Adaptive pathways and emerging strategies overcoming treatment resistance in castration resistant prostate cancer
2016-04-27CmeronArmstrongAllenGo
Cmeron M.Armstrong,Allen C.Go,b,c,*
aDepartment of Urology,University of California,Davis,Sacramento,CA,USA
bComprehensive Cancer Center,University of California,Davis,Sacramento,CA,USA
cVA Northern California Health Care System,Sacramento,CA,USA
Adaptive pathways and emerging strategies overcoming treatment resistance in castration resistant prostate cancer
Cameron M.Armstronga,Allen C.Gaoa,b,c,*
aDepartment of Urology,University of California,Davis,Sacramento,CA,USA
bComprehensive Cancer Center,University of California,Davis,Sacramento,CA,USA
cVA Northern California Health Care System,Sacramento,CA,USA
Prostate cancer;
Castration resistant prostate cancer;
Enzalutamide;
Abiraterone;
Docetaxel;
Drug resistance
The therapies available for prostate cancer patients whom progress from hormonesensitive to castration resistant prostate cancer include both systemic drugs,including docetaxel and cabazitaxel,and drugs that inhibit androgen signaling such as enzalutamide and abiraterone.Unfortunately,it is estimated that up to 30%of patients have primary resistance to these treatments and over time even those who initially respond to therapy will eventually develop resistance and their disease will continue to progress regardless of the presence of the drug.Determining the mechanisms involved in the development of resistance to these therapies has been the area of intense study and several adaptive pathways have been uncovered.Androgen receptor(AR)mutations,expression of AR-V7(or other constitutively active androgen receptor variants),intracrine androgen production and overexpression of androgen synthesis enzymes such as Aldo-Keto Reductase Family 1,Member C3(AKR1C3)are among the many mechanisms associated with resistance to anti-androgens.In regards to the taxanes, one of the key contributors to drug resistance is increased drug efflux through ATP Binding Cassette Subfamily B Member 1(ABCB1).Targeting these resistance mechanisms using different strategies has led to various levels of success in overcoming resistance to current therapies.For instance,targeting AR-V7 with niclosamide or AKR1C3 with indomethacin can improve enzalutamide and abiraterone treatment.ABCB1 transport activity can be inhibited by the dietary constituent apigenin and antiandrogens such as bicalutamide which in turn improves response to docetaxel.A more thorough understanding of how drug resistance develops will lead to improved treatment strategies.This review will cover the current knowledge of resistance mechanisms to castration resistant prostate cancer therapies and methods that have been identified which may improve treatment response.
©2016 Editorial Office of Asian Journal of Urology.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/ licenses/by-nc-nd/4.0/).
1.Introduction
Prostate cancer is the second leading cause of cancer related deaths and the most commonly diagnosed cancer in men with an estimated 220,800 new cases yearly in the United States[1,2].First line treatments for prostate cancer aim to reduce circulating androgen levels through the use of androgen deprivation therapies(ADT).This is accomplished using one of two methods:surgical bilateral orchiectomy which inhibits androgen synthesis by the testes or through the use of castration inducing drugs to reduce androgen levels and androgen receptor(AR)activation. While ADT is initially effective at reducing prostate cancer growth,after 2-3 years of treatment the majority of patients will progress to castration resistant prostate cancer (CRPC)and tumor growth will proceed even in the presence of castrate levels of androgen.At this point of disease progression,the number of therapeutic options is currently limited but is the focus of intense research to improve the outcome for patients[3].
Clinically,CRPC is defined as progression of prostate cancer in the presence of castrate levels of circulating testosterone[4,5].Often times,the AR is either overexpressed,hyper-activated,or both leading to the transcription of downstream target genes which ultimately promotes tumor progression despite the patient having negligible levels of androgen present.The mechanisms which lead to the development of CRPC from hormonesensitive prostate cancer are widely studied.The identified mechanisms,including AR ampli fication and mutation, AR co-activator and co-repressor modi fications,aberrant activation and/or post-translational modification,AR splice variants,and altered steroidogenesis,each results in an increase in AR activation and signaling.This can be due to an increased amount of androgen,enhanced response to existing androgen,and activation of the AR by non-classical ligands or no ligand at all among other methods[6-10].
Treatment of CRPC is currently achieved with the administration of taxanes,such as docetaxel and cabazitaxel,which interrupt the growth of fast-dividing cells through disruption of microtubule function,or with antiandrogen therapies including enzalutamide and abiraterone.The primary mechanism of anti-androgens is to inhibit AR activation either directly,by antagonizing the receptor, or indirectly by blocking androgen synthesis.Unfortunately, it is estimated that one third of patients given abiraterone and one fourth of patients given enzalutamide will fail to respond to initial treatment with these drugs[11,12]. Furthermore,within 24 months of initiating treatment, even those who initially respond to the drugs will develop resistance.
New methods by which treatment resistance develops in prostate cancer are constantly being identified.Due to the numerous dysregulated pathways that are implicated in prostate cancer drug resistance,elucidating ways to reverse this resistance becomes both increasingly complicated and important.This review will outline the current understanding of the major compensatory mechanisms that prostate cancer cells use to overcome the presence of the drugs(Fig.1).In addition,successful experimental strategies that have been observed to improve treatment response will be discussed(Fig.2).

Figure 1Approved(orange)and experimental(red)therapies for CRPC and their targets.AR,androgen receptor;ARE,androgenresponse element;AR-V,androgen receptor variants;CRPC,castration resistant prostate cancer;DHT,dihydrotestosterone;PSA, prostate specific antigen.
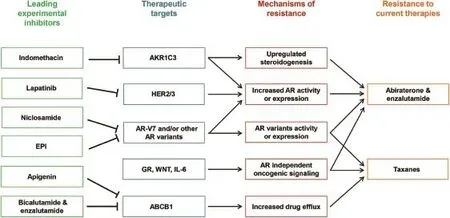
Figure 2An overview of CRPC drug resistance and promising experimental inhibitors that target resistance mechanisms.ABCB1, ATP Binding Cassette Subfamily B Member 1;AKR1C3,Aldo-Keto Reductase Family 1,Member C3;AR,androgen receptor;CRPC, castration resistant prostate cancer;GR,glucocorticoid receptor.
2.Current CRPC therapies
2.1.Anti-androgens
Anti-androgens seek to slow cancer cell growth by blocking activation of the AR.Despite the ability CRPC cells gain to bypass testosterone using the 5α-dione pathway to produce the more biologically active dihydrotestosterone(DHT), these cells still heavily rely on adrenal androgens which are converted to androstenedione by 3βHSD in the prostate or adrenal gland.DHT is then synthesized from androstenedione.Abiraterone acetate functions by reducing circulating androgens by inhibiting CYP17A1 and blocking the conversion of pregnenolone to DHT.The net result is a loss of androgen synthesis in peripheral tissues as well as a reduction in the precursors required for intratumoral androgen production. In addition to inhibition of CYP17A1,studies have observed that abiraterone can be converted into the more active Δ4-abiraterone(D4A)and this formofthe anti-androgen has also been shown to inhibit 3βHSD and SRD5A,two other enzymes involved in androgen synthesis.Furthermore,D4A has increased inhibition of prostate cancer xenograft growth compared to the parental abiraterone[13].
In regards to its efficacy,the COU-AA-302 trial showed a 4.4-month survival benefit with abiraterone in chemotherapy-naive CRPC patients and in patients who had progressed after docetaxel therapy,the phase III trial COUAA-301 demonstrated a 3.9-month survival benefit of abiraterone/prednisone over placebo/prednisone[11,14,15]. Despite these promising improvements in patient longevity, nearly a third of patients have primary resistance to abiraterone and even those who initially benefit from treatment will progress in their disease by 15 months of therapy[11].
As with abiraterone,enzalutamide also functions to reduce AR signaling.Instead of blocking production of its ligand,however,enzalutamide binds directly to the AR to inhibit its activation by androgens.Furthermore,enzalutamide inhibits AR translocation to the nucleus,coactivator recruitment,and binding of the AR to DNA,all of which reduce the activation of downstream AR target genes[16].Despite the fact that enzalutamide has been demonstrated to provide nearly 5 months improved survival compared to placebo treated individuals in CRPC patients who failed docetaxel treatment and is also effective in prechemotherapy hormone-naı¨ve prostate cancer patients,as many as one fourth of patients have primary resistance to enzalutamide and all patients had progressed by 24 months of initiating treatment[17,18].
2.2.Taxanes
Docetaxel and cabazitaxel both belong to a class of chemotherapeutics called taxanes.Docetaxel has traditionally been the first-line therapy for patients with CRPC.The introduction of enzalutamide and abiraterone,however, has led to a decrease docetaxel use as the primary treatment for CRPC.In addition to its use in CRPC,docetaxel has also proven to be effective in conjunction with ADT in hormone-naı¨ve prostate cancer patients with high volume or visceral metastases,providing a 17-month survival advantage over ADT alone[19].Docetaxel functions by binding free tubulin in cells which causes the formation of stable microtubules and prevents depolymerization, resulting in inhibition of mitosis and consequent induction of apoptosis[20-22].Interestingly,docetaxel has also been demonstrated to reduce AR expression in CRPC cells which could further slow the growth of prostate cancer cells[23]. Cabazitaxel,on the other hand,is primarily used in patients who have failed docetaxel therapy.The TROPIC trial observed a 2.4-month survival benefit over mitoxantrone in patients with metastatic CRPC whose disease had progressed on docetaxel[24].While both of these drugs are anti-mitotic and inhibit the division of proliferating cells through binding tubulin,unique mechanisms of action have been identified[25].
3.Mechanisms of resistance
While the drugs used for the treatment of CRPC have distinct methods of action and each has individualmechanisms of resistance,there is a surprising degree of cross-over in the pathways CRPC cells use to overcome drug treatment,particularly in the case of the anti-androgens. The resistance mechanisms can be broken up into several broad categories(Fig.1),a number of which will be discussed below.
3.1.Androgen receptor splice variants
AR splice variants can be formed by genome rearrangement and alternative splicing involving splicing factors such as hnRNPAs[26,27].Most commonly,AR variants lack the C-terminal ligand-binding domain and these truncated versions of AR are often ligand-independent and result in constitutive activation and uncontrolled downstream AR signaling[28-32].While AR variant expression is associated with poorer prognosis and the development of CRPC,the functional implications of AR variants are not yet fully understood,due in part to the lack of reliable variant specific antibodies[33].Analysis of in vitro prostate cancer cell lines has determined that nearly all CRPC lines display some level of AR variant expression and in fact,CWR22Rv1 cells have nearly equal expression of full length AR and AR variants.Furthermore,prostate cancer bone metastases have been found to have high AR variant expression[33].
Expression ofthese ARvariants is strongly associated with resistance to both abiraterone and enzalutamide,and though not as well studied,to docetaxel resistance as well. The most widely studied of these variants,AR-V7,appears to be of particular importance.It has been shown that AR-V7 expression in patients treated with enzalutamide or abiraterone correlates to a significantly lower prostate specific antigen(PSA)response,shorter progression-free and overall survival compared to men who do not express AR-V7[34].
Targeting AR variant expression is one way in which restoring sensitivity to anti-androgens can be achieved and a number of clinical trials are currently under way investigating various therapies to reduce AR variant expression and improve patient treatment response.Niclosamide,the anti-helminthic drug,has been demonstrated to preferably reduce expression of AR-V7 over full length AR,in enzalutamide resistant cells with comparatively high endogenous AR-V7 expression.Liu et al.[35,36]determined that niclosamide could induce AR-V7 protein degradation and reduce recruitment of AR-V7 to promoter regions of target genes resulting in reduced transcriptional activity and resensitize resistant cells to enzalutamide and abiraterone treatment.Furthermore,niclosamide had significant antitumor activity in a number of AR variant expressing CRPC cell lines such as enzalutamide resistant C4-2B cells(C4-2B MDVR)and CWR22Rv1 cells,as well as in an enzalutamide and abiraterone resistant CWR22Rv1 xenograft model.The combination of niclosamide with either enzalutamide or abiraterone produced maximal tumor inhibition in a CWR22Rv1 xenograft model.Based on these encouraging preclinical data,a phase II study with a lead-in safety phase of abiraterone in combination with niclosamide in a CRPC clinical trial was launched in 2016 at the University of California,Davis(NCT02807805).In this trial,recurrent or metastatic CRPC patients will receive abiraterone 1000 mg daily with prednisone 5 mg twice daily plus escalating doses of oral niclosamide/PDMX1001(400 mg twice daily,800 mg twice daily).Exploratory analysis of AR-V7 will also be conducted in this trial.
Other studies have also found that inhibiting AR variant expression can improve the response to enzalutamide; Nadiminty et al.[26]determined that downregulation of the splice factor hnRNPA1 reduced AR-V7 expression and consequently sensitized cells to treatment.Inhibition of HSP90 with onalespib was also observed to alter AR splicing and lower the expression of AR-V7[37].Furthermore, Yamashita et al.[38]were able to reduce CWR22Rv1 xenograft tumor growth by the addition of ASC-J9,a drug that degrades AR-V3 and full length AR.
Promising progress has also been made in developing drugs that target the N-terminus.This includes EPI and its derivatives.EPI covalently binds the N-terminal domain of both AR and its variants and inhibits transcriptional activity to inhibit prostate cancer cell growth in in vivo xenograft models[39,40].In vitro and in vivo studies have further demonstrated that EPI can inhibit the proliferation of enzalutamide resistant cells[41].Currently,a phase 1/2 clinical trial is underway(NCT02606123)investigating the use of EPI in men with metastatic CRPC who have progressed on enzalutamide or abiraterone[42].This study will determine the safety and tolerability of orally administered EPI and PSA response rate as the primary outcomes. Another class of drugs targeting the N-terminus of the AR, niphatenones,while able to inhibit transactivation of AR and its variants,also promoted the formation of glutathione adducts and therefore may not be as viable for prostate cancer therapy[43].
In regards to the taxanes,studies have demonstrated that AR-V7 can promote docetaxel resistance:Thadani-Mulero et al.[44]found that the AR variant ARV-567 was sensitive to microtubule stabilization induced by taxanes whereas AR-V7 was unaffected.In addition they showed that tumor xenografts expressing AR-V7 were resistant to docetaxel therapy while those with ARV-567 expression were highly sensitive to docetaxel.To compliment this fact, Zhang et al.[45]found that docetaxel resistant cell lines express higher levels of AR-V7 and that transfection of ARV7 into LNCaP cells protected them against docetaxel treatment.Interestingly,this group also saw an induction of docetaxel resistance when they transfected AR-V567 into the cells which contradicts what Thadani-Muleroand colleagues observed[44].To further complicate the taxane and AR variant connection,another study which measured AR-V7 expression in circulating tumor cells(CTC)of metastatic CRPC patients found that detection of AR-V7 in these cells was not correlated with primary resistance to taxanes [46].Furthermore,another study in CTC found that patients with nuclear CTC AR-V7 expression had increased survival benefit on taxanes compared to therapies directed AR signaling[47].The varying results from these studies suggest that the impact of AR-V7 on taxane resistance may be model-specific and more study in this area is needed.
3.2.Increased AR activation
Increased activation of the full length AR is also a welldocumented mechanism for promoting drug resistance,primarily to the anti-androgens.The observed increase in AR signaling that occurs when cells develop resistance can be due to a variety of methods including altered steroidogenesis or overexpression of the receptor itself.
Prolonged exposure to both enzalutamide and abiraterone incurs alterations in steroidogenesis.The resultant increase in androgen due to up-regulation of and mutations to enzymes involved in this complicated pathway promotes activation of the AR and is a likely contributor to both CRPC progression and anti-androgen resistance.Enzalutamide resistant prostate cancer cells had upregulated expression of androgen and its precursors including cholesterol,DHEA and progesterone.Additionally,genes involved in steroid biosynthesis are significantly over-expressed in enzalutamide resistant compared to enzalutamide-sensitive parental cells[48].Mostaghel et al.[49]detected up to a 4.5-fold increase in enzymes involved in steroidogenesis in abiraterone treated prostate cancer cells in vitro,including CYP17A1,AKR1C3,HSD17B3,and SDR5A2.Additionally,the hyperactive 1245C mutation of HSD3B1 has been observed in abiraterone-resistant xenograft models[50].Of the enzymes contributing to steroidogenesis,AKR1C3 is of particular import.Its activation contributes to both abiraterone and enzalutamide drug resistance in CRPC patients and it has been proposed as a biomarker for assessing prostate cancer progression[48,51].Liu et al.[48]found that indomethacin,a nonsteroidal anti-inflammatory drug, was capable of inhibiting AKR1C3 enzymatic activity and restored enzalutamide sensitivity in resistant prostate cancer cells.This suggests that targeting intracrine androgens improves enzalutamide therapy.Based on these promising preclinical studies,a single-arm phase II trial with a lead-in safety phase to determine the efficacy and toxicity of an indomethacin and enzalutamide combination in the treatment of CRPC will be launched at the University of California,Davis.
Upregulated AR activation can also be the result of mutations to the AR gene.It is estimated that 10%-30%of CRPC patients have AR mutations and these mutations can result in increased coactivator recruitment,and alter ligand specificity and affinity[52].The most commonly identified AR mutation,T878A,occurs most commonly in response to drugs targeting androgen synthesis,like abiraterone[53].This mutation,and others,are correlated to decreased ligand specificity of the AR allowing the receptor to activate in response to a broader range of molecules, including estrogen and glucocorticoids,that the wildtype AR is not responsive to[10,54,55].This could be of importance to patients receiving abiraterone since prednisone,a glucocorticoid,is co-administered with the anti-androgen to counterbalance some of its side effects.Also with abiraterone treatment,androgen precursors,including pregnenolone and progesterone,have been demonstrated to accumulate and some of these have also been identified to bind mutated AR and instigate downstream AR signaling [55-57].Furthermore,the F877L mutation of the AR is associated with changing ligand binding specificity of the AR to switch from agonist to antagonist activation,causing enzalutamide to activate the AR instead of inactivate it [58,59].The F877L mutation has also been identified in circulating cell-free DNA samples from patients whose disease had progressed while receiving enzalutamide or ARN-509,another anti-androgen structurally similar to enzalutamide[60].Interestingly,Korpal et al.[59]demonstrated that while the F877L mutation confers resistance to enzalutamide in vitro,cells expressing this mutation remain responsive to bicalutamide.
3.3.Increased AR expression
In addition to an upregulation in androgen synthesis pathways and AR mutation,increased AR activation can be attained through modulation of wildtype AR expression.In CRPC,the AR is commonly overexpressed however the method that drives this overexpression is not completely understood.One mechanism which has recently been determined is through upregulation of retinoic acid receptor-related orphan receptor γ(ROR-γ).ROR-γ was found to be upregulated in CRPC and could drive AR expression.ROR-γ recruited the AR co-activators SRC-1 and SRC-3 which in turn promoted AR transcription.Furthermore,treatment with ROR-γ antagonists suppressed prostate cancer xenograft growth and improved the response to enzalutamide[61].Also affecting AR expression,Gao et al. [62]observed that abiraterone treated patients had higher ErbB2 activity and this correlated with increased AR expression in the nucleus,suggesting a potential increase in AR signaling.They further went on to demonstrate that abiraterone resistant xenograft models had increased ErbB2 activity and in turn this led to stabilization of AR protein through PI3K/AKT signaling.By blocking ErbB2 using lapatinib in combination with abiraterone they were able to enhance treatment response in xenograft models.Mellinghoff et al.[63]determined that HER2 and HER3 signaling can increase AR signaling;knockdown of HER2 was found to inhibit transcription of the AR and both HER2 and HER3 stabilized the AR and promoted binding to androgenresponse elements(ARE).Another group,Shiota et al.[64], found that enzalutamide resistant tumors and cells have increased HER2 expression and that enzalutamide treatment induced HER2 expression in LNCaP cells.Furthermore,they determined that enzalutamide response could be enhanced by lapatinib through inhibition of the HER2 signaling axis.
The AR also plays a role in the response to taxanes.In fact,part of the mechanism of action attributed to taxanes is through modulation of the AR.Taxanes have been demonstrated to reduce AR expression,nuclear translocation,and transcriptional activity[23,65,66].These effects can be induced by docetaxel,but not cabazitaxel, treatment[65,67].Komura et al.[68]found that expression of lysine-specific demethylase 5D(KDM5D)is decreased in CRPC and low expression levels are associated with a poor patient prognosis.They further determined that knocking down KDM5D,which regulates AR transcriptional activity, induced docetaxel resistance in LNCaP cells,which are normally highly susceptible to docetaxel treatment,supporting a link between the AR and docetaxel sensitivity.
3.4.Androgen receptor co-regulators
A number of molecules have been identified that function as co-activators or co-repressors for the AR[69].These co-regulators help modulate AR transcriptional activity by acting on other molecules in the transcription complex through methylation,phosphorylation,ubiquitylation or acetylation,and can also act as molecular chaperones and help with recruitment of transcriptional machinery [70-72].The AR co-activator FKBP51 has been observed to be upregulated in relapsed LAPC-4 tumor xenografts in castrated mice resulting in increased activation of the AR in response to ligand[73].The p300/CBP and the steroid receptor co-activators(SRC)class of co-activators,which includes SRC-1,Tif-2,and SRC-3,are also associated with prostate cancer disease progression and SRC-1 and p300/ CBP have been linked to IL-6 induced androgenindependent AR activation[74,75].
AR co-activators can also mediate AR activation of truncated,ligand-independent AR splice variants.In particular,McGrath et al.[76]demonstrated that the coactivator FHL2(four and a half LIM protein 2)interacts with AR-V7.They determined that AR-V7 activation,as determined by ARE-luciferase reporter and in the absence of androgen,was enhanced by FHL2 expression and this response could not be abrogated by enzalutamide.
3.5.AR independent anti-androgen resistance
While most of the identified mechanisms inducing resistance to the anti-androgens are associated in one way or the other with increasing androgen signaling,there are also compensatory pathways that become activated that are independent of the AR and androgen synthesis.Downstream signaling of the glucocorticoid receptor(GR), another nuclear receptor like the AR,is increased by treatment with anti-androgens and treatment response to enzalutamide in prostate cancer patients is inversely correlated to GR expression.Furthermore,GR mRNA and protein expressions were found to be upregulated in antiandrogen resistant tumors and knockdown of the GR in resistant cells resensitized them to enzalutamide treatment in vitro[77].These effects are hypothesized to be a result of the commonality between the GR and AR allowing the GR to compensate for the reduced AR activity induced by anti-androgens.
IL-6 has also been proposed to play a role in the response to enzalutamide;Handle et al.[78]found that enzalutamide(as well as bicalutamide,another antiandrogen)up-regulates suppressor of cytokine 4 signaling 3(SOCS3)mRNA which in turn modulates IL-6/Stat3 signaling.When they knocked down SOCS3,they were able to reverse an IL-6/enzalutamide induced reduction in AR target genes.Further implicating IL-6/Stat3 in enzalutamide resistance,Liu et al.[79]found that overexpression of constitutively active Stat3 induced resistance to enzalutamide treatment whereas downregulation of Stat3 improved enzalutamide response and increased apoptosis. In another study,Liu et al.[80]also determined that the drug niclosamide can also down-regulate Stat3 target gene expression and resensitize enzalutamide resistant cells to treatment.Wnt/β-catenin signaling is another proliferative pathway that is upregulated in enzalutamide resistance and inhibition of this pathway has also been observed to increase enzalutamide sensitivity[81].
3.6.Altered drug efflux
A method primarily associated with docetaxel resistance involves overactivation or overexpression of multidrug resistance proteins(MDRP).These proteins,including ABCB1,serve as pumps on the cell membrane to excrete exogenous compounds,such as docetaxel,out of the cell. This results in a lower intracellular drug concentration and a loss of drug efficacy.Multiple studies have shown that docetaxel resistant cells express significantly increased levels of ABCB1 compared to docetaxel sensitive parental cells lines[82,83].Hour et al.[84]determined that the increase in ABCB1 observed in docetaxel resistant cells is likely due in part to the increased epidermal growth factor receptor(EGFR)expression also found in these cells.Others have observed that an increase in expression and phosphorylation of breast cancer resistance protein,another transporter protein,promotes docetaxel resistance as well [85].
Regulating these drug efflux pathways has been an area of intense study for resensitizing prostate cancer cells to docetaxel treatment.A number of phase I and II clinical trials have investigated the possibility of using MDRP inhibiting drugs,such as elacridar,in combination with chemotherapy.Despite phase I trials showing promise,only minimal clinical activity was observed in phase II trials [86,87].In vitro and in vivo studies have found that ABCB1 activity and/or expression can be reduced by a variety of dietary flavonoids including apigenin,naringenin,and genistein[83,88].Treatment of docetaxel resistant C4-2B cells with apigenin was observed to overcome ABCB1 mediated docetaxel resistance and resensitize cells to drug treatment by reducing ABCB1 expression[83].In a separate study,Zhu et al.[89]also determined that anti-androgens could reduce ABCB1 activity as assayed by Rhodamine 123 efflux.Furthermore,co-treatment in both AR-positive and AR-negative docetaxel resistant mouse xenograft models with bicalutamide and docetaxel was observed to signif icantly reduce tumor growth,indicating that this effect by bicalutamide is independent of AR status.
3.7.β-tubulin dysregulation
Also specific to taxane resistance,the presence of β-tubulin isoforms promotes both docetaxel and cabazitaxel resistance in prostate cancer.Specifically,taxanes have reduced efficiency for binding to the class III β-tubulin isoform[90,91].Studies have also found increased expression of class IV β-tubulin and mutations to class 1 β-tubulin which results in impaired polymerization in docetaxel resistant cells[92,93].Galletti et al.[94]found that ETS-related gene(ERG)overexpression in prostate cells leads to cabazitaxel resistance both in vitro and in vivo by interacting with β-tubulin and tubulin dimers.They further determined that cytoplasmic interruption of this interaction restores cabazitaxel sensitivity.Additionally,suppressed expression of β-tubulin isoform IVa by the synthetic estrogen diethylstilbestrol has been demonstrated to enhance tumor growth inhibition in combination with docetaxel in prostate cancer xenograft models[95].Others have demonstrated that the N-terminal domain of the ARinteracts with tubulin and targeting this domain with the small-molecule inhibitor EPI improved docetaxel effectiveness and reduced the number of cells displaying the epithelial-mesenchymal-transition(EMT)phenotype [96,97].
3.8.Cell survival/growth pathways and cytokines
Most prostate cancer cells that display resistance to one drug therapy or another have aberrant regulation of molecules involved in cell survival and death.Specifically, docetaxel resistance is associated with overexpression of signal transducers and activator of transcription(Stat)1, Stat3,clusterin,heat shock proteins(HSP),GATA2,and nuclear factor kappa B(NF-κB)[82,98-103].Reduced activity and expression of wildtype p53 has also been linked to docetaxel insensitivity[104].Furthermore,expression of pro-inflammatory cytokines,such as interleukin(IL)-6,IL-8 chemokine ligand 2(CCL2),transforming growth factor-β1 (TGF-β1)and macrophage inhibitory cytokine-1(MIC-1) have been shown to promote docetaxel resistance [105-109].
In many cases,correcting the aberrant expression of these molecules has been demonstrated to reintroduce sensitivity to docetaxel treatment.For instance,inhibition of IGF1R expression,a molecule downstream of GATA2 signaling,was observed to improve both docetaxel and cabazitaxel sensitivity in resistant cell lines[103].Modulating cytokine expression has also proven effective in vitro;reducing IL-6 and TNFα and inhibiting NF-κB expression using either synthetic or naturally occurring compounds results in an increased response to docetaxel in prostate cancer cells[82,110].
4.Conclusion
Resistance to the current therapies available for CRPC is inevitable.The variety of adaptive mechanisms by which this resistance occurs makes overcoming treatment resistance a challenging dilemma.Fortunately,numerous studies have identified several of these aberrantly functioning pathways and have put forth treatment strategies for how to best re-introduce sensitivity.With a more thorough understanding for how drug resistance occurs,novel therapies can be developed and tested for likely therapeutic benefits.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
This work is supported in part by grants NIH/NCI CA140468, CA168601,CA179970,DOD PC130062,Ralph de Vere White endowment,US Department of Veterans Affairs,Office of Research and Development VA Merits I01 BX002653,and by resources from the VA Northern California Health Care System,Sacramento,California.
[1]Ferlay J,Steliarova-Foucher E,Lortet-Tieulent J,Rosso S, Coebergh JW,Comber H,et al.Cancer incidence and mortality patterns in Europe:estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374-403.
[2]Siegel RL,Miller KD,Jemal A.Cancer statistics,2015.CA Cancer J Clin 2015;65:5-29.
[3]Harris WP,Mostaghel EA,Nelson PS,Montgomery B.Androgen deprivation therapy:progress in understanding mechanisms of resistance and optimizing androgen depletion.Nat Clin Pract Urol 2009;6:76-85.
[4]Cookson MS,Roth BJ,Dahm P,Engstrom C,Freedland SJ, Hussain M,et al.Castration-resistant prostate cancer:AUA Guideline.J Urol 2013;190:429-38.
[5]Saad F,Hotte SJ.Guidelines for the management of castrateresistant prostate cancer.Can Urol Assoc J 2010;4:380-4.
[6]Dehm SM,Schmidt LJ,Heemers HV,Vessella RL,Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance.Cancer Res 2008;68: 5469-77.
[7]Chang KH,Ercole CE,SharifiN.Androgen metabolism in prostate cancer:from molecular mechanisms to clinical consequences.Br J Cancer 2014;111:1249-54.
[8]Chang KH,Li R,Papari-Zareei M,Watumull L,Zhao YD, Auchus RJ,et al.Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc Natl Acad Sci U S A 2011;108:13728-33.
[9]Shtivelman E,Beer TM,Evans CP.Molecular pathways and targets in prostate cancer.Oncotarget 2014;5:7217-59.
[10]Steketee K,Timmerman L,Ziel-van der Made AC,Doesburg P, Brinkmann AO,Trapman J.Broadened ligand responsiveness of androgen receptor mutants obtained by random amino acid substitution of H874 and mutation hot spot T877 in prostate cancer.Int J Cancer 2002;100:309-17.
[11]de Bono JS,Logothetis CJ,Molina A,Fizazi K,North S,Chu L, et al.Abiraterone and increased survival in metastatic prostate cancer.N Engl J Med 2011;364:1995-2005.
[12]Scher HI,Fizazi K,Saad F,Taplin ME,Sternberg CN,Miller K, et al.Increased survival with enzalutamide in prostate cancer after chemotherapy.N Engl J Med 2012;367:1187-97.
[13]Li Z,Bishop AC,Alyamani M,Garcia JA,Dreicer R,Bunch D, et al.Conversion of abiraterone to D4A drives anti-tumour activity in prostate cancer.Nature 2015;523:347-51.
[14]Ryan CJ,Smith MR,de Bono JS,Molina A,Logothetis CJ,de Souza P,et al.Abiraterone in metastatic prostate cancer without previous chemotherapy.N Engl J Med 2013;368: 138-48.
[15]Ryan CJ,Smith MR,Fizazi K,Saad F,Mulders PF, Sternberg CN,et al.Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer(COUAA-302):final overall survival analysis of a randomised, double-blind,placebo-controlled phase 3 study.Lancet Oncol 2015;16:152-60.
[16]Sternberg CN,Petrylak DP,Madan RA,Parker C.Progress in the treatment of advanced prostate cancer.Am Soc Clin Oncol Educ Book 2014:117-31.
[17]Beer TM,Armstrong AJ,Rathkopf DE,Loriot Y,Sternberg CN, Higano CS,et al.Enzalutamide in metastatic prostate cancer before chemotherapy.N Engl J Med 2014;371:424-33.
[18]Sternberg CN,de Bono JS,Chi KN,Fizazi K,Mulders P, Cerbone L,et al.Improved outcomes in elderly patients with metastatic castration-resistant prostate cancer treated with the androgen receptor inhibitor enzalutamide:results from the phase III AFFIRM trial.Ann Oncol 2014;25:429-34.
[19]Sweeney CJ.ECOG:CHAARTED-ChemoHormonal therapy versus androgen ablation randomized trial for extensive disease in prostate cancer.Clin Adv Hematol Oncol 2006;4: 588-90.
[20]Dagher R,Li N,Abraham S,Rahman A,Sridhara R,Pazdur R. Approval summary:Docetaxel in combination with prednisone for the treatment of androgen-independent hormonerefractory prostate cancer.Clin Cancer Res 2004;10: 8147-51.
[21]McGrogan BT,Gilmartin B,Carney DN,McCann A.Taxanes, microtubules and chemoresistant breast cancer.Biochim Biophys Acta 2008;1785:96-132.
[22]Shelanski ML,Gaskin F,Cantor CR.Microtubule assembly in the absence of added nucleotides.Proc Natl Acad Sci U S A 1973;70:765-8.
[23]Kuroda K,Liu H,Kim S,Guo M,Navarro V,Bander NH. Docetaxel down-regulates the expression of androgen receptor and prostate-specific antigen but not prostate-specific membrane antigen in prostate cancer cell lines: implications for PSA surrogacy.Prostate 2009;69:1579-85.
[24]de Bono JS,Oudard S,Ozguroglu M,Hansen S,Machiels JP, Kocak I,et al.Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment:a randomised open-label trial.Lancet 2010;376:1147-54.
[25]de Leeuw R,Berman-Booty LD,Schiewer MJ,Ciment SJ, Den RB,Dicker AP,et al.Novel actions of next-generation taxanes benefit advanced stages of prostate cancer.Clin Cancer Res 2015;21:795-807.
[26]Nadiminty N,Tummala R,Liu C,Lou W,Evans CP,Gao AC. NF-kappaB2/p52:c-Myc:hnRNPA1 pathway regulates expression of androgen receptor splice variants and enzalutamide sensitivity in prostate cancer.Mol Cancer Ther 2015;14: 1884-95.
[27]Li Y,Alsagabi M,Fan D,Bova GS,Tewfik AH,Dehm SM. Intragenic rearrangement and altered RNA splicing of the androgen receptor in a cell-based model of prostate cancer progression.Cancer Res 2011;71:2108-17.
[28]Dehm SM,Tindall DJ.Alternatively spliced androgen receptor variants.Endocr Relat Cancer 2011;18:R183-96.
[29]Guo Z,Yang X,Sun F,Jiang R,Linn DE,Chen H,et al.A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth.Cancer Res 2009;69:2305-13.
[30]Hu R,Dunn TA,Wei S,Isharwal S,Veltri RW,Humphreys E, et al.Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer.Cancer Res 2009;69:16-22.
[31]Sun S,Sprenger CC,Vessella RL,Haugk K,Soriano K, Mostaghel EA,et al.Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant.J Clin Invest 2010;120:2715-30.
[32]Yang X,Guo Z,Sun F,Li W,Alfano A,Shimelis H,et al.Novel membrane-associated androgen receptor splice variant potentiates proliferative and survival responses in prostate cancer cells.J Biol Chem 2011;286:36152-60.
[33]Hornberg E,Ylitalo EB,Crnalic S,Antti H,Stattin P, Widmark A,et al.Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival.PLoS One 2011; 6:e19059.
[34]Antonarakis ES,Lu C,Wang H,Luber B,Nakazawa M, Roeser JC,et al.AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer.N Engl J Med 2014;371: 1028-38.
[35]Liu C,Lou W,Zhu Y,Nadiminty N,Schwartz CT,Evans CP,etal. Niclosamide inhibits androgen receptor variants expression and overcomes enzalutamide resistance in castration-resistant prostate cancer.Clin Cancer Res 2014;20:3198-210.
[36]Liu C,Armstrong C,Zhu Y,Lou W,Gao AC.Niclosamide enhances abiraterone treatment via inhibition of androgen receptor variants in castration resistant prostate cancer. Oncotarget 2016.http://dx.doi.org/10.18632/oncotarget.8493[Epub ahead of print].
[37]Ferraldeschi R,Welti J,Powers MV,Yuan W,Smyth T,Seed G, et al.Second-generation HSP90 inhibitor onalespib blocks mRNA splicing of androgen receptor variant 7 in prostate cancer cells.Cancer Res 2016;76:2731-42.
[38]Yamashita S,Lai KP,Chuang KL,Xu D,Miyamoto H,Tochigi T, et al.ASC-J9 suppresses castration-resistant prostate cancer growth through degradation of full-length and splice variant androgen receptors.Neoplasia 2012;14:74-83.
[39]Myung JK,Banuelos CA,Fernandez JG,Mawji NR,Wang J, Tien AH,et al.An androgen receptor N-terminal domain antagonist for treating prostate cancer.J Clin Invest 2013; 123:2948-60.
[40]Andersen RJ,Mawji NR,Wang J,Wang G,Haile S,Myung JK, et al.Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor.Cancer Cell 2010;17:535-46.
[41]Yang YC,Banuelos CA,Mawji NR,Wang J,Kato M,Haile S, et al.Targeting androgen receptor activation function-1 with EPI to overcome resistance mechanisms in castration-resistant prostate cancer.Clin Cancer Res 2016;22:4466-77.
[42]ESSAPharmaceuticals:Safety and anti-tumor study of oral EPI-506 for patients with metastatic castration-resistant prostate cancer.NLM Identifier:NCT02606123.
[43]Banuelos CA,Lal A,Tien AH,Shah N,Yang YC,Mawji NR, et al.Characterization of niphatenones that inhibit androgen receptor N-terminal domain.PLoS One 2014;9:e107991.
[44]Thadani-Mulero M,Portella L,Sun S,Sung M,Matov A, Vessella RL,et al.Androgen receptor splice variants determine taxane sensitivity in prostate cancer.Cancer Res 2014; 74:2270-82.
[45]Zhang G,Liu X,Li J,Ledet E,Alvarez X,Qi Y,et al.Androgen receptor splice variants circumvent AR blockade by microtubule-targeting agents.Oncotarget 2015;6:23358-71.
[46]Antonarakis ES,Lu C,Luber B,Wang H,Chen Y,Nakazawa M, et al.Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castrationresistant prostate cancer.JAMA Oncol 2015;1:582-91.
[47]Scher HI,Lu D,Schreiber NA,Louw J,Graf RP,Vargas HA, et al.Association of AR-V7 on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate cancer.JAMA Oncol 2016.http: //dx.doi.org/10.1001/jamaoncol.2016.1828[Epub ahead of print].
[48]Liu C,Lou W,Zhu Y,Yang JC,Nadiminty N,Gaikwad NW, et al.Intracrine Androgens and AKR1C3 activation confer resistance to enzalutamide in prostate cancer.Cancer Res 2015;75:1413-22.
[49]Mostaghel EA,Marck BT,Plymate SR,Vessella RL,Balk S, Matsumoto AM,et al.Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer:induction of steroidogenesis and androgen receptor splice variants.Clin Cancer Res 2011;17:5913-25.
[50]Cai C,Chen S,Ng P,Bubley GJ,Nelson PS,Mostaghel EA, et al.Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res 2011;71:6503-13.
[51]Tian Y,Zhao L,Zhang H,Liu X,Zhao L,Zhao X,et al.AKR1C3 overexpression may serve as a promising biomarker for prostate cancer progression.Diagn Pathol 2014;9:42.
[52]Waltering KK,Urbanucci A,Visakorpi T.Androgen receptor (AR)aberrations in castration-resistant prostate cancer.Mol Cell Endocrinol 2012;360:38-43.
[53]Taplin ME,Bubley GJ,Ko YJ,Small EJ,Upton M, Rajeshkumar B,et al.Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist.Cancer Res 1999;59:2511-5.
[54]Zhao XY,Malloy PJ,Krishnan AV,Swami S,Navone NM, Peehl DM,et al.Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor.Nat Med 2000;6:703-6.
[55]Culig Z,Hobisch A,Cronauer MV,Cato AC,Hittmair A, Radmayr C,et al.Mutant androgen receptor detected in an advanced-stage prostatic carcinoma is activated by adrenal androgens and progesterone.Mol Endocrinol 1993;7: 1541-50.
[56]Grigoryev DN,Long BJ,Njar VC,Brodie AH.Pregnenolone stimulates LNCaP prostate cancer cell growth via the mutated androgen receptor.J Steroid Biochem Mol Biol 2000; 75:1-10.
[57]Attard G,Reid AH,Auchus RJ,Hughes BA,Cassidy AM, Thompson E,et al.Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer.J Clin Endocrinol Metab 2012;97:507-16.
[58]Eisermann K,Wang D,Jing Y,Pascal LE,Wang Z.Androgen receptor gene mutation,rearrangement,polymorphism. Transl Androl Urol 2013;2:137-47.
[59]Korpal M,Korn JM,Gao X,Rakiec DP,Ruddy DA,Doshi S, et al.An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100(enzalutamide). Cancer Discov 2013;3:1030-43.
[60]Azad AA,Volik SV,Wyatt AW,Haegert A,Le Bihan S,Bell RH, et al.Androgen receptor gene aberrations in circulating cellfree DNA:biomarkers of therapeutic resistance in castrationresistant prostate cancer.Clin Cancer Res 2015;21:2315-24.
[61]Wang J,Zou JX,Xue X,Cai D,Zhang Y,Duan Z,et al.ROR-gamma drives androgen receptor expression and represents a therapeutic target in castration-resistant prostate cancer. Nat Med 2016;22:488-96.
[62]Gao S,Ye H,Gerrin S,Wang H,Sharma A,Chen S,et al.ErbB2 signaling increases androgen receptor expression in abiraterone-resistant prostate cancer.Clin Cancer Res 2016;22: 3672-82.
[63]Mellinghoff IK,Vivanco I,Kwon A,Tran C,Wongvipat J, Sawyers CL.HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability.Cancer Cell 2004;6:517-27.
[64]Shiota M,Bishop JL,Takeuchi A,Nip KM,Cordonnier T, Beraldi E,et al.Inhibition of the HER2-YB1-AR axis with Lapatinib synergistically enhances Enzalutamide anti-tumor efficacy in castration resistant prostate cancer.Oncotarget 2015;6:9086-98.
[65]Gan L,Chen S,Wang Y,Watahiki A,Bohrer L,Sun Z,et al. Inhibition of the androgen receptor as a novel mechanism of taxol chemotherapy in prostate cancer.Cancer Res 2009;69: 8386-94.
[66]Mistry SJ,Oh WK.New paradigms in microtubule-mediated endocrine signaling in prostate cancer.Mol Cancer Ther 2013;12:555-66.
[67]Al Nakouzi N,Le Moulec S,Albiges L,Wang C,Beuzeboc P, Gross-Goupil M,et al.Cabazitaxel remains active in patients progressing after docetaxel followed by novel androgen receptor pathway targeted therapies.Eur Urol 2015;68: 228-35.
[68]Komura K,Jeong SH,Hinohara K,Qu F,Wang X,Hiraki M, et al.Resistance to docetaxel in prostate cancer is associated with androgen receptor activation and loss of KDM5D expression.Proc Natl Acad Sci U S A 2016;113: 6259-64.
[69]Heemers HV,Tindall DJ.Androgen receptor(AR)coregulators:a diversity of functions converging on and regulating the AR transcriptional complex.Endocr Rev 2007;28: 778-808.
[70]Wolf IM,Heitzer MD,Grubisha M,DeFranco DB.Coactivators and nuclear receptor transactivation.J Cell Biochem 2008; 104:1580-6.
[71]Hermanson O,Glass CK,Rosenfeld MG.Nuclear receptor coregulators:multiple modes of modification.Trends Endocrinol Metab 2002;13:55-60.
[72]Agoulnik IU,Weigel NL.Androgen receptor coactivators and prostate cancer.Adv Exp Med Biol 2008;617:245-55.
[73]Ni L,Yang CS,Gioeli D,Frierson H,Toft DO,Paschal BM. FKBP51 promotes assembly of the Hsp90 chaperone complex and regulates androgen receptor signaling in prostate cancer cells.Mol Cell Biol 2010;30:1243-53.
[74]Ueda T,Mawji NR,Bruchovsky N,Sadar MD.Ligand-independent activation of the androgen receptor by interleukin-6 and the role of steroid receptor coactivator-1 in prostate cancer cells.J Biol Chem 2002;277:38087-94.
[75]Debes JD,Schmidt LJ,Huang H,Tindall DJ.p300 mediates androgen-independent transactivation of the androgen receptor by interleukin 6.Cancer Res 2002;62:5632-6.
[76]McGrath MJ,Binge LC,Sriratana A,Wang H,Robinson PA, Pook D,et al.Regulation of the transcriptional coactivator FHL2 licenses activation of the androgen receptor in castrate-resistant prostate cancer.Cancer Res 2013;73: 5066-79.
[77]Arora VK,Schenkein E,Murali R,Subudhi SK,Wongvipat J, Balbas MD,et al.Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 2013;155:1309-22.
[78]Handle F,Erb HH,Luef B,Hoefer J,Dietrich D,Parson W, et al.SOCS3 modulates the response to enzalutamide and is regulated by AR signaling and CpG methylation in prostate cancer cells.Mol Cancer Res 2016;14:574-85.
[79]Liu C,Zhu Y,Lou W,Cui Y,Evans CP,Gao AC.Inhibition of constitutively active Stat3 reverses enzalutamide resistance in LNCaP derivative prostate cancer cells.Prostate 2014;74: 201-9.
[80]Liu C,Lou W,Armstrong C,Zhu Y,Evans CP,Gao AC.Niclosamide suppresses cell migration and invasion in enzalutamide resistant prostate cancer cells via Stat3-AR axis inhibition.Prostate 2015;75:1341-53.
[81]Lee E,Ha S,Logan SK.Divergent androgen receptor and beta-catenin signaling in prostate cancer cells.PLoS One 2015;10:e0141589.
[82]O’Neill AJ,Prencipe M,Dowling C,Fan Y,Mulrane L, Gallagher WM,et al.Characterisation and manipulation of docetaxel resistant prostate cancer cell lines.Mol Cancer 2011;10:126.
[83]Zhu Y,Liu C,Nadiminty N,Lou W,Tummala R,Evans CP, et al.Inhibition of ABCB1 expression overcomes acquired docetaxel resistance in prostate cancer.Mol Cancer Ther 2013;12:1829-36.
[84]Hour TC,Chung SD,Kang WY,Lin YC,Chuang SJ,Huang AM, et al.EGFR mediates docetaxel resistance in human castration-resistant prostate cancer through the Aktdependent expression of ABCB1(MDR1).Arch Toxicol 2015; 89:591-605.
[85]Xie Y,Xu K,Linn DE,Yang X,Guo Z,Shimelis H,et al.The 44-kDa Pim-1 kinase phosphorylates BCRP/ABCG2 and thereby promotes its multimerization and drug-resistant activity in human prostate cancer cells.J Biol Chem 2008;283:3349-56.
[86]van Zuylen L,Sparreboom A,van der Gaast A,Nooter K, Eskens FA,Brouwer E,et al.Disposition of docetaxel in the presence of P-glycoprotein inhibition by intravenous administration of R101933.Eur J Cancer 2002;38:1090-9.
[87]Fracasso PM,Goldstein LJ,de Alwis DP,Rader JS, Arquette MA,Goodner SA,et al.Phase I study of docetaxel in combination with the P-glycoprotein inhibitor,zosuquidar,in resistant malignancies.Clin Cancer Res 2004;10:7220-8.
[88]Michaelis M,Rothweiler F,Nerreter T,SharifiM, Ghafourian T,Cinatl J.Karanjin interferes with ABCB1, ABCC1,and ABCG2.J Pharm Pharm Sci 2014;17:92-105.
[89]Zhu Y,Liu C,Armstrong C,Lou W,Sandher A,Gao AC.Antiandrogens inhibit ABCB1 efflux and ATPase activity and reverse docetaxel resistance in advanced prostate cancer. Clin Cancer Res 2015;21:4133-42.
[90]Ploussard G,Terry S,Maille P,Allory Y,Sirab N,Kheuang L, et al.Class III beta-tubulin expression predicts prostate tumor aggressiveness and patient response to docetaxelbased chemotherapy.Cancer Res 2010;70:9253-64.
[91]Terry S,Ploussard G,Allory Y,Nicolaiew N,Boissiere-Michot F,Maille P,et al.Increased expression of class III beta-tubulin in castration-resistant human prostate cancer. Br J Cancer 2009;101:951-6.
[92]Hara T,Ushio K,NishiwakiM,Kouno J,ArakiH,HikichiY,et al.A mutation in beta-tubulin and a sustained dependence on androgen receptor signalling in a newly established docetaxelresistantprostate cancercellline.CellBiolInt2010;34:177-84.
[93]Makarovskiy AN,Siryaporn E,Hixson DC,Akerley W.Survival of docetaxel-resistant prostate cancer cells in vitro depends on phenotype alterations and continuity of drug exposure. Cell Mol Life Sci 2002;59:1198-211.
[94]Galletti G,Matov A,Beltran H,Fontugne J,Miguel Mosquera J, Cheung C,et al.ERG induces taxane resistance in castrationresistant prostate cancer.Nat Commun 2014;5:5548.
[95]Montgomery RB,Bonham M,Nelson PS,Grim J,Makary E, Vessella R,et al.Estrogen effects on tubulin expression and taxane mediated cytotoxicity in prostate cancer cells.Prostate 2005;65:141-50.
[96]Zhu ML,Horbinski CM,Garzotto M,Qian DZ,Beer TM, Kyprianou N.Tubulin-targeting chemotherapy impairs androgen receptor activity in prostate cancer.Cancer Res 2010;70:7992-8002.
[97]Martin SK,Banuelos CA,Sadar MD,Kyprianou N.N-terminal targeting of androgen receptor variant enhances response of castration resistant prostate cancer to taxane chemotherapy.Mol Oncol 2014.http://dx.doi.org/10.1016/j.molonc.2014.10.014.pii:S1574-7891(14)00263-4,[Epub ahead of print].
[98]Gan L,Wang J,Xu H,Yang X.Resistance to docetaxelinduced apoptosis in prostate cancer cells by p38/p53/p21 signaling.Prostate 2011;71:1158-66.
[99]Domingo-Domenech J,Oliva C,Rovira A,Codony-Servat J, Bosch M,Filella X,et al.Interleukin 6,a nuclear factorkappaB target,predicts resistance to docetaxel in hormoneindependent prostate cancer and nuclear factor-kappaB inhibition by PS-1145 enhances docetaxel antitumor activity. Clin Cancer Res 2006;12:5578-86.
[100]Patterson SG,Wei S,Chen X,Sallman DA,Gilvary DL, Zhong B,et al.Novel role of Stat1 in the development of docetaxel resistance in prostate tumor cells.Oncogene 2006; 25:6113-22.
[101]Zemskova M,Sahakian E,Bashkirova S,Lilly M.The PIM1 kinase is a critical component of a survival pathway activated by docetaxel and promotes survival of docetaxel-treated prostate cancer cells.J Biol Chem 2008;283:20635-44.
[102]Codony-Servat J,Marin-Aguilera M,Visa L,Garcia-Albeniz X, Pineda E,Fernandez PL,et al.Nuclear factor-kappa B and interleukin-6 related docetaxel resistance in castrationresistant prostate cancer.Prostate 2013;73:512-21.
[103]Vidal SJ,Rodriguez-Bravo V,Quinn SA,Rodriguez-Barrueco R, Lujambio A,Williams E,et al.A targetable GATA2-IGF2 axis confers aggressiveness in lethal prostate cancer.Cancer Cell 2015;27:223-39.
[104]Liu C,Zhu Y,Lou W,Nadiminty N,Chen X,Zhou Q,et al. Functional p53 determines docetaxel sensitivity in prostate cancer cells.Prostate 2013;73:418-27.
[105]Singh RK,Lokeshwar BL.Depletion of intrinsic expression of Interleukin-8 in prostate cancer cells causes cell cycle arrest, spontaneous apoptosis and increases the efficacy of chemotherapeutic drugs.Mol Cancer 2009;8:57.
[106]Qian DZ,Rademacher BL,Pittsenbarger J,Huang CY, Myrthue A,Higano CS,et al.CCL2 is induced by chemotherapy and protects prostate cancer cells from docetaxelinduced cytotoxicity.Prostate 2010;70:433-42.
[107]Shiota M,Kashiwagi E,Yokomizo A,Takeuchi A,Dejima T, Song Y,et al.Interaction between docetaxel resistance and castration resistance in prostate cancer:implications of Twist1,YB-1,and androgen receptor.Prostate 2013;73: 1336-44.
[108]Marin-Aguilera M,Codony-Servat J,Kalko SG,Fernandez PL, Bermudo R,Buxo E,et al.Identification of docetaxel resistance genes in castration-resistant prostate cancer.Mol Cancer Ther 2012;11:329-39.
[109]Mimeault M,Johansson SL,Batra SK.Marked improvement of cytotoxic effects induced by docetaxel on highly metastatic and androgen-independent prostate cancer cells by downregulating macrophage inhibitory cytokine-1.Br J Cancer 2013;108:1079-91.
[110]Niu L,Deng J,Zhu F,Zhou N,Tian K,Yuan H,et al.Antiinflammatory effect of Marchantin M contributes to sensitization of prostate cancer cells to docetaxel.Cancer Lett 2014;348:126-34.
Received 6 July 2016;received in revised form 22 July 2016;accepted 1 August 2016
Available online 22 August 2016
*Corresponding author.Department of Urology,University of California Davis Medical Center,4645 2nd Ave,Research III,Suite 1300, Sacramento,CA 95817,USA.
E-mail address:acgao@ucdavis.edu(A.C.Gao).
Peer review under responsibility of Second Military Medical University.
http://dx.doi.org/10.1016/j.ajur.2016.08.001
2214-3882/©2016 Editorial Office of Asian Journal of Urology.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
杂志排行
Asian Journal of Urology的其它文章
- Novel immunotherapy approaches for metastatic urothelial and renal cell carcinoma
- Specific bone region localization of osteolytic versus osteoblastic lesions in a patient-derived xenograft model of bone metastatic prostate cancer
- Cultured circulating tumor cells and their derived xenografts for personalized oncology
- Intrinsic subtypes and bladder cancer metastasis
- Mismatch repair enzyme expression in primary and castrate resistant prostate cancer
- Developing immunotherapy strategies in the treatment of prostate cancer
