The evolving landscape of prostate cancer stem cell:Therapeutic implications and future challenges
2016-04-27EunJinYunGingLoJerTsongHsieh
Eun-Jin Yun,U-Ging Lo,Jer-Tsong Hsieh*
Department of Urology,UT Southwestern Medical Center,Dallas,TX,USA
The evolving landscape of prostate cancer stem cell:Therapeutic implications and future challenges
Eun-Jin Yun,U-Ging Lo,Jer-Tsong Hsieh*
Department of Urology,UT Southwestern Medical Center,Dallas,TX,USA
Prostate cancer;
Prostate cancer(PCa)is the most common cause of malignancy in males and the second leading cause of cancer mortality in United States.Current treatments for PCa include surgery,radiotherapy,and androgen-deprivation therapy.Eventually,PCa relapses to an advanced castration-resistant PCa(CRPC)that becomes a systematic disease and incurable. Therefore,identifying cellular components and molecular mechanisms that drive aggressive PCa at early stage is critical for disease prognosis and therapeutic intervention.One potential strategy for aggressive PCa is to target cancer stem cells(CSCs)that are identified by several unique characteristics such as immortal,self-renewal,and pluripotency.Also,CSC is believed to be a major factor contributing to resistance to radiotherapy and conventional chemotherapies.Moreover,CSCs are thought to be the critical cause of metastasis,tumor recurrence and cancer-related death of multiple cancer types,including PCa.In this review,we discuss recent progress made in understanding prostate cancer stem cells(PCSCs).We focus on the therapeutic strategies aimed at targeting specific surface markers of CSCs,the key signaling pathways in the maintenance of self-renewal capacity of CSCs,ATP-binding cassette(ABC) transporters that mediate the drug-resistance of CSCs,dysregulated microRNAs expression profiles in CSCs,and immunotherapeutic strategies developed against PCSCs surface markers. ©2016 Editorial Office of Asian Journal of Urology.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/ licenses/by-nc-nd/4.0/).
1.Introduction
Prostate cancer(PCa)is the most frequently diagnosed cancer and has the second highest mortality rate among men in the United States.It is also listed as one of the five most diagnosed cancers worldwide,especially in western developed countries[1].Recently,the incidence and mortality rate of PCa rose significantly in several Asian countriessuch as Japan,Korea and China[1].At the early stage,PCa is multi-focal and can be managed effectively by surgery, high-intensity focused ultrasound therapy(HIFU),or radiotherapy.However,the advanced-stage of PCa,characterized by acquisition of invasive phenotypes,leading to bone metastases,is generally incurable[2].It is becoming increasingly clear that heterogeneous tumor cell populations,which may arise from different cancer stem cells (CSC)subpopulations,are hierarchically organized.CSCs were first described in acute myeloid leukemia[3],and later,were also identified in various solid tumors including PCa[4].CSCs are defined as small subsets of cells within a tumor that are highly tumorigenic with unlimited selfrenewal capacity and can also regenerate non-tumorigenic progeny.Particularly,CSCs seem to be more resistant than differentiated tumor cells to conventional therapies[5,6]. Therefore,the CSC theory is likely to facilitate the understanding of tumor progression and expedite the development of effective therapeutic strategies targeting CSCs.
Here,we review the current knowledge of CSCs and the relationship between CSCs and metastatic castrationresistant prostate cancer(CRPC).In addition,we will address the therapeutic implications and challenges for targeting CSCs and discuss emerging and innovative approaches for the treatment of CRPC.Understanding the link between CSCs and metastatic CRPC will facilitate the development of novel therapeutic approaches and improve the clinical outcomes of PCa patients.
2.Normal prostate stem cells and CSCs
Stem cells have been defined as cells that have the ability to perpetuate themselves through self-renewal and to differentiate into mature tissue types[7,8].Self-renewal is crucial to stem cell function,because it is required for stem cells to persist for the lifetime of the animal.Moreover, while stem cells from different organs may vary in their developmental potential,all stem cells must self-renew and regulate the relative balance between self-renewal and differentiation.While CSCs are not necessarily derived from normal stem cells,this fraction of tumor cells possesses many functional similarities with normal stem cells(NSCs) such as self-renewal capacity and pleuripotency[9]. Therefore,understanding the regulation of NSC is also fundamental to understanding the regulation of CSCs.
The normal prostate epithelial stem cell differentiates into three epithelial cell types,basal,luminal,and rare neuroendocrine[10].Basal cells express high molecular weight cytokeratin(CK)including CK5 and CK14,and also Bcl-2,CD44,p63 but not androgen receptor(AR)[11-13]. Terminally differentiated luminal cells express low molecular weight CK such as CK8,CK18 and also express AR[12]. Luminal cells secrete prostate-specific antigen(PSA)and prostate-specific alkaline phosphatase(PAP)into the glandular lumen in an androgen-dependent manner.Unlike the majority of secretory luminal cells,neuroendocrine(NE) cells express synaptophysin and chromogranin A,but not AR or PSA and also secrete neuropeptides including serotonin, bombesin,calcitonin and somatostatin[10,14].
Androgen-deprivation of rodent models provides evidence for the presence of normal prostate stem cells (NPSCs)within the basal compartment of the prostate gland.Basal cells preferentially survive during castration whereas most of the luminal cells are lost through programmed cell death[15,16].However,the cellular origin of the NPSCs is not always certain.For example,the luminal population of castration-resistant Nkx3.1-expressing cells (CARNs)express CK18 and AR but not basal markers[17]and these cells exhibit stem cell characteristics and can regenerate prostatic tissue after androgen replacement [17].In addition,the cellular origins of PCa remain a subject of debate.In mouse models,it has been shown that basal and luminal populations can both serve as cells of origin for PCa[18,19],however,only basal cells have been shown to be efficient targets for transformation in human PCa[20].Also,in vivo,PCa with stem cell characteristics survive castration,express a luminal progenitor phenotype with low AR expression,and possess tumor-initiating potential after androgen replacement[21].In contrast,PCSCs may also be derived from NSCs with malignant transformation because CRPC is androgen-independent and basal cells can be identified from a majority of metastasis [22].In addition,some key molecules that normally regulate self-renewal and survival of NSCs(e.g.p63,Bcl-2,and hTERT)are preferentially localized in basal compartment [12,14].However,human PCa has a markedly luminal phenotype which has led to the idea that PCa arises from a fully differentiated luminal cell.Shen et al.[23]demonstrated that CARNs,which are a luminal stem cell population,are a cell type of origin for certain types of PCa.Also, a recent study showed that both populations of CD49fhibasal cells and CD26hiluminal cells generated a mixture of CK5+basal cells and CK8+luminal cells.However,only purified luminal cells could generate organoids with a glandular architecture under established organoid culture conditions,suggesting that luminal stem cells are capable of regenerating the normal glandular architecture of human prostate[24].
Another possible origin of PCSCs might be through cell fusion between stem cells and other types of cells including differentiated cells,stromal cells,or inflammatory cells[25].Cell fusion may allow for the combination of self-renewal properties of NSCs with the accumulated mutations in differentiated cells to attain a fully neoplastic transformation.As an example,it has been shown that bone marrow derived cells can fuse with neoplastic epithelium to promote tumor development and metastasis through creation of CSCs[26].Although the origin of PCSCs still remains controversial,it is also possible that PCa might have acquired CSCs features through genetic/epigenetic changes.
3.Isolation and identi fication of PCSCs
Hematopoietic stem cell markers have provided the paradigms for identifying and isolating CSCs in solid tumors including PCa[27,28].In prostate,the biomarkers CD44, stem cell antigen(Sca-1),CD133(prominin-1),and ABCG2 are commonly used for fluorescence-activated cell sorting (FACS)to isolate NSCs[14,19,29].A unique PCSC marker(s) has not yet been found,although certain markers of“stemness”that are generally present in various NSCs andCSCs are also found in PCSCs(Table 1).CD44 is a cell surface receptor involved in cell-cell interactions and in cell adhesion and migration[30].CD44+PCa cells displayed increased proliferation,tumorigenicity,and metastatic potential compared to CD44-cells[31].Furthermore, CD44+cells could initiate serially passed prostaspheres and serially transplantable tumors suggesting high self-renewal potential[31].Another study also showed a similar result; CD44+/CD24-cells formed prostaspheres consistently in vitro,and CD44+/CD24-xenografts initiated tumors in vivo with the injection of small number of cells[32].The Sca-1 is expressed by stem or progenitor cells in various tissues,and has been used to enrich for murine hematopoietic stem cells[33].Sca-1+cells exhibited increased proliferated capacity,with a subpopulation of Sca-1+cells also expressing Bcl-2 and integrin α6[18].Another stem cell marker,CD133,is expressed in hematopoietic stem cells and has long been used to identify CSCs.CD133+prostate cells display stem cell features such as prostasphere formation and development of prostatic-like acini in immunocompromised male mice[29].In addition,CD133+cells also co-express CK14 or hTERT and gave rise to more and larger branching ducts consisting of luminal and basal epithelial cells compared to CD133-cells[34].The ATP-binding cassette(ABC)membrane transporter,ABCG2, expressed in these cells,enables the efflux of Hoechst 33342 dye suggesting the association of these cells with multidrug resistance[35].Aldehyde dehydrogenease (ALDH)is an enzyme involved in intracellular retinoic acid production;in PCSCs,high expression of ALDH1A1 was found to be positively correlated with Gleason score and pathologic stage but inversely correlated with overall survival,indicating that it may be a potential PCSC marker [36].Combining multiple markers has also improved the isolation of PCSCs.CD44+α2β1highCD133+cell populations possessed a significant capacity for self-renewal and could regenerate the phenotypically mixed populations of nonclonogenic cells[37].Another study also demonstrated that the CD44+α2β1highpopulations from LAPC-9 tumor xenografts showed tumorigenic potential[38].
As mentioned,the most well-known CSC markers are commonly associated with a variety of NSCs.Therefore, these markers could be utilized,in combination with negative selection against differentiation markers,to isolate and characterize CSCs from different malignancies. Most importantly,the identification of PCSC specific marker would facilitate the development of tailored drugs to cure PCa.
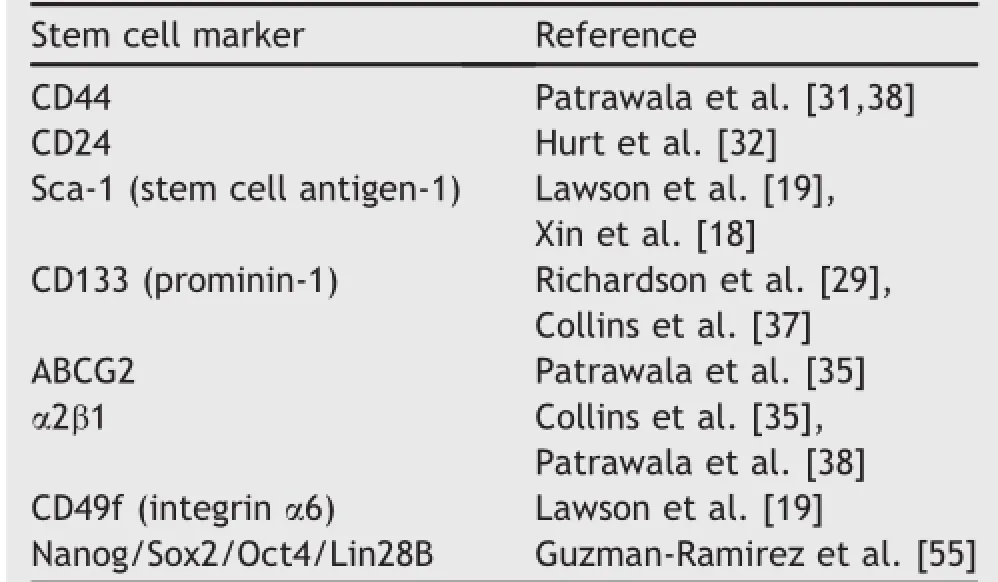
Table 1Markers associated with PCSC.
4.Conventional therapeutic approaches and CSCs
Androgen deprivation therapy(ADT)is the gold standard regimen for metastatic PCa patients.However,ADT only targets androgen-sensitive PCa cells,and most patients relapse and eventually progress to CRPC after the initial response[39].Conventional anticancer therapies including chemotherapy,radiation and immunotherapy also kill rapidly growing differentiated tumor cells[5].Since current anticancer therapies fail to eradicate CSCs,only half of the patients respond to chemotherapy and even those who initially respond to treatment eventually become resistant [40].
In general,CRPC exhibits a phenotype similar to the NSCs [41],suggesting that a clonal expansion of small PCSC population from the original tumor and/or de-differentiation of PCa leads to PCSC.It has been shown that NSCs from various tissues tend to be more resistant to chemotherapy than mature cell types from same tissues[42].The mechanisms for this are not clear yet,however,several lines of evidence are suggestive of possible mechanisms.For instance,the dormant status of PCSCs has been thought to reduce their susceptibility to chemotherapy.ABC transporters are membrane transporters that can efflux various distinct and structurally unrelated small molecules out of cells[35].Like hematopoietic stem cells,NPSCs and PCSCs appear to express high levels of ABC transporters;such as ABCG2,which can also serve as markers for PCSCs[35].The presence of these proteins can increase the changes of efflux of cytotoxic chemotherapeutics from the cells,thereby making them resistant.High expression of ABC transporters in PCSCs can be determined by dye exclusion assay with Hoechst 33342[43].Within a solid tumor,there are small populations of cells,called side population(SP)that demonstrate increased efflux of the Hoechst dye[43].Accumulated evidence showed that this cell population possesses similar characteristics to CSCs.Zhang et al.[44]showed prostaspheres exhibiting CSC phenotype were highly chemoresistant and had high levels of expression of a member of the ABC transporter family,ABCG2.Moreover,expression of ABCG2 is regulated by many CSC associated signaling pathways such as Hedgehog,Notch and PTEN/PI3K/AKT pathway [45-47].In addition,apoptotic signaling pathways are also deregulated in CSCs[48]with high expression of antiapoptotic molecules and reduced expression of proapoptotic genes such as,Bcl-2,Bcl-XLand Mcl-1[48].In a CD133+glioma,CSCs express a high level of Bcl-2 and Bcl-XL, and high expression of Mcl-1 correlates with resistance to Bcl-2 inhibitor[49].In PCa,our recent data indicate that cluster in,an anti-apoptotic chaperone,is responsible for generating chemo-resistant PCa cells that exhibit CSC phenotypes[50].Also,CSCs exhibit preferential activation of the DNA damage repair system that may also contribute to chemoresistance as well as radioresistance[6].Several studies have demonstrated that CSCs enriched underselective culture conditions,enhanced DNA damage repair signaling such as those through the ATR-Chk1 and Chk2 [6,51].
Collectively,current chemotherapy and radiotherapy fail to eliminate the PCSC population because they do not aim for the“Achilles’heel”of PCSC.Therefore,it is necessary to develop CSC therapy by specifically targeting PCSCs.Direct targeting PCSCs along with fast-growing progeny may increase therapeutic efficacy and prevent tumor recurrence.
5.Therapies targeting CSCs
A small population of CSCs,which are involved in tumor initiation,maintenance,metastasis and recurrence,often contribute to the treatment failure and cancer relapse after chemotherapy,radiotherapy and other conventional therapies against cancer.Therefore,it is of critical importance to develop therapeutic strategies that can specifically eradicate CSC populations and prevent tumor relapse after long term clinical treatment.CSCs possess several unique properties such as presenting of specific surface markers,slow cell division rate,efficient DNA repair machinery,highly expressed drug-efflux pumps,distinct miRNA expression profile,as well as existing in a hypoxia and acidosis microenvironment.Based on these CSCs characteristics,multiple strategies have been developed to eliminate the CSC niche in the tumor by targeting CSC surface markers,manipulating signaling pathways that would lead to CSC apoptosis and differentiation,inhibiting drug-efflux pumps,modulating critical miRNA levels,and adjusting the crosstalk with tumor microenvironment stimulants.
5.1.Targeting CSC markers
PCa also contains rare stem-like cell populations responsible for tumor formation and maintenance.Identification of specific markers presented by these PCSCs is therefore, important for precise isolation of PCa tumor-initiating cells. In particular,α2 and α6 integrins with CD44 and CD133 are often identified in PCSCs.By using patient-derived PCa tissue sample,Colombel et al.[52]found that tumors with higher level of c-met,integrin α2 and α6 were positively associated with the occurrence of bone metastasis in PCa patients.Meanwhile,by examining bone marrow samples from different stages of PCa patients,Ricci et al.[53]also identified that the presence of c-met-,integrin a2-and α6-, and CD45-positive cells is significantly associated with the risk of metastasis and cancer lethality in PCa patients.Both studies indicate that bone metastases could be the end result of PCSCs dissemination from primary tumors[52,53]. Moreover,Guzel et al.[54]demonstrated that SOX2,OCT4, KLF4 and ABCG2 expression were highly enhanced in recurrent human PCa tissue,compared to non-recurrent ones,suggesting that the increased stem cell-like PCa cell population may contribute to chemoresistance and recurrence of PCa.In an earlier study,Collins et al.[37]identified and characterized a population of PCSCs in human PCa tumors that possess significant self-renewal capacity and express CSCs markers such as CD44,α2β1 integrin and CD133.Moreover,Guzma´n-Ramı´rez et al.[55]demonstrated that the prostaspheres propagated from human PCa tumors exhibit CSCs markers such as CD133,Nestin,and CD44 in addition to NSC markers such as Oct-4,Nanog, Bmi-1,and Jagged-1.Overall,these studies suggest that the enriched neoplastic cells in the prostasphere derived from human PCa specimens possess self-renewal and clonogenic potential.
5.2.Targeting key signaling pathways involving CSCs maintenance
CSCs typically display highly activation of one or more conserved signal transduction pathways involved in organ development and tissue homeostasis including the Hedgehog(HH),Notch,and Wnt pathways[56].Increasing evidence demonstrates that those developmental signal pathways can interact with other cellular signaling pathways such as PI3K,MAPK,and NF-κB.And deregulation of these self-renewal pathways often occur in CSCs,which allow for drug resistance and relapse.Current cancer treatment strategies using nanoformulation of small molecules may provide advantages for selectively targeting self-renewal mechanisms,in particular,HH,Wnt and Notch signaling pathways in CSCs(Fig.1).
Activation of HH pathway has been found to be associated with basal cell carcinoma,glioma and medulloblastoma[57].SONIC Hedgehog(SHH)signaling,mediated through several components including transmembrane proteins Patched1(PTCH1)and Smoothened(SMOH),to ultimately activate the GLI zinc-finger transcription factors,is involved in organism development.Increasing evidence has demonstrated that dysregulation of SHH signaling occurs in multiple cancer types including PCa.It is also affirmed that SHH signaling has a critical role in progression of PCa.Sanchez et al.[58]demonstrated that SHH-GLI signaling components are highly elevated in PCa, compared to normal prostatic epithelia.Moreover,interference with the SHH-GLI pathways by using cyclopamine or anti-SHH antibodies can inhibit proliferation of GLI+/PSA+primary prostate tumor cultures,suggesting that this autocrine regulation of SHH signaling may be critical to sustain PCa growth.
Notch is a signaling mechanism that regulates cell fate specification,embryogenesis pattern formation,stem cell maintenance and initiation of differentiation in many tissues.Aberrant activation of Notch signaling may lead to uncontrolled proliferation,restricted differentiation and prevention of apoptosis in CSCs.Many studies have documented a consistent deregulation of Notch signaling components in PCa cell lines,genetically engineered mouse PCa models and human PCa specimens.It has been shown that several Notch signaling components including Notch2, Notch3,Notch4 and Jagged-1 are expressed in PCa cell lines such as LNCaP,C4-2B,DU145,and PC3.In addition,the study done by Zhang et al.[59]showed that Jagged-1 contributed to PCa cell growth by regulation of CDK2 kinase activity.Moreover,Zhu et al.[60]demonstrated that jagged-1 and Notch-1 were highly elevated in metastatic and high grade PCa tumors.Moreover,Domingo-Domenech et al.[61]demonstrated that docetaxel-resistant CRPCcells had higher activity of Notch and HH signaling,while inhibition of both Notch(by DBZ)and HH(by cyclopamine) signaling led to depletion of docetaxel-resistant DU145 and CWR22Rv1 cancer cells through inhibition of Akt and Bcl-2. Also,Notch pathway has been found to be associated with embryonal brain tumor by the depletion of CSCs via suppression of notch signaling[62].
Wnt signaling is an evolutionarily conserved developmental pathway,which determines cell fate and controls stem cells during development.Bisson and Prowse[63] demonstrated that Wnt signaling is critical for prostasphere formation and self-renewal of PCa cells.In addition to Wnt3,they observed a significant increase of β-catenin, keratin 18,CD133 and CD44 expression in PCa,accompanied by increased prostasphere size and enhanced selfrenewal capacity.This evidence suggests that Wnt signaling regulates the self-renewal of PCSCs.Moreover, Lee et al.[64]demonstrated that a small molecule nuclear β-catenin inhibitor(C3)can suppress both AR and β-catenin signaling pathway,accompanied by diminished AR/β-catenin target gene expression and ablation of PCa cell growth.
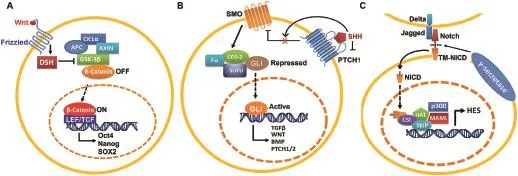
Figure 1Self-renewal pathways in CSCs(A)Wnt signaling pathway,(B)SONIC Hedgehog signaling pathway,and(C)Notch signaling pathway.CSCs,cancer stem cells.
5.3.Targeting ABC transporters involving in CSCsdrug resistance
One of the mechanisms for CSCs to confer resistance to chemotherapy is by enhanced expression of the ABC transporter,which can allow CSCs to pump small molecules such as chemotherapeutic drugs by ATP hydrolysis out of the cells.For example,Zhang et al.[44]demonstrated that the PCa cell-derived tumor spheres with elevated levels of CSCs markers(Gli1,Bmi1 and CD44),had also developed chemo-resistance due to the elevated levels of ABCG2.
5.4.Manipulation of miRNA
Emerging evidence demonstrates that CSCs are the critical drivers of tumor progression and metastasis.MicroRNAs (miRNAs)may play a crucial role in repressing or promoting metastasis by regulating CSCs.Accumulating studies have shown that deregulation of miRNA is associated with the tumor initiation and progression of PCa(Table 2).In particular,the emergence of CRPC could be attributed to the existence of PCSCs.Therefore,targeting miRNA regulation has become a novel strategy to dissect the initiation of PCa.
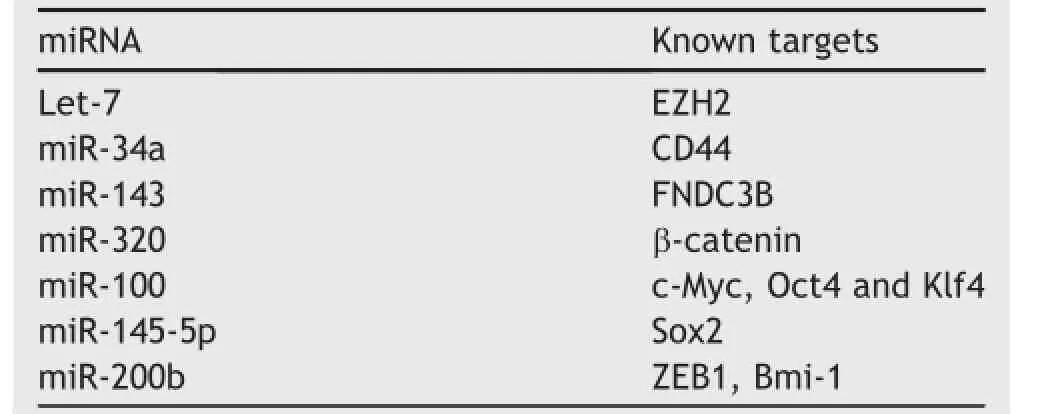
Table 2List of tumor suppressive miRNAs related with PCSC.
By profiling miRNA expression,Liu et al.[65]found that several tumor suppressive miRNAs,such as miR-34a,let-7b, miR-106a and miR-141,were significantly down-regulated in various stemprogenitor cell populations in PCa.Along with this finding,they also demonstrated that let-7 can inhibit PCa cell clonal expansion and tumor regeneration. Kong et al.[40]found an inverse correlation between let-7 and EZH2 in PCa.Let-7 can suppress clonogenic ability and sphere-forming capacity of PCa cells by inhibiting EZH2 expression.
There is conflicting evidence,however,on the role of some miRNAs in PCSC growth and expansion.PCSCs with clonogenic,tumor-initiating and metastatic capacities,like many other CSCs,are enriched in the CD44+cell population.Liu et al.[66]demonstrated that miR-34a,a p53-regulated miRNA,was able to suppress CD44 mRNA expression,thus,systemic delivery of miR-34a could inhibit PCa metastasis and regeneration.However,Cheng et al. [76]found that mice with prostate epithelia-specific activation of miR-34a and p53,show expansion of the PCSC compartment,accompanied by a development of early invasive adenocarcinoma and high-grade prostatic intraepithelial neoplasia.Consistently,loss of both miR-34 and p53 led to the acceleration of MET-dependent growth,selfrenewal,and motility of prostate stemprogenitor cells.
On the other hand,using microarray and reverse transcription polymerase chain reaction analysis,Fan et al.[67] profiled the miRNA expression in the prostate PC-3 sphere vs.adherent cells and found that miR-143 level was increased during the differentiation of PCSCs,which promoted PCa metastasis by repressing Fibronectin type III domain containing 3B(FNDC3B)expression.
In addition,Hsieh et al.[68]found that miR-320 is significantly down-regulated in PCa.By profiling the global gene expression in PCa cells transfected with miR-320,they found both CSC markers and downstream target genes of Wnt/β-catenin pathway are significantly repressed.On the contrary,knockdown of miR-320 significantly increases the CSC-like properties,such as tumor sphere formation,chemoresistance and tumorigenicity.This study demonstrates that miR-320 is a key negative regulator in PCSC and has the potential to become a novel therapeutic agent for PCa.
Wang et al.[77]reported that miR-100 negatively regulated migration,invasion,epithelial-to-mesenchymal transition,colony formation,spheroid formation and expression of the stem cell factors including c-Myc,Oct4 and Klf4 in PC-3 and DU145 cells.On the other hand,Huang et al.[69] reported that miR-143 and miR-145 could suppress in vitro tumor sphere formation and the expression of CSC markers factors(CD133,CD44,Oct4,c-Myc and Klf4)in PC-3 cells, which also correlated with the inhibition of bone invasion of PC-3 cells in vivo.Overall,these findings demonstrate that miR-143 and miR-145 play a crucial role in the bone metastasis by PCa by regulating CSC characteristics.
Furthermore,Yu et al.[70]showed that overexpressing miR-200b can suppress PCa cell proliferation and migration and enhance sensitivity to docetaxel by targeting B-cell-specific Moloney murine leukemia virus insertion site 1(Bmi-1).
5.5.Immunological targeting of CSCs
Recently,adoptive T-cell immunotherapy using autologous or allogeneic tumor reactive lymphocytes has been applied to treat cancer patients with refractory metastatic melanoma.This approach has demonstrated efficient tumor regression[71].Since tumor-infiltrating lymphocytes with tumor-specific receptors can be generated from cancer patients,researchers can utilize this machinery and improve adoptive T cell therapy by introducing specific antigen receptors,either tumor-specific T-cell receptors or chimeric antigen receptors(CAR,composed of an antibodybased external receptor structure and intracellular T-cell signaling domains),into these circulating lymphocytes.
Since CARs can induce T cells to attack tumors in a MHC-unrestricted manner,the application of adoptive T cell therapy has been expanded to treat multiple cancers such as lymphoma,chronic lymphocytic leukemia,melanoma, and neuroblastoma[72-74].Since CSCs are more resistant to radiotherapy and chemotherapy,researchers have begun to examine the possibility of immunotherapies targeting CSCs.In a recent study,Deng et al.[75]developed a novel immunotherapy strategy targeting a specific CSC antigen, epithelial cell adhesion molecule(EpCAM).They introduced EpCAM-specific CARs into the human peripheral blood lymphocytes(PBLs),and demonstrated that CAR-expressing PBLs can not only kill PC3 tumor with high level of EpCAM, but can also significantly inhibit the growth of PC3 tumor with low EpCAM.
6.Conclusion
Therapeutic resistance continues to be a problem in the treatment of PCa and PCSCs are likely an important factor in the development of therapeutic resistance,resulting in poor survival of PCa patients.The concept of CSCs reflecting hierarchic tumor organization has underlying important clinical implications and is supported by many efforts unveiling potential mechanisms associated with CSCs. Despite the progress,there are key issues that need to be addressed,such as identification of PCSC markers,understanding the interaction between PCSCs and their microenvironment.It is therefore,critical to gain a better understanding of the mechanisms involved in the mechanisms of resistance of PCSC to conventional therapies which would result in innovative therapeutic approaches.
Conflicts of interest
The authors declare no conflict of interest.
[1]Torre LA,Siegel RL,Ward EM,Jemal A.Global cancer incidence and mortality rates and trends-an update.Cancer Epidemiol Biomarkers Prev 2016;25:16-27.
[2]Partin AW,Kattan MW,Subong EN,Walsh PC,Wojno KJ, Oesterling JE,et al.Combination of prostate-specific antigen, clinical stage,and Gleason score to predict pathological stage of localized prostate cancer.A multi-institutional update. JAMA 1997;277:1445-51.
[3]Bonnet D,Dick JE.Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell.Nat Med 1997;3:730-7.
[4]Maitland NJ,Collins AT.Inflammation as the primary aetiological agent of human prostate cancer:a stem cell connection?J Cell Biochem 2008;105:931-9.
[5]Al-Hajj M,Becker MW,Wicha M,Weissman I,Clarke MF. Therapeutic implications of cancer stem cells.Curr Opin Genet Dev 2004;14:43-7.
[6]Bao S,Wu Q,McLendon RE,Hao Y,Shi Q,Hjelmeland AB,et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response.Nature 2006;444: 756-60.
[7]Spangrude GJ,Heimfeld S,Weissman IL.Purification and characterization of mouse hematopoietic stem cells.Science 1988;241:58-62.
[8]Morrison SJ,Shah NM,Anderson DJ.Regulatory mechanisms in stem cell biology.Cell 1997;88:287-98.
[9]Clarke MF,Dick JE,Dirks PB,Eaves CJ,Jamieson CH,Jones DL, et al.Cancer stem cells-perspectives on current status and future directions:AACR Workshop on cancer stem cells.Cancer Res 2006;66:9339-44.
[10]Abate-Shen C,Shen MM.Molecular genetics of prostate cancer.Genes Dev 2000;14:2410-34.
[11]Bonkhoff H,Remberger K.Widespread distribution of nuclear androgen receptors in the basal cell layer of the normal and hyperplastic human prostate.Virchows Arch 1993;422:35-8.
[12]Liu AY,True LD,LaTray L,Nelson PS,Ellis WJ,Vessella RL, et al.Cell-cell interaction in prostate gene regulation and cytodifferentiation.Proc Natl Acad Sci USA 1997;94: 10705-10.
[13]Signoretti S,Waltregny D,Dilks J,Isaac B,Lin D,Garraway L, et al.p63 is a prostate basal cell marker and is required for prostate development.Am J Pathol 2000;157:1769-75.
[14]Tang DG,Patrawala L,Calhoun T,Bhatia B,Choy G,Schneider-Broussard R,et al.Prostate cancer stem/progenitor cells:identification,characterization,and implications.Mol Carcinog 2007;46:1-14.
[15]Kyprianou N,Isaacs JT.Identification of a cellular receptor for transforming growth factor-beta in rat ventral prostate and its negative regulation by androgens.Endocrinology 1988;123: 2124-31.
[16]De Marzo AM,Meeker AK,Epstein JI,Coffey DS.Prostate stem cell compartments:expression of the cell cycle inhibitor p27Kip1 in normal,hyperplastic,and neoplastic cells.Am J Pathol 1998;153:911-9.
[17]Wang ZA,Mitrofanova A,Bergren SK,Abate-Shen C, Cardiff RD,Califano A,et al.Lineage analysis of basal epithelial cells reveals their unexpected plasticity and supports a cell-of-origin model for prostate cancer heterogeneity. Nat Cell Biol 2013;15:274-83.
[18]Xin L,Lawson DA,Witte ON.The Sca-1 cell surface marker enriches for a prostate-regenerating cell subpopulation that can initiate prostate tumorigenesis.Proc Natl Acad Sci USA 2005;102:6942-7.
[19]Lawson DA,Xin L,Lukacs RU,Cheng D,Witte ON.Isolation and functional characterization of murine prostate stem cells. Proc Natl Acad Sci USA 2007;104:181-6.
[20]Goldstein AS,Huang J,Guo C,Garraway IP,Witte ON.Identification of a cell of origin for human prostate cancer.Science 2010;329:568-71.
[21]Germann M,Wetterwald A,Guzman-Ramirez N,van der Pluijm G,Culig Z,Cecchini MG,et al.Stem-like cells with luminal progenitor phenotype survive castration in human prostate cancer.Stem Cells 2012;30:1076-86.
[22]Smith BA,Sokolov A,Uzunangelov V,Baertsch R,Newton Y, Graim K,et al.A basal stem cell signature identifies aggressive prostate cancer phenotypes.Proc Natl Acad Sci USA 2015;112: E6544-52.
[23]Wang X,Kruithof-de Julio M,Economides KD,Walker D,Yu H, Halili MV,et al.A luminal epithelial stem cell that is a cell of origin for prostate cancer.Nature 2009;461:495-500.
[24]Karthaus WR,Iaquinta PJ,Drost J,Gracanin A,van Boxtel R, Wongvipat J,et al.Identification of multipotent luminal progenitor cells in human prostate organoid cultures.Cell 2014; 159:163-75.
[25]Wang JC.Good cells gone bad:the cellular origins of cancer. Trends Mol Med 2010;16:145-51.
[26]Rizvi AZ,Swain JR,Davies PS,Bailey AS,Decker AD, Willenbring H,et al.Bone marrow-derived cells fuse with normal and transformed intestinal stem cells.Proc Natl Acad Sci USA 2006;103:6321-5.
[27]Kasper S.Exploring the origins of the normal prostate and prostate cancer stem cell.Stem Cell Rev 2008;4:193-201.
[28]Taylor RA,Risbridger GP.The path toward identifying prostatic stem cells.Differentiation 2008;76:671-81.
[29]Richardson GD,Robson CN,Lang SH,Neal DE,Maitland NJ, Collins AT.CD133,a novel marker for human prostatic epithelial stem cells.J Cell Sci 2004;117:3539-45.
[30]Oxley SM,Sackstein R.Detection of an L-selectin ligand on a hematopoietic progenitor cell line.Blood 1994;84:3299-306.
[31]Patrawala L,Calhoun T,Schneider-Broussard R,Li H,Bhatia B, Tang S,et al.Highly purified CD44+prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells.Oncogene 2006;25:1696-708.
[32]Hurt EM,Kawasaki BT,Klarmann GJ,Thomas SB,Farrar WL. CD44+CD24-prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. BJC 2008;98:756-65.
[33]Holmes C,Stanford WL.Concise review:stem cell antigen-1: expression,function,and enigma.Stem Cells 2007;25:1339-47.
[34]Mundy GR.Metastasis to bone:causes,consequences and therapeutic opportunities.Nat Rev Cancer 2002;2:584-93.
[35]Patrawala L,Calhoun T,Schneider-Broussard R,Zhou J, Claypool K,Tang DG.Side population is enriched in tumorigenic,stem-like cancer cells,whereas ABCG2+and ABCG2-cancer cells are similarly tumorigenic.Cancer Res 2005;65: 6207-19.
[36]Li Y,Cozzi PJ,Russell PJ.Promising tumor-associated antigens for future prostate cancer therapy.Med Res Rev 2010;30: 67-101.
[37]Collins AT,Berry PA,Hyde C,Stower MJ,Maitland NJ.Prospective identification of tumorigenic prostate cancer stem cells.Cancer Res 2005;65:10946-51.
[38]Patrawala L,Calhoun-Davis T,Schneider-Broussard R,Tang DG. Hierarchical organization of prostate cancer cells in xenograft tumors:the CD44+α2β1+cell population is enriched in tumorinitiating cells.Cancer Res 2007;67:6796-805.
[39]SharifiN,Kawasaki BT,Hurt EM,Farrar WL.Stem cells in prostate cancer:resolving the castrate-resistant conundrum and implications for hormonal therapy.Cancer Biol Ther 2006; 5:901-6.
[40]Kong D,Heath E,Chen W,Cher ML,Powell I,Heilbrun L,et al. Loss of let-7 up-regulates EZH2 in prostate cancer consistent with the acquisition of cancer stem cell signatures that are attenuated by BR-DIM.PLoS One 2012;7:e33729.
[41]Carson 3rd CC.Carcinoma of the prostate:overview of the most common malignancy in men.N C Med J 2006;67:122-7.
[42]Harrison DE,Lerner CP.Most primitive hematopoietic stem cells are stimulated to cycle rapidly after treatment with 5-fluorouracil.Blood 1991;78:1237-40.
[43]Deeley RG,Westlake C,Cole SP.Transmembrane transport of endo-and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins.Physiol Rev 2006;86:849-99.
[44]Zhang L,Jiao M,Li L,Wu D,Wu K,Li X,et al.Tumorspheres derived from prostate cancer cells possess chemoresistant and cancer stem cell properties.J Cancer Res Clin Oncol 2012;138: 675-86.
[45]Bhattacharya S,Das A,Mallya K,Ahmad I.Maintenance of retinal stem cells by Abcg2 is regulated by notch signaling.J Cell Sci 2007;120:2652-62.
[46]Bleau AM,Hambardzumyan D,Ozawa T,Fomchenko EI, Huse JT,Brennan CW,et al.PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells.Cell stem cell 2009;4:226-35.
[47]Sims-Mourtada J,Izzo JG,Ajani J,Chao KS.Sonic Hedgehog promotes multiple drug resistance by regulation of drug transport.Oncogene 2007;26:5674-9.
[48]Dean M,Fojo T,Bates S.Tumour stem cells and drug resistance.Nat Rev Cancer 2005;5:275-84.
[49]Tagscherer KE,Fassl A,Campos B,Farhadi M,Kraemer A, Bock BC,et al.Apoptosis-based treatment of glioblastomas with ABT-737,a novel small molecule inhibitor of Bcl-2 family proteins.Oncogene 2008;27:6646-56.
[50]Sung SY,Liao CH,Wu HP,Hsiao WC,Wu IH,Jinpu,et al.Loss of let-7 microRNA upregulates IL-6 in bone marrow-derived mesenchymal stem cells triggering a reactive stromal response to prostate cancer.PLoS One 2013;8:e71637.
[51]Eriksson D,Stigbrand T.Radiation-induced cell death mechanisms.Tumour Biol 2010;31:363-72.
[52]Colombel M,Eaton CL,Hamdy F,Ricci E,van der Pluijm G, Cecchini M,et al.Increased expression of putative cancer stem cell markers in primary prostate cancer is associated with progression of bone metastases.Prostate 2012;72: 713-20.
[53]Ricci E,Mattei E,Dumontet C,Eaton CL,Hamdy F,van der Pluije G,et al.Increased expression of putative cancer stem cell markers in the bone marrow of prostate cancer patients is associated with bone metastasis progression.Prostate 2013; 73:1738-46.
[54]Guzel E,Karatas OF,Duz MB,Solak M,Ittmann M,Ozen M. Differential expression of stem cell markers and ABCG2 in recurrent prostate cancer.Prostate 2014;74:1498-505.
[55]Guzma´n-Ramı´rez N,Voller M,Wetterwald A,Germann M, Cross NA,Rentsch CA,et al.In vitro propagation and characterization of neoplastic stem/progenitor-like cells from human prostate cancer tissue.Prostate 2009;69:1683-93.
[56]Takebe N,Miele L,Harris PJ,Jeong W,Bando H,Kahn M,et al. Targeting Notch,Hedgehog,and Wnt pathways in cancer stem cells:clinical update.Nat Rev Clin Oncol 2015;12:445-64.
[57]Kar S,Deb M,Sengupta D,Shilpi A,Bhutia SK,Patra SK.Intricacies of hedgehog signaling pathways:a perspective in tumorigenesis.Exp Cell Res 2012;318:1959-72.
[58]Sanchez P,Hernandez AM,Stecca B,Kahler AJ,DeGueme AM, Barrett A,et al.Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling.Natl Acad Sci USA 2004;101:12561-6.
[59]Zhang Y,Wang Z,Ahmed F,Banerjee S,Li Y,Sarkar FH.Downregulation of Jagged-1 induces cell growth inhibition and S phase arrest in prostate cancer cells.Int J Cancer 2006;119: 2071-7.
[60]Zhu H,Zhou X,Redfield S,Lewin J,Miele L.Elevated Jagged-1 and Notch-1 expression in high grade and metastatic prostate cancers.Am J Transl Res 2013;5:368-78.
[61]Domingo-Domenech J,Vidal SJ,Rodriguez-Bravo V,Castillo-Martin M,Quinn SA,Rodriguez-Barrueco R,et al.Suppression of acquired docetaxel resistance in prostate cancer through depletion of notch-and hedgehog-dependent tumor-initiating cells.Cancer cell 2012;22:373-88.
[62]Fan X,Matsui W,Khaki L,Stearns D,Chun J,Li YM,et al.Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors.Cancer Res 2006;66:7445-52.
[63]Bisson I,Prowse DM.WNT signaling regulates self-renewal and differentiation of prostate cancer cells with stem cell characteristics.Cell Res 2009;19:683-97.
[64]Lee E,Madar A,David G,Garabedian MJ,Dasgupta R,Logan SK. Inhibition of androgen receptor and beta-catenin activity in prostate cancer.Proc Natl Acad Sci USA 2013;110:15710-5.
[65]Liu C,Kelnar K,Vlassov AV,Brown D,Wang J,Tang DG. Distinct microRNA expression profiles in prostate cancer stem/progenitor cells and tumor-suppressive functions of let-7.Cancer Res 2012;72:3393-404.
[66]Liu C,Kelnar K,Liu B,Chen X,Calhoun-Davis T,Li H,et al.The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44.Nat Med 2011;17: 211-5.
[67]Fan X,Chen X,Deng W,Zhong G,Cai Q,Lin T.Up-regulated microRNA-143 in cancer stem cells differentiation promotes prostate Cancer cells metastasis by modulating FNDC3B expression.BMC Cancer 2013;13:61.
[68]Hsieh IS,Chang KC,Tsai YT,Ke JY,Lu PJ,Lee KH,et al. MicroRNA-320 suppresses the stem cell-like characteristics of prostate cancer cells by downregulating the Wnt/betacatenin signaling pathway.Carcinogenesis 2013;34:530-8.
[69]Huang S,Guo W,Tang Y,Ren D,Zou X,Peng X.miR-143 and miR-145 inhibit stem cell characteristics of PC-3 prostate cancer cells.Oncol Rep 2012;28:1831-7.
[70]Yu J,Lu Y,Cui D,Li E,Zhu Y,Zhao Y,et al.miR-200b suppresses cell proliferation,migration and enhances chemosensitivity in prostate cancer by regulating Bmi-1.Oncol Rep 2014;31:910-8.
[71]Dudley ME,Wunderlich JR,Yang JC,Sherry RM,Topalian SL, Restifo NP,et al.Adoptive cell transfer therapy following nonmyeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma.J Clin Oncol 2005;23:2346-57.
[72]June CH.Adoptive T cell therapy for cancer in the clinic.J Clin Invest.2007;117:1466-76.
[73]Morgan RA,Dudley ME,Wunderlich JR,Hughes MS,Yang JC, Sherry RM,et al.Cancer regression in patients after transfer of genetically engineered lymphocytes.Science 2006;314: 126-9.
[74]Park JR,Digiusto DL,Slovak M,Wright C,Naranjo A,Wagner J, et al.Adoptive transfer of chimeric antigen receptor redirected cytolytic T lymphocyte clones in patients with neuroblastoma.Mol Ther 2007;15:825-33.
[75]Deng Z,Wu Y,Ma W,Zhang S,Zhang YQ.Adoptive T-cell therapy of prostate cancer targeting the cancer stem cell antigen EpCAM.BMC Immunol 2015;16:1.
[76]Cheng CY,Hwang CI,Corney DC,Flesken-Nikitin A,Jiang L, Oner GM,et al.miR-34 cooperates with p53 in suppression of prostate cancer by joint regulation of stem cell compartment. Cell Rep 2014;6:1000-7.
[77]Wang M,Ren D,Guo W,Wang Z,Huang S,Du H,et al.Loss of miR-100 enhances migration,invasion,epithelial-mesenchymal transition and stemness properties in prostate cancer cells through targeting Argonaute 2.Int J Oncol 2014;45: 362-72.
Received 7 June 2016;received in revised form 8 September 2016;accepted 8 September 2016
Available online 20 September 2016
*Corresponding author.
E-mail address:jt.hsieh@utsouthwestern.edu(J.T.Hsieh).
Peer review under responsibility of Second Military Medical University.
http://dx.doi.org/10.1016/j.ajur.2016.09.006
2214-3882/©2016 Editorial Office of Asian Journal of Urology.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Cancer stem cell;
Normal stem cell
杂志排行
Asian Journal of Urology的其它文章
- Novel immunotherapy approaches for metastatic urothelial and renal cell carcinoma
- Specific bone region localization of osteolytic versus osteoblastic lesions in a patient-derived xenograft model of bone metastatic prostate cancer
- Cultured circulating tumor cells and their derived xenografts for personalized oncology
- Intrinsic subtypes and bladder cancer metastasis
- Mismatch repair enzyme expression in primary and castrate resistant prostate cancer
- Developing immunotherapy strategies in the treatment of prostate cancer
