Non-invasive actionable biomarkers for metastatic prostate cancer
2016-04-27JunLuo
Jun Luo
Department of Urology and James Buchanan Brady Urological Institute,Johns Hopkins University, Baltimore,MD,USA
Review
Non-invasive actionable biomarkers for metastatic prostate cancer
Jun Luo
Department of Urology and James Buchanan Brady Urological Institute,Johns Hopkins University, Baltimore,MD,USA
Prostate cancer;
Androgen receptor;
AR-V7;
Biomarker;
CRPC
In the current clinical setting,many disease management options are available for men diagnosed with prostate cancer.For metastatic prostate cancer,first-line therapies almost always involve agents d esigned to inhibit androgen receptor(AR)sign aling. Castration-resistant prostate cancers(CRPCs)that arise following first-line androgen deprivation therapies(ADT)may continue to respond to additional lines of AR-targeting therapies (abiraterone and enzalutamide),chemotherapies(docetaxel and cabazitaxel),bonetargeting Radium-223 therapy,and immunotherapy sipuleucel-T.The rapidly expanding therapies for CRPC is expected to transform this lethal disease into one that can be managed for prolonged period of time.In the past 3 years,a number of promising biomarkers that may help to guide treatment decisions have been proposed and evaluated,including androgen receptor splice variant-7(AR-V7),a truncated AR lacking the ligand-binding domain(LBD)and mediate constitutively-active AR signaling.Putative treatment selection markers such as ARV7 may further improve survival benefit of existing therapies and help to accelerate development of new agents for metastatic prostate cancer.In the metastatic setting,it is important to consider compatibility between the putative biomarker with non-invasive sampling.In this review,biomarkers relevant to the setting of metastatic prostate cancer are discussed with respect to a number of key attributes critical for clinical development of non-invasive,actionable markers.It is envisioned that biomarkers for metastatic prostate cancer will continue to be discovered,developed,and refined to meet the unmet needs in both standard-of-care and clinical trial settings.
©2016 Editorial Office of Asian Journal of Urology.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/ licenses/by-nc-nd/4.0/).
1.Introduction
Prostate cancer is a commonly diagnosed disease in the Western world and metastatic prostate cancer remains one of the leading causes of cancer death in many countries[1].In geographical regions where prostate-specific antigen(PSA)screening is not routinely used,prostate cancers are less frequent,and are typically diagnosed in later stages of the disease spectrum.Upon diagnosis of localized prostate cancer,treatment decisions can be made on the basis of the risk levels[2],and patients commonly receive local therapies with curative intention, including a variety of surgical procedures and radiation treatments.The diagnosis of metastatic prostate cancer may follow local treatment failures,or occur as newly diagnosed disease without prior local therapies(Fig.1).In the USA,for example,roughly about 25%men diagnosed with metastatic prostate cancer are newly diagnosed patients,while about 75%present as recurrent disease following local therapy failures[3].For all men with metastatic castration-sensitive prostate cancer(mCSPC), the stand-of-care first-line treatment has been androgendeprivation therapies established in the 1940s by Huggins and Hodges[4].
Androgen deprivation therapies(ADT)are clinically effective in controlling the disease until the development of metastatic castration-resistant prostate cancer (mCRPC).Prior to 2004,there was no approved therapies for mCRPC.Today,there are six FDA-approved drugs for mCRPC,including androgen receptor(AR)-directed therapies(abiraterone acetate and enzalutamide)[5-8],taxane chemotherapies(docetaxel and cabazitaxel)[9,10], immunotherapy(sipuleucel-T)[11],and the bonetargeting radiopharmaceutical radium-223[12].Fig.1 illustrates an overall summary of the prostate cancer disease spectrum and the treatment landscape.Among these mCRPC therapies,abiraterone and enzalutamideare second-line AR-targeting therapies that were developed based on the concept that AR signaling remains a therapeutic target in mCRPC,due to overexpression of the AR protein and the presence of intra-prostatic male hormones sufficient in driving AR-dependent tumor growth[13].
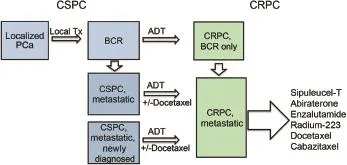
Figure 1A brief summary of the disease spectrum and treatment options for prostate cancer.ADT,androgendeprivation therapy;BCR,biochemical recurrence;CRPC, castration-resistant prostate cancer;CSPC,castration-sensitive prostate cancer;PCa,prostate cancer;Tx,treatment.
The survival benefit of each individual therapies for mCRPC has been established in definitive clinical trials [5-9,11,12].While the development of these new agents for mCRPC prolonged patient survival,a number of challenges in this new treatment landscape have emerged. First,not all men benefit from the therapies.Second,the economic burden imposed by the new agents is a concern especially when the benefit is unpredictable.Third, almost invariably,treated men will stop responding and develop resistance shortly after initiation of the therapies.In most cases,the clinical benefit for a single agent is short-lived and a decision needs to be made for the subsequent line of therapy.Prior exposure to one or more of the six therapies may lead to shortened duration of response(if any)to subsequent therapies.This possible“cross-resistance”is not well understood and the optimal treatment sequencing remains a challenge in the clinic. Lastly,while there is a clear need to develop new agents to overcome drug resistance in mCRPC,clinical trial design has become more challenging.These challenges underscore the critical importance in clinical development of biomarkers for mCRPC to facilitate treatment selection.
Molecular information gleaned from patient tissue specimens has traditionally been the prime target for developing biomarkers for prostate cancer.Indeed,the molecular taxonomy of primary prostate cancer and a comprehensive mapping of potentially actionable targets have deepened our understanding of prostate cancer and provided essential tools needed to combat the disease [14,15].In most metastatic prostate cancer cases,however,a tissue-based biomarker strategy may not be feasible for a number of reasons.Metastatic biopsies involve invasive procedures that are expensive and may miss target lesions,and the specimens may not represent the overall tumor burden due to potential tumor heterogeneity, although a recent study suggested limited intra-patient heterogeneity[16].Metastatic biopsies are currently not part of routine clinical care of patients and sequential biopsy sampling procedures will be very difficult to implement.Liquid biopsies that can be sampled non-invasively and evaluated during the course of treatment are the solution to this problem.It is well established that in patients with metastatic prostate cancer,tumor cells(CTC)can enter the circulation,and that circulating cell-free tumor DNA(CtDNA)representing the tumor genome can be captured[17].
In this review,we will highlight the most promising biomarkers for mCRPC that have been evaluated in clinical studies so far(see Table 1 for a summary of the markers to be discussed),focusing on the latest evidence supporting clinical development of CTC-and CtDNA-based detection of AR aberrations as biomarkers for metastatic prostate cancer.Because both CTC and circulating tumor DNA(CtDNA) can be obtained through a non-invasive procedure(a simple blood draw),related markers can be evaluated sequentially in a setting relevant to the treatments available to men with mCRPC.If successfully developed and fully validated analytically and clinically,the implementation of these markers has the potential to improve the therapeutic benefit of existing therapies,and facilitate clinical development of new agents to overcome resistance.
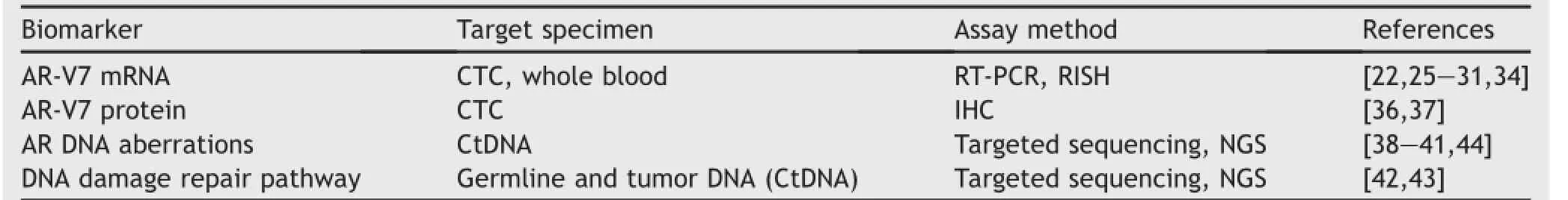
Table 1Summary of non-invasive actionable biomarkers for mCRPC evaluated in clinical studies.
2.Development of a CTC-based AR-V7 mRNA test
AR-V7 was initially discovered and reported in 2009[18]and has been characterized as one of the most important AR splice variants due to its relative abundance and frequency of detection in mCRPC,its constitutively active function, and compatibility with highly specific detection.Due to space limitations,readers are directed to a few recent reviews for evidence supporting these assertions[19-21]. Most importantly,we recently reported preliminary findings from a prospective clinical study evaluating whether detection of AR-V7 in blood samples before treatment,by a CTC-based mRNA test,was associated with lack of response to abiraterone and enzalutamide[22].In this study,we enrolled a total of 71 men with mCRPC initiating either abiraterone or enzalutamide,of which 62 were determined to be positive for CTC and suitable for CTC-based evaluation of AR-V7 transcript.Among the CTC-positive samples collected at treatment baseline(n=62),18 were positive for AR-V7 mRNA.Strikingly,none of AR-V7 patients achieved meaningful PSA response,as defined by at least 50%drop in serum PSA levels as a result of the therapy during clinical follow-up.The median PSA-progression-free survival(PSAPFS)in the AR-V7-positive cohort was 1.4 months,which was significantly shorter than that in the AR-V7-negative cohort(6.1 months).We also evaluated radiographic progression-free survival(rPFS)and observed significantly shorter rPFS in the AR-V7 positive group in comparison to the AR-V7 negative group(2.1 months vs.6.4 months). Overall survival(OS)was also worse in the AR-V7 positive men.Although our study was limited by small sample size, the data suggest that AR-V7 was a negative prognostic marker in men receiving abiraterone and enzalutamide,two AR-targeting drugs that conceptually would be ineffective in suppressing AR signaling mediated by AR-V7[23,24].
Among the subjects(n=42)that were sampled sequentially during the course of treatment,we did not observe a change in AR-V7 status in follow-up samples in subjects(n=16)tested positive at the baseline.However, six patients with a negative AR-V7 result at the baseline were AR-V7 positive in follow-up samples.The treatment outcome of patients who experienced a“conversion”of their AR-V7 status was intermediate,worse than those showing consistently negative results but better than those showing persistent positive results[22].
In a subsequent follow-up study,we evaluated whether AR-V7 status can also predict treatment outcome in men receiving taxane chemotherapies[25].We enrolled a total of 44 men initiating either docetaxel or cabazitaxel,of which 37 were determined to the CTC-positive.Among the 37 CTC-positive men,17 were initially AR-V7 positive at the baseline.We observed~41%PSA response rate in the 17 AR-V7 positive patients,and 65%PSA response rate in the 20 ARV7 negative patients.This finding suggests that taxane chemotherapies,unlike AR-targeting therapies,may remain efficacious in AR-V7-positive men.Indeed,our preliminary analysis showed that in AR-V7 positive men,taxane chemotherapy was more efficacious than the AR-targeting agents on the basis of outcome measures including PSA-PFS and rPFS,while no statistically significant difference in treatment outcome by therapies were observed in the AR-V7 negative men[25].Interestingly,a subset of AR-V7 positive patients had no dateable AR-V7 following taxane chemotherapies.Future studies are warranted to further evaluate the clinical utility of sequential AR-V7 measurements, especially given that serial measurements across many different treatments are clinically feasible[26].Collectively, studies conducted in a single-institution setting suggest that predictive utility of AR-V7 is specific to AR-targeting agents and a positive detection of AR-V7 may still be compatible with treatments with taxane chemotherapies.These pilot studies[22,25,26]have generated data supporting expanded evaluation of AR-V7 as a treatment selection marker in the standard-of-care setting.
3.Confirmatory studies involving detection of ARV7 transcript in blood
The pioneering study by Antonarakis et al.[22]also established AR-V7 as a measurable target in blood samples, and have since motivated a number of confirmatory studies with or without enrichment of CTCs.Steinestel et al.[27] evaluated 37 CTC-positive prostate cancer patients,of which 18 were AR-V7 positive,using a CTC-based test similar to that described in Antonarakis et al.[22].Among the 22 patients treated with abiraterone or enzalutamide and evaluated for PSA response,14 were AR-V7 positive and eight were AR-V7 negative.The authors reported a significant difference in PSA response rates between AR-V7 positive and AR-V7 negative men(8%vs.63%,respectively, for AR-V7 positive and AR-V7 negative patients).It is worth noting that the same group subsequently identified four AR-V7 positive patients with PSA response(defined by greater than 50%PSA drop)from a total of 21 AR-V7 positive men,suggesting a positive AR-V7 test may not always predict lack of PSA response(defined by greater than 50% PSA drop)to abiraterone or enzalutamide[28].Onstenk et al.[29]measured AR-V7 transcript in blood samples with more than 10 CTCs from men initiating cabazitaxel,and detected AR-V7 in 55%patients(16/29).Consistent withour findings[25],AR-V7 status was not associated with treatment outcome as evaluated by PSA response,PFS,or OS in this cohort of men treated with cabazitaxel.
Most recently,two studies demonstrated the feasibility of detecting AR-V7 mRNA transcript in blood without the CTC enrichment step[30,31].In the study by Liu et al.[30], a total of 46 CRPC patients were evaluated for AR-V7 by the whole blood RNA assay.AR-V7 was detected in 68%of the samples.Among patients compatible with evaluation of biomarker association with history of treatment,AR-V7 status was strongly associated with prior treatment with second-line AR-targeting therapies(abiraterone,enzalutamide,and ketoconazole).Another AR splice variant, ARV567ES[32],was also detected in a smaller proportion (~30%)of patient samples,most of which were concurrently positive for AR-V7.This study did not evaluate association between AR-V7 status and treatment outcome.In another study by Todenhofer et al.[31],a cut-off value was established from an initial cohort of 27 mCRPC and 33 noncancer controls for AR-V7 positivity determination.A validation cohort of 37 men initiating abiraterone was evaluated for association between baseline biomarker status determined by the whole-blood RNA assay with treatment outcome.AR-V7 was positive in four samples and negative in 33 samples in this validation cohort.Positive AR-V7 status was associated with worse treatment outcome,as evaluated by PSA response rate(0%vs.42%,p=0.27,in AR-V7 positive vs.AR-V7 negative men),PSA-PFS(0.7 months vs. 4.0 months,p<0.001,in AR-V7 positive vs.AR-V7 negative men),and median OS(5.5 months vs.22.1 months, p<0.001,in AR-V7 positive vs.AR-V7 negative men).
4.AR-V7 mRNA detection by RISH
In our initial clinical study[22],we also reported a tissuebased mRNA test for AR-V7 by RNA in situ hybridization (RISH).In a very limited sample set for which both metastatic biopsy and CTC samples were collected at the same time points,tissue RISH data were concordant with CTC findings from the same patient[22].Guedes et al.[33]recently evaluated the analytical validity of this tissue-based AR-V7 RISH in an expanded sample set.Notably,AR-V7 mRNA was mostly undetectable in pre-ADT specimens.A recent study by Saylor et al.[34]further conformed this finding.In the study by Saylor et al.[34],AR-V7 was positive by RISH in all 12 mCRPC specimens collected after treatment with secondline AR targeting agents,but only one out of 30 prostate cancer specimens from untreated men(i.e.,radical prostatectomy specimens)was positive.In this study,an additional set of 22 specimens collected from metastatic prostate cancer prior to first-line ADT was analyzed.Although the sample size was small,AR-V7 was more frequently detected in those with shorter duration of response to first-line ADT (6/9 positive vs.4/13 positive in short responder vs.long responders),and there was a trend for positive association between AR-V7 detection and OS and disease-specific survival in this pre-ADT cohort.Collectively,these findings suggest that RISH-based AR-V7 test may be feasible in clinical settings where tissue samples can be made available. Although metastatic biopsies are not routinely collected from men with metastatic prostate cancer,it is possible that RISH may be applied to CTC samples,upon further refinement of the RISH test following immobilization of nucleated cells(including CTC)onto standard slides.
5.AR-V7 protein detections in CTCs
AR-V7 protein detection requires an AR-V7-specific antibody.A mouse monoclonal anti-AR-V7 antibody was described and characterized in our initial studies[18,23,35] and has been made available to the research community. This antibody has not been optimized for immunohistochemistry(IHC)and used in diagnostic settings[20].Most recently,Welti et al.[36]conducted analytical and clinical evaluation of a new anti-AR-V7 rabbit monoclonal antibody using tissue specimens.In analytical studies,the antibody detected the AR-V7 protein but also a non-specific molecule in some cell lines and tissue samples.In biopsy specimens derived frommen with CSPCand CRPC(both before and after second-line AR-targeting therapies),there was a significant and progressive increase of AR-V7 IHC score in CRPC(n=37) (when compared to CSPC)and CRPC collected after treatment with abiraterone and enzalutamide(when compared to CRPCcollected before abiraterone and enzalutamide).An antibody against the AR N-terminal domain(AR-NTD)was also used in the study.In CRPC(n=37),AR-V7 protein levels and AR-V7/AR-NTD ratios was associated with shorter OS. Using the rabbit anti-AR-V7 monoclonal antibody, Scher et al.[37]evaluated AR-V7 protein expression in liquid biopsies from men with mCRPC initiating treatments with abiraterone,enzaltamide,or taxane chemotherapies.To make the CTC samples compatible with IHC, the EPIC Sciences(San Diego,California)CTC platform was used.The EPIC platform involves immobilization of blood cells(including CTC)after red blood cell lysis, ensuring all CTCs are captured.This is followed by CTC identification and IHC using the anti-AR-V7 monoclonal antibody.In this study,AR-V7 protein positive CTCs were found in 18%of blood samples collected from men with mCRPC at treatment baseline(n=191).Positive detection rates varied according to number of lines of therapy patients received at the time of sample collection, ranging from 3%(2 of 67)prior to first-line therapy,18%(9 of 50)prior to second-line therapy,and 31%(23 of 74) prior to prior to third and subsequent lines of therapies. Consistent with clinical association between AR-V7 and treatment outcome demonstrated in our previous studies [22,25],detection of AR-V7 positive CTC was associated with worse outcome in all measures,including PSA response,rPFS,time on therapy,and OS,in men treated with AR-targeting therapies(n=128).However,AR-V7 detection was not associated with most of these outcome measures in men treated with taxane chemotherapies(n=63)(following collection of baseline samples).In AR-V7 positive men,those treated with taxanes had longer median survival than those treated with AR-targeting therapies,even though taxanes are administrated in later stages of disease with a significantly higher AR-V7 positive rate(~29%,18 of 63 samples)at treatment baseline relative to the detection rate in men initiating AR-targeting agents(~13%,16 of 128 samples).In AR-V7 negative men,such difference in outcome by treatmentcategory was not observed.Therefore,both AR-V7 mRNA and protein detection in CTC has been associated with treatment-specific outcome.Collectively,these studies support further development of AR-V7 as a non-invasive treatment-specific biomarker in men underdoing standard-of-care treatments for mCRPC.
6.Detection of AR amplification and AR mutation
The AR gene is located in the X chromosome and normally has only one copy in a male genome.However,the AR gene is very commonly and specifically amplified/mutated in mCRPC,suggesting that these AR DNA aberrations are acquired alterations following ADT.AR amplification may contribute to elevated levels of ARprotein thatmay sensitize AR to lower levels of androgens in treated patients,and mutated AR may be activated by weak androgenic ligands, nonandrogenic ligands,or anti-androgens.Both AR mutations and AR amplification/gain can be detected in CtDNA samples from men with mCRPC,and utilized to evaluate clinical association and treatment induced tumor evolution [38].Recently,a larger scale study focused on these AR aberrations in patients receiving abiraterone[39].In this study, next-generation sequencing(NGS)data were collected from a total of 274 CtDNA samples from 97 patients.Because CtDNA represented a fraction of total cell-free DNA(CfDNA), a cut-off of7.5%was used to qualify the samples for analysis, leading to data points from 217 informative samples from 80 patients.Among the 217 samples with CtDNAfraction greater than 7.5%,81 were positive for AR gain,and 26 were positive for AR mutations,including L702H,T878A/S,H875Y,and W742C/L.Notably,detection of AR gain and mutations appearto be mutually exclusive,with only three cases having both AR gain and AR mutation.Overall,114 of 217 samples were positive for these AR aberrations.Among the 80 baseline samples,36 were positive for AR aberrations.These AR aberration-positive patients were less likely to have PSA response and had shorter OS and PFS compared to patients without any of the AR aberrations.
Azad et al.[40]recently reported detection of AR gain/ amplification in 45%(28/62)CtDNA samples from 62 mCRPC patients progressing on abiraterone(n=29),enzalutamide (n=19),and other systemic therapies.They also detected AR ligand-binding domain(LBD)mutations in 11 of 62 patients(18%).Among these 62 patients,39 were treated with enzalutamide as the subsequent line of therapy following the sampling time points for AR analysis,allowing investigation of association between pretreatment AR biomarker status and clinical outcomes on enzalutamide.They reported lower rates of PSA response(PSA decline greater than 30%)(20%vs.60%,p=0.013)in patients with detectable AR aberrations(n=19)relative to those without AR aberrations(n=20).In this cohort,median clinical/radiographic PFS after enzalutamide was also worse in AR marker positive patients(2.3 months)when compared with AR marker negative patients(7.0 months)(p<0.001,log-rank test).
In a follow-up study from the same group Wyatt et al.[41] examined AR DNA alterations in CtDNA samples from 65 men receiving enzalutamide.Array comparative genomic hybridization was used to detect AR copy number alterations and targeted deep sequencing was used to detect AR mutations.Samples were collected at treatment baseline (n=65),at 12 weeks post baseline without disease progression(n=30),and at the time of progression(n=30). AR copy number alterations and/or AR mutations were detected in 48%(31 of 65)and 60%(18 of 30)of baseline and progression samples,while such events were rarely detected in samples collected at 12 weeks,possibly due to lack of detectable CtDNA in the majority of responding patients. Consistent with findings from Romanel et al.[39],detection of AR amplification and mutation was mutually exclusive in this cohort,suggesting that AR mutation and AR amplification may be independent drivers of CRPC progression.In this study,AR alterations detected in CtDNA was associated with worse treatment outcome,and there was an increase in detection frequency in progression samples.
Collectively,these findings suggest that detection of AR DNA aberrations(AR amplification/gain/mutation)can be achieved non-invasively and is associated with worse treatment outcome.However,more studies are warranted to determine whether the difference in outcome between groups with positive and negative CtDNA AR aberration status is treatment-specific,i.e.,whether the biomarker status is also associated with outcome in the setting of taxane chemotherapies.
7.Detection of DNA damage repair pathway genes
Recently a number of actionable mutations are characterized in details in metastatic biopsies from men with mCRPC [15].One of the most exciting findings from this study is the detection of mutations involving the DNA damage response pathway in both germline and tumor samples at frequencies that were substantially higher than anticipated.It is now clear that patients harboring these mutations have a favorable response pattern if treated with poly(ADP)-ribose polymerase(PARP)inhibitors or other agents targeting the DNA damage response pathway under the concept of“synthetic lethality”[42].Although initial reports relied on tissues for mutation detection[15,42],such mutations may be detected by a non-invasive method in mCRPC patients using CtDNA as the biosource.Importantly,in a significant proportion of tumor samples with such mutations,a germline mutation may be present and can be readily evaluated using germline DNA even before full manifestation of the disease(e.g.,at the time of initial diagnosis)[43].Therefore,it is possible that genetic testing may provide an opportunity to eradicate the lethal tumor clones early on (e.g.,at the time of diagnosis)with agents targeting the DNA damage response/repair pathway in newly diagnosed patients harboring these actionable mutations.
8.Future directions for biomarker research in metastatic prostate cancer
In spite of the advances that have been made,biomarker research and clinical translation is still a nascent field.Going forward,a few frontiers in biomarker studies of metastatic prostate cancer are identified in order to realize the potential of liquid biopsies and technological advances for the development of non-invasive treatment-specific biomarkers for metastatic prostate cancer.First,comprehensiveexamination of multiple biosources and analytes in largesize,well-defined patient cohorts may further define the treatment selections markers.Platforms that allow integrated studies such as those demonstrated by Sperger et al. [44]may help to achieve this goal.Second,in the setting of potent AR-targeting therapies,it is critical to develop noninvasive neuroendocrine/small cell prostate cancer markers.Neuroendocrine/small-cell prostate carcinomas are resistant to standard AR agents.Recent studies provided a molecular signature that may allow development of biomarkers for this increasingly important subgroup of tumors [45].In particular,non-invasive markers may be developed by targeting RNA(expression level,variant detection,noncoding RNA,etc.),or DNA(mutation,methylation),and may utilize a variety of biosources(CtDNA,CTC,exosomem, platelets).Last,since many agents approved for mCRPC patients are undergoing evaluation in patients with mCSPC, it is reasonable to expect that the treatment landscape for mCSPC may undergo changes similar to that for mCRPC in the years to come.Indeed,docetaxel added to first-line ADT has recently become the standard-of-care for mCSPC given the survival benefit especially in high-volume patients[46]. Collectively,the changing clinical landscape for metastatic prostate cancer and the limited utility of existing prognostic markers highlight an urgent need to develop biomarkers that can inform treatment selection in all men with metastatic prostate cancer.
9.Conclusion
In this review,recent evidence supporting the clinical utility of biomarkers compatible with non-invasive sampling in the setting of mCRPC was presented.Biomarker candidates included AR-V7,AR amplification/gain/mutation,and mutated DNA damage response genes.A few frontiers of biomarker research were identified.Well-designed biomarkers studies will require substantial investment in marker identification and qualification,assay platform,and access to clinical resources.Although the AR-V7 test represent one example of a rationally qualified marker beginning to benefit men with mCRPC,substantial efforts in discovery,validation, and implementation are still needed in order to further optimize treatment selection.Discovery will be facilitated by technological advances in NGS and the ability to analyze small amount of biomolecules obtained from liquid biopsies. Validation studies may include both analytical validation as well as clinical utility studies in prospective trials,and may require the development of novel testing platforms.While commercial interest and regulatory approval may facilitate clinical implementation of a test in specific intended use settings,laboratory-developed tests(LDTs)may also be implemented in clinical testing labs prior to FDA approval.It is fully envisioned that non-invasive actionable biomarkers will become indispensable assets in maximizing the benefit of existing therapies as well as clinical development of new agents for metastatic prostate cancer.
Conflicts of interest
The author has served as a paid consultant/advisor for Sun Pharma,Astellas,Gilead,and Sanof i;has received research funding to his institution from Orion,Mirati,Astellas, Sanof i,and Gilead;and has received royalties from technologies licensed to A&G Pharmaceutical and Tokai Pharmaceuticals.
Acknowledgments
Due to limited scope of the review,many published studies that formed the basis for viewpoints expressed in this review were not cited.The author wishes to thank all investigators who contributed to the knowledge and insight in the topic covered in this review.The author’s laboratory is currently funded by a Prostate Cancer Foundation grant,an NIH grant R01 CA185297,and US Department of Defense Prostate Cancer Research Program grants W81XWH-13-2-0093 and W81XWH-15-2-0050.
[1]Siegel RL,Miller KD,Jemal A.Cancer statistics.CA Cancer J Clin 2016;2016:7-30.
[2]Heidenreich A,Bastian PJ,Bellmunt J,Bolla M,Joniau S,van der Kwast T,et al.EAU guidelines on prostate cancer.part 1: screening,diagnosis,and local treatment with curative intent-update 2013.Eur Urol 2014;65:124-37.
[3]Scher HI,Solo K,Valant J,Todd MB,Mehra M.Prevalence of prostate cancer clinical states and mortality in the United States:estimates using a dynamic progression model.PLoS One 2015;10:e0139440.
[4]Huggins C,Hodges CV.Studies on prostatic cancer.I.The effect of castration,of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941.J Urol 2002;167(2 Pt 2):948-52.
[5]Beer TM,Armstrong AJ,Rathkopf DE,Loriot Y,Sternberg CN, Higano CS,et al.Enzalutamide in metastatic prostate cancer before chemotherapy.N Engl J Med 2014;371:424-33.
[6]de Bono JS,Logothetis CJ,Molina A,Fizazi K,North S,Chu L, et al.Abiraterone and increased survival in metastatic prostate cancer.N Engl J Med 2011;364:1995-2005.
[7]Ryan CJ,Smith MR,de Bono JS,Molina A,Logothetis CJ,de Souza P,et al.Abiraterone in metastatic prostate cancer without previous chemotherapy.N Engl J Med 2013;368: 138-48.
[8]Scher HI,Fizazi K,Saad F,Taplin ME,Sternberg CN,Miller K, et al.Increased survival with enzalutamide in prostate cancer after chemotherapy.N Engl J Med 2012;367:1187-97.
[9]de Bono JS,Oudard S,Ozguroglu M,Hansen S,Machiels JP, Kocak I,et al.Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment:a randomised open-label trial. Lancet 2010;376:1147-54.
[10]Tannock IF,de Wit R,Berry WR,Horti J,Pluzanska A,Chi KN, et al.Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer.N Engl J Med 2004;351: 1502-12.
[11]Kantoff PW,Higano CS,Shore ND,Berger ER,Small EJ, Penson DF,et al.Sipuleucel-T immunotherapy for castrationresistant prostate cancer.N Engl J Med 2010;363:411-22.
[12]Parker C,Nilsson S,Heinrich D,Helle SI,O’Sullivan JM, Fossa SD,et al.Alpha emitter radium-223 and survival in metastatic prostate cancer.N Engl J Med 2013;369:213-23.
[13]Nakazawa M,Antonarakis ES,Luo J.Androgen receptor splice variants in the era of enzalutamide and abiraterone.Horm Cancer 2014;5:265-73.
[14]Cancer Genome Atlas Research Network.Electronic address scmo,cancer genome atlas research N.The molecular taxonomy of primary prostate cancer.Cell 2015;163:1011-25.
[15]Robinson D,Van Allen EM,Wu YM,Schultz N,Lonigro RJ, Mosquera JM,et al.Integrative clinical genomics of advanced prostate cancer.Cell 2015;161:1215-28.
[16]Kumar A,Coleman I,Morrissey C,Zhang X,True LD,Gulati R, et al.Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer.Nat Med 2016;22:369-78.
[17]Hegemann M,Stenzl A,Bedke J,Chi KN,Black PC, Todenhofer T.Liquid biopsy:ready to guide therapy in advanced prostate cancer?BJU Int 2016 Jul 19.http: //dx.doi.org/10.1111/bju.13586[Epub ahead of print].
[18]Hu R,Dunn TA,Wei S,Isharwal S,Veltri RW,Humphreys E, et al.Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer.Cancer Res 2009;69:16-22.
[19]Antonarakis ES,Armstrong AJ,Dehm SM,Luo J.Androgen receptor variant-driven prostate cancer:clinical implications and therapeutic targeting.Prostate Cancer Prostatic Dis 2016; 19:231-41.
[20]Luo J.Development of AR-V7 as a putative treatment selection marker for metastatic castration-resistant prostate cancer.Asian J Androl 2016;18:580-5.
[21]Lu C,Luo J.Decoding the androgen receptor splice variants. Transl Androl Urol 2013;2:178-86.
[22]Antonarakis ES,Lu C,Wang H,Luber B,Nakazawa M, Roeser JC,et al.AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer.N Engl J Med 2014;371: 1028-38.
[23]Hu R,Lu C,Mostaghel EA,Yegnasubramanian S,Gurel M, Tannahill C,et al.Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer.Cancer Res 2012;72:3457-62.
[24]Li Y,Chan SC,Brand LJ,Hwang TH,Silverstein KA,Dehm SM. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res 2013;73:483-9.
[25]Antonarakis ES,Lu C,Luber B,Wang H,Chen Y,Nakazawa M, et al.Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castrationresistant prostate cancer.JAMA Oncol 2015;1:582-91.
[26]Nakazawa M,Lu C,Chen Y,Paller CJ,Carducci MA, Eisenberger MA,et al.Serial blood-based analysis of AR-V7 in men with advanced prostate cancer.Ann Oncol 2015;26: 1859-65.
[27]Steinestel J,Luedeke M,Arndt A,Schnoeller TJ,Lennerz JK, Wurm C,et al.Detecting predictive androgen receptor modifications in circulating prostate cancer cells.Oncotarget 2015 Apr 23[Epub ahead of print].
[28]Bernemann C,Schnoeller TJ,Luedeke M,Steinestel K, Boegemann M,Schrader AJ,et al.Expression of AR-V7 in circulating tumour cells does not preclude response to next generation androgen deprivation therapy in patients with castration resistant prostate cancer.Eur Urol 2016 Jul 25.http://dx.doi.org/10.1016/j.eururo.2016.07.021.pii: S0302-2838(16)30426-2.[Epub ahead of print].
[29]Onstenk W,Sieuwerts AM,Kraan J,Van M,Nieuweboer AJ, Mathijssen RH,et al.Efficacy of cabazitaxel in castrationresistant prostate cancer is independent of the presence of AR-V7 in circulating tumor cells.Eur Urol 2015;68:939-45.
[30]Liu X,Ledet E,Li D,Dotiwala A,Steinberger A,Feibus A,et al.A whole blood assay for AR-V7 and ARv567es in patients with prostate cancer.J Urol 2016 Jul 20.http: //dx.doi.org/10.1016/j.juro.2016.06.095.pii:S0022-5347(16) 30914-4.[Epub ahead of print].
[31]Todenhofer T,Azad A,Stewart C,Gao J,Eigl BJ,Gleave ME, et al.AR-V7 transcripts in whole blood RNA of patients with metastatic castration resistant prostate cancer correlate with response to Abiraterone acetate.J Urol 2016 Jul 16.http: //dx.doi.org/10.1016/j.juro.2016.06.094.pii:S0022-5347(16) 30860-6.[Epub ahead of print].
[32]Sun S,Sprenger CC,Vessella RL,Haugk K,Soriano K, Mostaghel EA,et al.Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant.J Clin Invest 2010;120:2715-30.
[33]Guedes LB,Morais CL,Almutairi F,Haffner MC,Zheng Q, Isaacs JT,et al.Analytic validation of RNA In Situ Hybridization(RISH)for AR and AR-V7 expression in human prostate cancer.Clin Cancer Res 2016;22:4651-63.
[34]Saylor PJ,Lee RJ,Arora KS,Deshpande V,Hu R,Olivier K,et al. Branched chain RNA in situ hybridization for androgen receptor splice variant AR-V7 as a prognostic biomarker for metastatic castration-sensitive prostate cancer.Clin Cancer Res 2016 Jul 20.pii:clincanres.0237.2016.[Epub ahead of print].
[35]Hu R,Isaacs WB,Luo J.A snapshot of the expression signature of androgen receptor splicing variants and their distinctive transcriptional activities.Prostate 2011;71:1656-67.
[36]WeltiJ,Rodrigues DN,Sharp A,Sun S,Lorente D,Riisnaes R,etal. Analytical validation and clinical qualification of a new immunohistochemical assay for androgen receptor splice Variant-7 protein expression in metastatic castration-resistant prostate cancer.Eur Urol 2016 Apr 22.http://dx.doi.org/10.1016/j. eururo.2016.03.049.pii:S0302-2838(16)30027-6.[Epub ahead of print].
[37]Scher HI,Lu D,Schreiber NA,Louw J,Graf RP,Vargas HA, et al.Association of AR-V7 on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate cancer.JAMA Oncol 2016 Jun 4. http://dx.doi.org/10.1001/jamaoncol.2016.1828[Epub ahead of print].
[38]Carreira S,Romanel A,Goodall J,Grist E,Ferraldeschi R, Miranda S,et al.Tumor clone dynamics in lethal prostate cancer.Sci Transl Med 2014;6:254ra125.
[39]Romanel A,Gasi Tandefelt D,Conteduca V,Jayaram A, Casiraghi N,Wetterskog D,et al.Plasma AR and abirateroneresistant prostate cancer.Sci Transl Med 2015;7:312re10.
[40]Azad AA,Volik SV,Wyatt AW,Haegert A,Le Bihan S,Bell RH, et al.Androgen receptor gene aberrations in circulating cellfree DNA:biomarkers of therapeutic resistance in castrationresistant prostate cancer.Clin Cancer Res 2015;21:2315-24.
[41]Wyatt AW,Azad AA,Volik SV,Annala M,Beja K,McConeghy B, et al.Genomic alterations in cell-free DNA and enzalutamide resistance in castration-resistant prostate cancer.JAMA Oncol 2016 May 5.http://dx.doi.org/10.1001/jamaoncol.2016.0494 [Epub ahead of print].
[42]Mateo J,Carreira S,Sandhu S,Miranda S,Mossop H,Perez-Lopez R,et al.DNA-repair defects and olaparib in metastatic prostate cancer.N Engl J Med 2015;373:1697-708.
[43]Pritchard CC,Mateo J,Walsh MF,De Sarkar N,Abida W, Beltran H,et al.Inherited DNA-repair gene mutations in men with metastatic prostate cancer.N EnglJMed 2016;375:443-53.
[44]Sperger JM,Strotman LN,Welsh A,Casavant BP,Chalmers Z, Horn S,et al.Integrated analysis of multiple biomarkers from circulating tumor cells enabled by exclusion-based analyte isolation.Clin Cancer Res 2016 Jul 11.pii:clincanres.1021.2016.[Epub ahead of print].
[45]Beltran H,Prandi D,Mosquera JM,Benelli M,Puca L,Cyrta J, et al.Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer.Nat Med 2016;22:298-305.
[46]Sweeney CJ,Chen YH,Carducci M,Liu G,Jarrard DF, Eisenberger M,et al.Chemohormonal therapy in metastatic hormone-sensitive prostate cancer.N Engl J Med 2015;373: 737-46.
Received 1 September 2016;accepted 1 September 2016
Available online 13 September 2016
E-mail address:jluo2@jhmi.edu.
Peer review under responsibility of Second Military Medical University.
http://dx.doi.org/10.1016/j.ajur.2016.09.003
2214-3882/©2016 Editorial Office of Asian Journal of Urology.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
杂志排行
Asian Journal of Urology的其它文章
- Novel immunotherapy approaches for metastatic urothelial and renal cell carcinoma
- Specific bone region localization of osteolytic versus osteoblastic lesions in a patient-derived xenograft model of bone metastatic prostate cancer
- Cultured circulating tumor cells and their derived xenografts for personalized oncology
- Intrinsic subtypes and bladder cancer metastasis
- Mismatch repair enzyme expression in primary and castrate resistant prostate cancer
- Developing immunotherapy strategies in the treatment of prostate cancer
