bone metastases derived from prostate cancer.In this setting,disappearance of lesions on bone scan has been observed[42].In mRCC,cabozantinib was compared to everolimus in a phase III clinical trial,demonstrating an improvement in progression-free survival(PFS),response rate and OS[14].In the overall study population,progression-free survival(PFS)was 7.4 months with cabozantinib versus 3.9 months with everolimus(p<0.001).Subset analyses of patients with bone metastases show persistence of this PFS benefit(7.4 months with cabozantinib vs.2.7 months with everolimus)[43]. Cabozantinib was also noted to exert a greater reduction in markers of bone turnover,such as N-telopeptide.
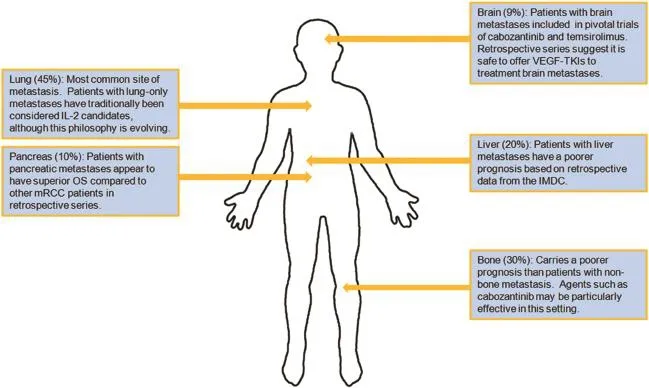
Figure 1Unique considerations for sites of metastasis in mRCC.IL-2,interleukin-2;IMDC,International mRCC Database Consortium;mRCC,metastatic renal cell carcinoma;OS,overall survival;VEGF-TKIs,VEGF-tyrosine kinase inhibitors.
5.2.Brain metastases
As noted previously,brain metastases occur in roughly 9%of patients with mRCC.The central nervous system(CNS)is thought to represent a sanctuary site for RCC;preclinical studies show incomplete penetration with agents such as sunitinib and sorafenib[44].For front-line therapy,the pivotal phase III study of temsirolimus did permit patients with brain metastases-a distinction from previously published front-line trials of VEGF-directed therapies.In patients with mRCC and poor-risk features,temsirolimus was compared to IFN-α and the combination of both agents. Treatment with temsirolimus monotherapy led to an improvement in OS as compared to IFN-α.Although subset analyses pertaining to patients with brain metastases are not available,the inclusion of this subpopulation provides some justification for offering temsirolimus front-line.
There have been retrospective reviews assessing the safety and efficacy of VEGF-TKIs in mRCC with brain metastasis.In one single institution study,65 patients with brain metastases from mRCC were identified.The preponderance (80%)received VEGF-directed therapy front-line with a very limited number of neurologic adverse events.In total, neurologic adverse events were noted in five patients,and includedradiation necrosisand brain metastasishemorrhage.
In the second-line setting,the current clinical debate surrounds use of either cabozantinib or nivolumab.Like cabozantinib,nivolumab was assessed in a phase III comparison against everolimus in patients with prior VEGF-directed therapy[12].Nivolumab was noted to improve both OS and response rate as compared to everolimus. However,it is worthy to note that the phase III assessment of nivolumab did not include patients with brain metastases,while the phase III assessment of cabozantinib did. Thus,in the second-line setting,cabozantinib may be the preferred approach for patients with CNS disease.
6.Conclusion
An understanding of the biology of RCC metastasis will most surely drive advances in systemic therapy in both the adjuvant and metastatic setting.As noted herein,there are multiple studies that are currently addressing the potential role of VEGF-and mTOR-inhibitors as adjuvant treatment. This is a derivative approach based on the observed activity of these agents in the metastatic setting.Although themajority of adjuvant studies have yet to report,the emerging data from the S-TRAC and ASSURE studies(both exploring VEGF-TKIs)have produced contrasting results. Cautious interpretation of the data will surely be necessary before these approaches are implemented clinically.The potential interplay of immune cells in generating metastases might prompt speculation that adjuvant PD-1 or PD-L1 inhibition could be feasible as adjuvant treatment.These studies will likely be emerging in the near future.
Once RCC does spread,it is critical to note that varying sites of metastasis may carry distinct implications for prognosis.While patients with bone,liver and brain metastases may carry an inferior prognosis relative to the overall population of mRCC patients,patients with pancreatic metastases may have a superior prognosis.At present,there is insufficient evidence to take a tailored approach for each metastatic site.Subset analyses from phase III studies do afford an opportunity to understand the relative benefit of systemic agents in unique settings(e.g., cabozantinib in bone metastases).Furthermore,review of eligibility criteria from these phase III studies do allow the practicing clinician to understand the applicability of data-for instance,patients with brain metastases were not consistently permitted entry in front-line trials.Prospective studies that assess these unique populations may ultimately provide the most relevant insights.
Many gaps remain in our understanding of RCC metastasis.For instance,although the distinct biology of RCC histologies was acknowledged,we have little sense of how this might affect patterns of metastasis.The varied clinical outcomes for different histological subtypes certainly underscores that each may progress through distinct mechanisms.Furthermore,although much emphasis has been placed on optimizing treatment of disease at certain anatomic sites(e.g.,bone and brain metastases),it would be helpful to know if other sites of metastases(e.g., pancreatic metastases,adrenal metastases and so on) progress through distinct mechanisms.A better biological understanding of these clinical circumstances will move us towards the goal of personalizing therapy across these settings.
Conflicts of interest
Dr.Pal receives honoraria from Novartis,Medivation and Astellas Pharma,and receives consulting fees from Pfizer, Novartis,Aveo,Genentech,Exelixis,BMS,Astellas and GSK.
[1]National comprehensive cancer network clinical practice guidelines:renal cell carcinoma.Available at:http://www. nccn.org;[accessed 15.08.16].
[2]Fyfe G,Fisher RI,Rosenberg SA,Sznol M,Parkinson DR, Louie AC.Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy.J Clin Oncol 1995;13:688-96.
[3]Motzer RJ,Bacik J,Murphy BA,Russo P,Mazumdar M.Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma.J Clin Oncol 2002;20:289-96.
[4]Pal SK,Kortylewski M,Yu H,Figlin RA.Breaking through a plateau in renal cell carcinoma therapeutics:development and incorporation of biomarkers.Mol Cancer Ther 2010;9: 3115-25.
[5]Sternberg CN,Davis ID,Mardiak J,Szczylik C,Lee E, Wagstaff J,et al.Pazopanib in locally advanced or metastatic renal cell carcinoma:results of a randomized phase III trial.J Clin Oncol 2010;28:1061-8.
[6]Motzer RJ,Escudier B,Oudard S,Hutson TE,Porta C, Bracarda S,et al.Efficacy of everolimus in advanced renal cell carcinoma:a double-blind,randomised,placebo-controlled phase III trial.Lancet 2008;372:449-56.
[7]Motzer RJ,Hutson TE,Tomczak P,Michaelson MD,Bukowski RM, Rixe O,et al.Sunitinib versus interferon alfa in metastatic renal-cell carcinoma.N Engl J Med 2007;356:115-24.
[8]Hudes G,Carducci M,Tomczak P,Dutcher J,Figlin R, Kapoor A,et al.Temsirolimus,interferon alfa,or both for advanced renal-cell carcinoma.New Engl J Med 2007;356: 2271-81.
[9]Rini BI,Escudier B,Tomczak P,Kaprin A,Szczylik C, Hutson TE,et al.Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma(AXIS):a randomised phase 3 trial.Lancet 2011;378:1931-9.
[10]Rini BI,Halabi S,Rosenberg JE,Stadler WM,Vaena DA, Archer L,et al.Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma:final results of CALGB 90206. J Clin Oncol 2010;28:2137-43.
[11]Pal SK,Choueiri TK,Karam JA,Heng DY.Metastatic renal cell carcinoma:contending with a sea change in therapy.Urol Oncol 2015;33:507-8.
[12]Motzer RJ,Escudier B,McDermott DF,George S,Hammers HJ, Srinivas S,et al.Nivolumab versus everolimus in advanced renal-cell carcinoma.New Engl J Med 2015;373(19):1803-13.
[13]Motzer RJ,Hutson TE,Glen H,Michaelson MD,Molina A, Eisen T,et al.Lenvatinib,everolimus,and the combination in patients with metastatic renal cell carcinoma:a randomised, phase 2,open-label,multicentre trial.Lancet Oncol 2015;16: 1473-82.
[14]Choueiri TK,Escudier B,Powles T,Tannir NM,Mainwaring PN, Rini BI,et al.Cabozantinib versus everolimus in advanced renal cell carcinoma(METEOR):final results from a randomised,open-label,phase 3 trial.Lancet Oncol 2016;17(7): 917-27.
[15]Pal SK,Figlin RA.Targeted therapies for renal cell carcinoma: understanding their impact on survival.Target Oncol 2010;5: 131-8.
[16]Kim WY,Kaelin WG.Role of VHL gene mutation in human cancer.J Clin Oncol 2004;22:4991-5004.
[17]Zhou L,Liu XD,Sun M,Zhang X,German P,Bai S,et al.Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma.Oncogene 2016;35(21):2687-97.
[18]Linehan WM,Spellman PT,Ricketts CJ,Creighton CJ,Fei SS, Davis C,et al.Comprehensive molecular characterization of papillary renal-cell carcinoma.New Engl J Med 2016;374: 135-45.
[19]Davis CF,Ricketts CJ,Wang M,Yang L,Cherniack AD,Shen H, et al.The somatic genomic landscape of chromophobe renal cell carcinoma.Cancer Cell 2014;26:319-30.
[20]Pal SK,Choueiri TK,Wang K,Khaira D,Karam JA,Van Allen E, et al.Characterization of clinical cases of collecting duct carcinoma of the kidney assessed by comprehensive genomic profiling.Eur Urol 2016;70(3):516-21.
[21]Shuch B,Bratslavsky G,Linehan WM,Srinivasan R.Sarcomatoid renal cell carcinoma:a comprehensive review of the biology and current treatment strategies.Oncologist 2012;17:46-54.
[22]Malouf GG,Ali SM,Wang K,Balasubramanian S,Ross JS, Miller VA,et al.Genomic characterization of renal cellcarcinoma with sarcomatoid dedifferentiation pinpoints recurrent genomic alterations.Eur Urol 2016;70:348-57.
[23]Pal SK,He M,Tong T,Wu H,Liu X,Lau C,et al.RNA-seq reveals aurora kinase-driven mTOR pathway activation in patients with sarcomatoid metastatic renal cell carcinoma.Mol Cancer Res 2015;13:130-7.
[24]Gerlinger M,Rowan AJ,Horswell S,Larkin J,Endesfelder D, Gronroos E,et al.Intratumor heterogeneity and branched evolution revealed by multiregion sequencing.N Engl J Med 2012;366:883-92.
[25]Callea M,Albiges L,Gupta M,Cheng SC,Genega EM,Fay AP, et al.Differential expression of PD-L1 between primary and metastatic sites in clear cell renal cell carcinoma.Cancer Immunol Res 2015;3(10):1158-64.
[26]Grange C,Tapparo M,Collino F,Vitillo L,Damasco C, Deregibus MC,et al.Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche.Cancer Res 2011;71:5346-56.
[27]Lopez-Lago MA,Posner S,Thodima VJ,Molina AM,Motzer RJ, Chaganti RS.Neutrophil chemokines secreted by tumor cells mount a lung antimetastatic response during renal cell carcinoma progression.Oncogene 2013;32:1752-60.
[28]Finke J,Ko J,Rini B,Rayman P,Ireland J,Cohen P.MDSC as a mechanism of tumor escape from sunitinib mediated antiangiogenic therapy.Int Immunopharmacol 2011;11:856-61.
[29]Razorenova OV,Finger EC,Colavitti R,Chernikova SB, Boiko AD,Chan CK,et al.VHL loss in renal cell carcinoma leads to up-regulation of CUB domain-containing protein 1 to stimulate PKC{δ}-driven migration.Proc Natl Acad Sci U S A 2011;108:1931-6.
[30]Aubert S,Fauquette V,Hemon B,Lepoivre R,Briez N, Bernard D,et al.MUC1,a new hypoxia inducible factor target gene,is an actor in clear renal cell carcinoma tumor progression.Cancer Res 2009;69:5707-15.
[31]Struckmann K,Mertz K,Steu S,Storz M,Staller P,Krek W, et al.pVHL co-ordinately regulates CXCR4/CXCL12 and MMP2/MMP9 expression in human clear-cell renal cell carcinoma.J Pathol 2008;214:464-71.
[32]Pal SK,Haas NB.Adjuvant therapy for renal cell carcinoma: past,present,and future.Oncologist 2014;19:851-9.
[33]Haas NB,Manola J,Uzzo RG,Flaherty KT,Wood CG,Kane C, et al.Adjuvant sunitinib or sorafenib for high-risk,non-metastatic renal-cell carcinoma(ECOG-ACRIN E2805):a doubleblind,placebo-controlled,randomised,phase 3 trial.Lancet 2016;387:2008-16.
[34]Pfizer announces positive top-line results from phase 3 S-TRAC trial of SUTENT®(sunitinib)as adjuvant therapy in patients at high risk of recurrent renal cell carcinoma.Available at: http://www.pfizer.com;[accessed 15.08.16].
[35]Eggener SE,Yossepowitch O,Kundu S,Motzer RJ,Russo P.Risk score and metastasectomy independently impact prognosis in patients with recurrent renal cell carcinoma.J Urol 2008;180: 873-8.
[36]Bianchi M,Sun M,Jeldres C,Shariat SF,Trinh QD,Briganti A, et al.Distribution of metastatic sites in renal cell carcinoma: a population-based analysis.Ann Oncol 2012;23:973-80.
[37]McKay RR,Kroeger N,Xie W,Lee JL,Knox JJ,Bjarnason GA, et al.Impact of bone and liver metastases on patients with renal cell carcinoma treated with targeted therapy.Eur Urol 2014;65:577-84.
[38]Vickers MM,Al-Harbi H,Choueiri TK,Kollmannsberger C, North S,MacKenzie M,et al.Prognostic factors of survival for patients with metastatic renal cell carcinoma with brain metastases treated with targeted therapy:results from the international metastatic renal cell carcinoma database consortium.Clin Genitourin Cancer 2013;11:311-5.
[39]Hegde U,Patel V,Kaloudis E.Durable spontaneous regression of lung metastases from renal cell carcinoma after incomplete use of multiple kinase inhibitor sorafenib.Int J Urol 2013;20: 644-5.
[40]Nicholls MF,Siddons AH.Spontaneous disappearance of lung metastases in a case of kidney carcinoma(hypernephroma). Brit J Surg 1960;47:531-3.
[41]Nishida H,Shirai T,Hayashi K,Takeuchi A,Tanzawa Y, Mizokami A,et al.Cryotreatment against metastatic renal cell bone tumour reduced multiple lung metastases.Anticancer Res 2011;31:2927-30.
[42]Smith DC,Smith MR,Sweeney C,Elfiky AA,Logothetis C, Corn PG,et al.Cabozantinib in patients with advanced prostate cancer:results of a phase II randomized discontinuation trial.J Clin Oncol 2013;31:412-9.
[43]Escudier BJ,Powles T,Motzer RJ,Olencki T,Aren OR, Oudard S,et al.Efficacy of cabozantinib(C)vs.everolimus(E) in patients(pts)with advanced renal cell carcinoma(RCC)and bone metastases(mets)from the phase III METEOR study.J Clin Oncol 2016;34:4558.ASCO Meeting Abstracts.
[44]Dudek AZ,Raza A,Chi M,Singhal M,Oberoi R,Mittapalli RK, et al.Brain metastases from renal cell carcinoma in the era of tyrosine kinase inhibitors.Clin Genitourin Cancer 2013;11: 155-60.
Received 19 August 2016;accepted 19 August 2016
Available online 30 August 2016
*Corresponding author.
E-mail address:spal@coh.org(S.K.Pal).
Peer review under responsibility of Second Military Medical University.
1Both authors contributed equally to this work.
http://dx.doi.org/10.1016/j.ajur.2016.08.006
2214-3882/©2016 Editorial Office of Asian Journal of Urology.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
杂志排行
Asian Journal of Urology的其它文章
- Developing immunotherapy strategies in the treatment of prostate cancer
- Novel immunotherapy approaches for metastatic urothelial and renal cell carcinoma
- Intrinsic subtypes and bladder cancer metastasis
- Cultured circulating tumor cells and their derived xenografts for personalized oncology
- Specific bone region localization of osteolytic versus osteoblastic lesions in a patient-derived xenograft model of bone metastatic prostate cancer
- Mismatch repair enzyme expression in primary and castrate resistant prostate cancer
