健康婴儿的闪光视觉诱发电位检查
2016-04-25VladislavVoitenkovAndreyKlimkinNataliaSkripchenko
Vladislav Voitenkov, Andrey Klimkin, Natalia Skripchenko
(作者单位:俄罗斯,圣彼得堡 197022,儿童感染科学研究院,神经生理科)
健康婴儿的闪光视觉诱发电位检查
Vladislav Voitenkov, Andrey Klimkin, Natalia Skripchenko
(作者单位:俄罗斯,圣彼得堡 197022,儿童感染科学研究院,神经生理科)
摘要
关键词:视觉诱发电位;正常数值;婴儿
Abstract
•AIM:To evaluate visual evoked potentials(VEPs) in healthy infants.
•METHODS:Thirty-four neurologically and ophthalmologically healthy infants aged 2-10mo were enrolled. Flash VEPs were implemented, with main peak P3 latency, amplitude and differences of latency and amplitude between sides being investigated.
•RESULTS:In all cases main peak was registered. Its average latency was 138-140 ms and main amplitude 7-9 μV. Latency was variable.
•CONCLUSION:We propose that latency or amplitude deviation in this population may not happen due to pathological condition, but merely due to ongoing myelination of visual pathways and cortex modeling. Caution needed in attributing the main cortical peak as P3 or some other waves numbers. Cortical nature of the main flash visual evoked potentials peak in infants aged 1-3mo is doubtful.
KEYWORDS:•visual evoked potentials;normative data;infants
Citation:Voitenkov V, Klimkin A, Skripchenko N. Flash visual evoked potentials in healthy infants.GuojiYankeZazhi(IntEyeSci) 2016;16(4):614-616
INTRODUCTION
Investigation of visual system in children, especially in infants, may be challenging and often needs implementation of neurophysiologic methods, such as visual evoked potentials(VEPs)[1-2]. Maturation of the visual pathways takes months after the birth, with its final stages being at 3-5y. Newborns prefer visual patterns resembling a human face;6wk old infants already start to distinguish internal configuration of the mother’s face, detect the living object’s movements and prefer human movements[3-4]. It is argued that visual processing in newborns aged less than 2mo goes on subcortical level, as visual cortex is quite immature yet[1].
In infants, it’s more reasonable to use flash visual evoked potentials(fVEPs), as in this case there is no need in fixed stare on checkerboard pattern, routinely used in pattern VEPs[5]. Wave continuity P2-N2-P3 is supposed to be the main fVEPs complex, with its average latency in adults being 124-130 ms, and amplitude=7-8 μV[5]. In preterm infants fVEPs waves, which may be analyzed properly, start to appear at 24wk postmenstrual age[6].
In first year of life fVEPs parameters in healthy infants, acquired in different laboratories, vary significantly. Some authors reports mean P2 latency 100 ms and N3 150 ms[7];main cortical peak latency 100-170 ms and amplitude 2-15 μV[8];average P100 latency(pattern VEPs) 179-180 ms[9];mean P2 latency 170±16 ms and amplitude 6.26±5.46 μV[10], P3 latency 145 ms[11];P2 latency 155±30 ms and amplitude 5.9±4.3 ms[12], mean N2 latency 185.98±31 ms and its amplitude 1.78±1.5 μV[13]. As it may be seen from the data summarized in Table 1, difference in values is vast;have to be noted, that different authors attributed same waves on the fVEPs as different P and N peaks, thus contributing to differences of the data presented.
As may be seen from the data presented, main issue is not only the latency of the cortical wave;amplitude also vary in the range 1.78-15 μV, according to different authors. Thus, despite seemingly well-established normative data for fVEPs, in infants this data needs further exploration.
SUBJECTS AND METHODS
Our study was conducted among healthy children(n=34, 68eyes). Age varied from 32 to 306d, average 130.5±86.11;21 males, 13 females.
Table 1Latencies and amplitudes of the main cortical wave in healthy infants, according to different authors
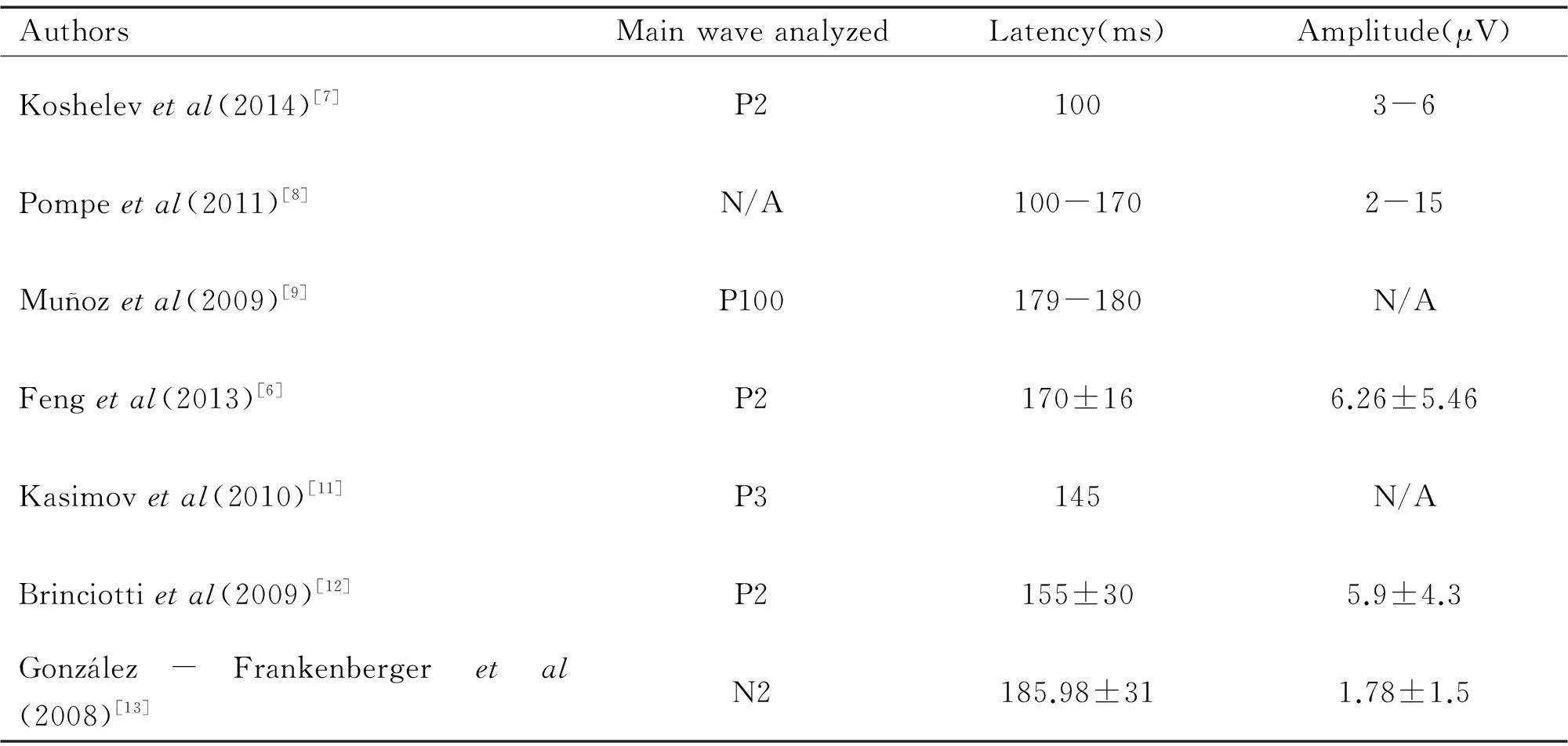
AuthorsMainwaveanalyzedLatency(ms)Amplitude(μV)Koshelevetal(2014)[7]P21003-6Pompeetal(2011)[8]N/A100-1702-15Muñozetal(2009)[9]P100179-180N/AFengetal(2013)[6]P2170±166.26±5.46Kasimovetal(2010)[11]P3145N/ABrinciottietal(2009)[12]P2155±305.9±4.3Gonzlez-Frankenbergeretal(2008)[13]N2185.98±311.78±1.5
Study population was neurologic and ophthalmologic healthy infants of either sex. Healthy infant’s criteria were children with normal general health and with normal pupillary light reflex, normal pupillary diameter, no visual complaints of the parents and medical staff, no refractive errors, no neurologic disturbances(all underwent thorough clinical examination by neurologist). Additional neurosonography and electroencephalogram(EEG) recordings were performed on every infant in the group;all children with EEG and sonographic anomalies were excluded.
Exclusion criteria were history of head injury, stroke, medical illness(general and ophthalmic) and any medications including mydriatic and miotic drugs.
All patients underwent flash visual evoked potentials recording on Neiro-MEP EMG/EP system(Neurosoft Company, Ivanovo, Russia).
The scalp electrodes were placed relative to known landmarks, in proportion to the size of the head, according to the international 10-20 system:active electrode at Oz, reference electrode at Cz and ground electrode at Fpz. All electrode sites were cleaned with abrasive gel to reduce the skin resistance. Recording was done placing the electrodes on the subject’s scalp according to 10/20 international system. Stimulation system was light-emitting diode matrix, red color of the flash(640 nm), monocular stimulation, and duration of stimulus 5 ms, frequency 1 Hz, analysis epoch 400 ms. Latency and amplitude of the main cortical wave complex(N2/P3), its asymmetry between the eyes were evaluated.
All patients’ parents were fully informed of the purpose of the study;it was approved by the Local Ethical Committee;written informed consent was obtained from all patients’ parents.All evaluations were performed in neurophysiology laboratory of the Scientific Research Institute of Children’s Infections, St.Petersburg, Russia.
RESULTS
N2/P3 complex was registered in all 23 children. Amplitudes and latencies of this complex is presented in the Table 2.
On the individual level asymmetry of the latencies more than 10 ms was registered in 11 cases(30%). Significant difference of the amplitudes was not registered in any case. Typical fVEPs of the healthy infant, male aged 72d, is presented in Figure 1.
Table 2Amplitudes and latencies of the N2/P3 complex in the group

ParameterODOSP3latency(ms)138.9±15.6140.8±17.2P3amplitude(μV)8.48±5.467.91±5.06Latenciesasymmetry(ms)6.75±4.19Amplitudesasymmetry(μV)1.9±1.4
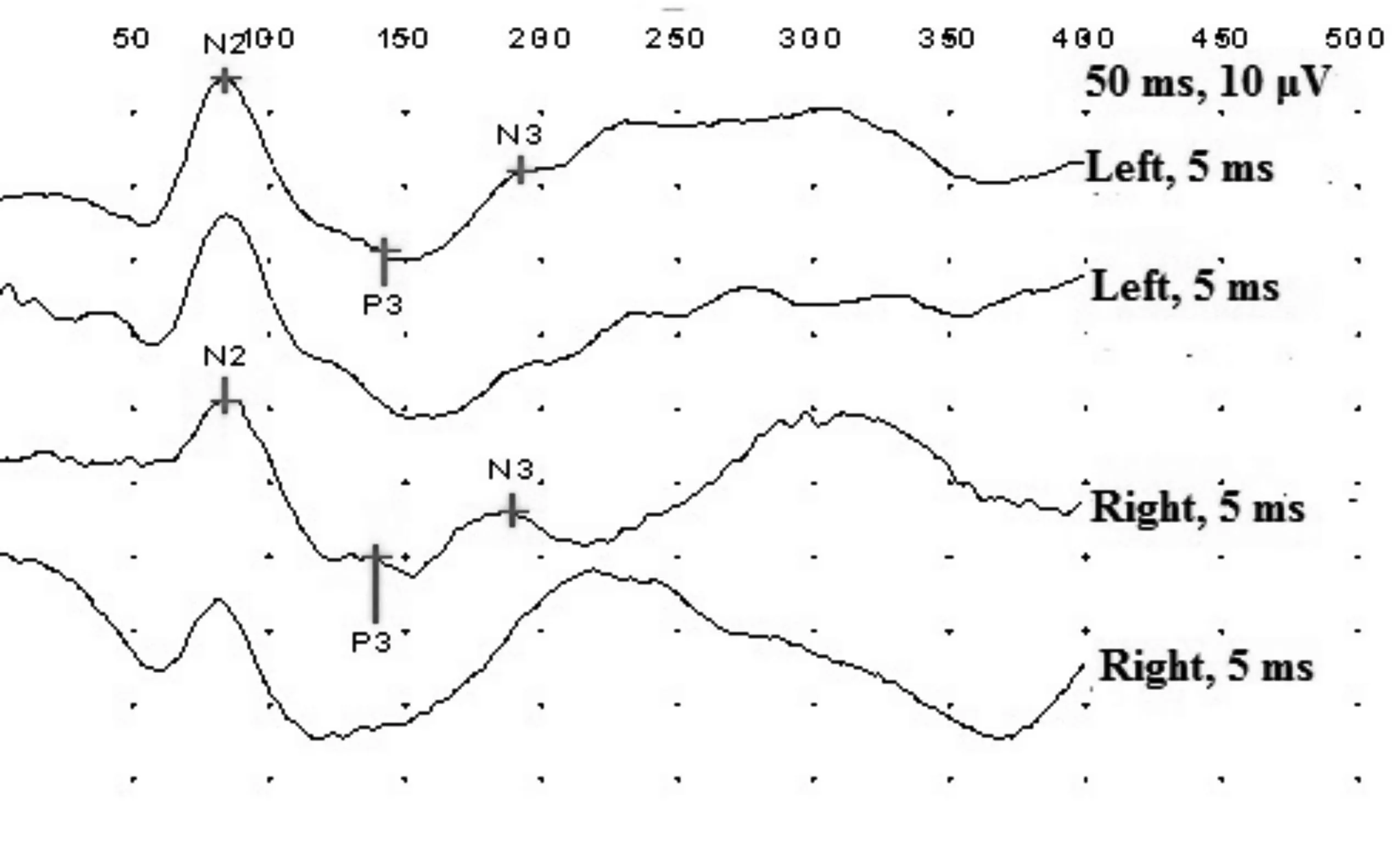
Figure 1VEPs in healthy male patient, age 72d.
DISCUSSION
Latency asymmetry which was seen in our population may happen due to incomplete myelination of the visual pathways[9,14]. Apart from myelination, important role for the proper functioning of the visual system plays development of temporal lobe and basal ganglia;in infants this process varies between healthy individuals[1,15]. Absence of the significant difference of the amplitudes between the eyes may be due to small size of the head of the infant or possibly unspecific conduction of the excitation between the brain parts. Also it has to be stressed that there are no solid proof of the cortical genesis of the so-called cortical waves on the fVEPs in the infants of the first 3mo of life. It was demonstrated that in 100% of the infants with periventricular leukomalacia main waves of fVEPs were registered, then in children with subcortical lesions no proper peaks were seen. Also fVEPs were insufficient in detecting parieto-occipital lobe involvement in infants after significant hypoglycaemia as compared to healthy controls, which may point to the subcortical levels of generation of the main peaks of these evoked potentials in the first months of life[16].
Thus we can assume that in healthy children of the first months of life(7 of our infants were younger than 90d) N2-P3 complex may appear due to subcortical centers functioning. In lots of animals which have no cortex at all visual system still functions properly;since 1930s one of the main questions in this field was discussion about the possibility of “midbrain vision” in human[17]. It was argued that infants with yet undeveloped higher centers of visual cortex may see due to activation of phylogenetically ancient pathway that has specific functional properties and that interacts with cortical processes[18]. Also this pathway may play significant role in vision in adults[19].
Variability of latencies in our population of healthy infants demonstrates that normative data in infants have to be treated with caution. Deviation of latency(or amplitude) from the normative parameters not always have to mean some pathologic process on the way;we assume that more significant is the absence of the waves(unilateral or bilateral). One recent work argues that delay of myelination may be the main cause of latency differences in infants[20].
Our attribution of the main waves complex which we registered as N2/P3, as was demonstrated, may be a matter of debate;anyway, it is certainly the wave complex which reflects the main processing of the visual information in the infant’s brain. Its absence may lead to the conclusion that visual system formation is disrupted in some way. In all cases the dynamic investigation is recommended.Pattern visual evoked potentials seems to be more reliable and easy to interpret than fVEPs, so perhaps its implementation in pediatrics as early as possible may be recommended[21].
Thus, in all healthy infants aged less than 1y main cortical wave complex may be registered. Its average latency in our population was 138-140 ms and average amplitude 7-9 μV. Latency or/and amplitude deviation from the established normative data may not be due to pathologic conditions, but may be seen in healthy infants as well. In evaluation of fVEPs in infants caution is needed:real pathologic finding may be not the latency lengthening or amplitudes drop, but presence or absence of the main cortical complex(may be attributed as N2/P3).
REFERENCES
1 Mercuri E, Baranello G, Romeo DM, Cesarini L, Ricci D. The development of vision.EarlyHumDev2007;83(12):795-800
2 Ortibus EL, De Cock PP, Lagae LG. Visual perception in preterm children:what are we currently measuring?PediatrNeurol2011;45(1):1-10
3Bushneil IWR, Sai F, Mullin JT. Neonatal recognition of the mother’s face.BrJDevPsychol1989;7(1):3-15
4Yoon JM, Johnson SC. Biological motion displays elicit social behavior in 12-month-olds.ChildDev2009;80(4):1069-1075
5 Holder GE, Celesia GG, Miyake Y, Tobimatsu S, Weleber RG;International Federation of Clinical Neurophysiology. International Federation of Clinical Neurophysiology:recommendations for visual system testing.ClinNeurophysiol2010;121(9):1393-1409
6 Feng JJ, Wang TX, Yang CH, Wang WP, Xu X. Flash visual evoked potentials at 2-year-old infants with different birth weights.WorldJPediatr2010;6(2):163-168
7 Koshelev DI, Galautdinov MF, Vachmyanina AA. The practice of the application of the flash visual evoked potentials for the visual system’s evaluation.VestnikOGU2014;12(173):181-187
8 Pompe MT, Kranjc BS, Brecelj J. The study of chromatic and achromatic VEP in the first year of life.ActaOphthalmol2009;87(s244):0
10 Feng JJ, Wang WP, Guo SJ, Liu ZW, Xu X. Flash visual evoked potentials in preterm infants.Ophthalmology2013;120(3):489-494
11 Kasimov EM, Salmanova SZ, Guseinova SK, Alieva NZ. Some aspects of transformation of visual development delay syndrome into syndrome of visual impairment in children with perinatal encephalopathy.RussianMedJ2010;11(2):47-49
12 Brinciotti M, Matricardi M, Colatrella A, Torcia F, Fallucca F, Napoli A. Visual evoked potentials in infants of diabetic mothers:relations to clinical and metabolic status during pregnancy and delivery.ClinNeurophysiol2009;120(3):563-568
14 Mikó-Baráth E, Markó K, Budai A, Török B, Kovacs I, Jandó G. Maturation of cyclopean visual evoked potential phase in preterm and full-term infants.InvestOphthalmolVisSci2014;55(4):2574-2583
15 De Regnier RA. Neurophysiologic evaluation of brain function in extremely premature newborn infants.SeminPerinatol2008;32(1):2-10
16 Hu L, Gu Q, Zhu Z, Yang C, Chen C, Cao Y, Zhou W. Flash visual evoked potentials are not specific enough to identify parieto-occipital lobe involvement in term neonates after significant hypoglycaemia.ActaPaediatr2014;103(8):e329-333
17 Bing R, Bruckner R. GehirnundAuge. Grundriβ der Ophthalmo-Neurologie, 3.Aufl. Basel:Schwabe 1954
18 Tamietto M, de Gelder B. Neural bases of the non-conscious perception of emotional signals.NatRevNeurosci2010;11(10):697-709
19 Visconti di Oleggio Castello M, Gobbini MI. Familiar Face Detection in 180 ms.PLoSOne2015;10(8):e0136548
20 Jethani J, Jethani M. Flash visual evoked potentials in patients with periventricular leucomalacia in children less than1y of age.IndianJOphthalmol2013;61(11):634-635
21Voitenkov V, Skripchenko N, Klimkin A. Visual pathways involvement in clinically isolated syndrome in children.IntJOphthalmol2015;8(2):382-384
DOI:10.3980/j.issn.1672-5123.2016.4.06
通讯作者:Vladislav Voitenkov. vlad203@inbox.ru
目的:评估健康婴儿的视觉诱发电位(VEP)。
方法:选取34例神经系统及眼部均健康的、年龄为2~10月龄的婴儿,运用闪光VEP检查并比较双眼P3波潜伏期与振幅。
结果:所有婴儿的P3波数据均被记录。其平均潜伏期为138~140ms,振幅为7~9μV。潜伏期不稳定。
结论:病理状态下,婴儿的潜伏期或振幅可能不会出现变异。而由于视路和皮层的髓鞘发育会使其出现变异,应注意类似于P3波的皮层波或其他波出现。因此,使用闪光VEP检测年龄为1~3月龄的婴儿是不准确的。
引用:Voitenkov V, Klimkin A, Skripchenko N.健康婴儿的闪光视觉诱发电位检查.国际眼科杂志2016;16(4):614-616
