冠心病患者替格瑞洛相关呼吸困难并发症发生的临床分析
2016-04-21王绪云席少枝陈韵岱
王绪云,席少枝,刘 佳,荆 晶,陈韵岱,尹 彤
冠心病患者替格瑞洛相关呼吸困难并发症发生的临床分析
王绪云,席少枝,刘 佳,荆 晶,陈韵岱,尹 彤*
(解放军总医院心内科,北京 100853)
在冠心病(CAD)患者中分析与替格瑞洛呼吸困难有关的影响因素,及该并发症对患者的替格瑞洛依从性和预后的影响。募集2014年1月至2015年2月于解放军总医院心内科接受替格瑞洛治疗的CAD患者。统计患者院内及出院6个月替格瑞洛相关呼吸困难的发生情况,分析该并发症的影响因素。通过6个月随访观察,在持续服用替格瑞洛的患者中,分析替格瑞洛相关呼吸困难的发生对联合缺血和出血终点事件的影响。共募集647例CAD患者。6个月内,发生替格瑞洛相关呼吸困难85例(13.14%),其中43例发生在1周内,35例发生在1个月内,7例发生在1个月后。因呼吸困难停用替格瑞洛者15例。多因素相关分析发现,患者合用替罗非班发生替格瑞洛相关呼吸困难的风险增加(OR:2.45,95%CI:1.41~4.27,=0.001),而合用他汀类药物(OR:0.20,95%CI:0.04~0.92,=0.04)、或质子泵抑制剂(OR:0.56,95% CI:0.32~0.97,=0.04)发生替格瑞洛相关呼吸困难的风险降低。对6个月随访期内持续服用替格瑞洛的患者(353例)分析发现,联合缺血和出血终点事件在替格瑞洛相关呼吸困难者与未发生者之间无显著性差异。CAD患者中替格瑞洛相关呼吸困难多见于服药1个月内,合并用药可能是影响替格瑞洛相关呼吸困难发生的主要相关因素。替格瑞洛相关呼吸困难的发生会影响患者的依从性,但可能不影响患者的预后。
替格瑞洛;替格瑞洛相关呼吸困难;影响因素;依从性;预后
双联抗血小板治疗已成为急性冠脉综合征(acute coronary syndrome,ACS)和经皮冠状动脉介入治疗(percutaneous coronary intervention,PCI)患者预防缺血事件形成的基石[1,2]。替格瑞洛(ticagrelor)是一种新型的可逆性P2Y12抑制剂,与氯吡格雷(clopidogrel)相比,可以显著改善ACS患者的预后[3]。因此,在最新国内及国外的美国心脏病学会(American College of Cardiology,ACC)/欧洲心脏病学会(European Society of Cardiology,ESC)治疗指南中,替格瑞洛均被高级别推荐用于冠心病(coronary artery disease,CAD)患者抗血小板治疗[1,2,4]。
大量的研究发现,替格瑞洛可通过抑制红细胞对腺苷的摄取增加血浆腺苷浓度,从而发挥腺苷介导的冠脉血流增加、抑制血小板聚集的作用,但同时伴随替格瑞洛相关呼吸困难的发生[5,6]。PALTO研究亚组分析提示,1个月随访期内,替格瑞洛相关呼吸困难的发生率为22.56%,因此原因停药率为9.15%[7],由此可见,替格瑞洛相关呼吸困难已成为临床不可忽视的问题。替格瑞洛在国内于2012年获批应用于临床,由于应用时间尚短,临床普及不够广泛,因此,与替格瑞洛相关呼吸困难发生、影响因素和预后有关的数据甚少[8,9]。鉴于此,本研究旨在分析CAD患者中替格瑞洛相关呼吸困难的发生情况、临床环境影响因素、及该并发症对患者依从性和预后的影响,以期为替格瑞洛的临床应用进一步提供循证医学证据。
1 对象与方法
1.1 研究对象
连续募集2014年1月至2015年2月于解放军总医院心内科住院,并接受替格瑞洛90mg 2次/d联合阿司匹林(aspirin)100mg 1次/d进行抗栓治疗的CAD患者。排除标准:年龄<18岁,存在替格瑞洛治疗禁忌证,有心脏停搏病史,有严重的呼吸困难病史,血小板计数<100×109/L,合并癌症或存在多器官功能衰竭且生存期<1个月的终末期患者,存在严重的肝肾功能不全。本研究符合赫尔辛基宣言,并通过了伦理委员会的批准。向所有入选患者说明情况,并签署知情同意书。
1.2 研究方法
记录所有入选患者的基线资料。对所有患者自服用替格瑞洛起随访观察6个月,记录替格瑞洛相关呼吸困难及联合心血管缺血和出血终点事件的发生情况。替格瑞洛相关呼吸困难的判断依据前期文献报道的标准判断流程[10],包括:呼吸困难发生在患者服用替格瑞洛后,且多发生在安静时,与活动无关,患者的运动耐量不受其影响;既往无类似症状发生,且不伴有贫血、哮喘、端坐呼吸、夜间阵发性呼吸困难、胸痛及胸部紧缩感等特点;心肺查体及心电图、超声心动图、胸部X线、呼吸功能、血浆N末端B型脑钠肽前体(N-terminal probrain natriuretic peptide,NT-proBNP)等客观检查指标无明显异常;除外心肺疾病所引起的呼吸困难;停用替格瑞洛后,即可明显好转的呼吸困难。联合心血管缺血终点事件包括主要缺血(心源性死亡、非致死性心肌梗死和缺血性脑卒中)和次要缺血(明确或可能的支架内血栓、冠状动脉血管重建)终点事件。心源性死亡定义为由明确的心血管因素或除外任何非心血管因素导致的死亡。非致死性心肌梗死定义依据最新的ACC/ESC指南诊断标准,包括非ST段抬高型心肌梗死(non-ST segment elevated myocardial infarction,N-STEMI)和ST段抬高型心肌梗死(ST segment elevated myocardial infarction,STEMI)[1,2]。缺血性脑卒中定义为由于缺血事件导致神经功能病灶性缺失,且症状持续≥24h或导致死亡。明确的支架内血栓定义为PCI手术后发生的和靶血管相关的心肌梗死或死亡,经过造影证实有完全或部分性冠状动脉血栓性闭塞。可能的支架内血栓定义为PCI术后1个月内死亡或者心肌梗死,但没有经过造影证实。冠状动脉血管重建定义为患者再次因ACS入院后,因病情需要行PCI或冠状动脉搭桥手术。联合出血终点事件包括最新定义的心肌梗死溶栓治疗临床试验(thrombolysis in myocardial infarction,TIMI)主要出血和次要出血事件[11,12],其中TIMI主要出血事件包括:颅内出血,血红蛋白下降>5g/dl的临床显著性出血,7d内死亡的致死性出血;TIMI次要出血事件包括:临床显著性出血(包括影像表现),血红蛋白下降3~5g/dl,需要就医的以及未满足上述条件的显著性出血。出血终点事件中不包括冠状动脉搭桥或外科手术引起的出血。上述随访均由专门经过培训的医师在门诊或经电话完成,所有住院和随访期间替格瑞洛相关的呼吸困难及联合缺血和出血终点事件的发生均由≥2位副主任医师专家确诊。
1.3 统计学处理
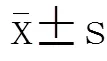
2 结 果
2.1 患者的基线资料
647例患者中,男性446例(68.93%),年龄32~88(61.18±10.31)岁。纳入研究的冠心病患者中,稳定型冠心病患者69例(10.66%),不稳定型心绞痛患者461例(71.25%),N-STEMI患者32例(4.95%),STEMI患者86例(13.29%),共有454例(70.17%)患者接受药物洗脱支架置入治疗(表1)。
2.2 替格瑞洛相关呼吸困难的发生情况
6个月随访期内依据诊断标准判定且经过临床专家确诊后,共有85例(13.14%)患者发生替格瑞洛相关呼吸困难,其中43例(50.59%)发生在用药1周内,35例(41.18%)发生在用药1个月内,7例(8.24%)发生在用药1个月后。因替格瑞洛呼吸困难而停药的患者15例(2.32%)。STEMI(24.71%11.57%,=0.002)及合并应用替罗非班(60.00%38.97%,<0.001)的患者在替格瑞洛呼吸困难组的比例明显高于无替格瑞洛呼吸困难组,而不稳定型心绞痛患者的比例在无替格瑞洛呼吸困难组明显高于替格瑞洛呼吸困难组(73.13%58.82%,=0.007)。余临床资料,两组之间差异均无统计学意义(均>0.05;表1)。
2.3 替格瑞洛相关呼吸困难影响因素分析
对可能影响替格瑞洛呼吸困难的因素进行多元回归分析发现,合并应用替罗非班使替格瑞洛呼吸困难的发生风险增高(OR:2.45,95%CI:1.41~4.27,=0.001),而合并使用他汀类药物(OR:0.20,95%CI:0.04~0.92,=0.04)及质子泵抑制剂(OR:0.56,95%CI:0.32~0.97,=0.04)均使替格瑞洛呼吸困难的发生风险降低(表2)。
2.4 替格瑞洛相关呼吸困难与临床转归的相关性分析
647例患者中,完成6个月随访且随访期内持续服用替格瑞洛的患者共353例(54.56%),其中替格瑞洛呼吸困难患者52例(14.73%),无替格瑞洛呼吸困难患者301例(85.27%)。两组患者在6个月随访期内联合缺血(3.85%2.66%,=0.98)和出血(19.23%16.94%,=0.69)终点事件的发生率差异均无统计学意义(表3)。
3 讨 论
本研究分析了我院心内科经替格瑞洛抗栓治疗的CAD患者在住院和出院6个月期间,替格瑞洛相关呼吸困难的发生率、影响因素、以及该并发症对替格瑞洛依从性和患者预后的影响。结果发现,替格瑞洛相关呼吸困难在CAD患者中的发生率为13.14%,其中停药发生率为2.32%;合并应用替罗非班、他汀类药物和质子泵抑制剂可能影响替格瑞洛相关呼吸困难的发生风险;替格瑞洛相关呼吸困难并不影响患者的预后。
前期研究报道,替格瑞洛引发的呼吸困难发生率在10%~38%,其中停药发生率介于0.1%~9%之间[3,13,14]。本研究发现替格瑞洛相关呼吸困难的发生率为13.14%,与PLATO研究结果相似(13.8%);但因替格瑞洛呼吸困难停药发生率(2.32%)高于PLATO研究(0.9%)[3]。与前期国内研究相比,本研究中替格瑞洛相关呼吸困难发生率及因此停药率均较高[8]。以上差异考虑与纳入研究的人群、样本量及随访时间的不同有关。随着替格瑞洛在临床的广泛应用,其相关呼吸困难的发生应得到重视,因为该并发症可能是导致替格瑞洛依从性差的主要原因之一。替格瑞洛相关呼吸困难多为一过性的轻到中度发作,患者多可耐受[15],因此,除了少数患者发生不能耐受的呼吸困难需要考虑停用替格瑞洛外,其他患者应避免因停用替格瑞洛造成的抗血小板疗效降低、继而增加引发冠状动脉血栓形成的风险。
本研究发现,合并应用替罗非班与替格瑞洛呼吸困难的发生具有独立相关性。替罗非班是可逆的强效血小板糖蛋白Ⅱb/Ⅲa受体拮抗剂[16],研究提示,替格瑞洛与替罗非班合用并未增加患者出血风险[17],这可能与二者均是可逆性的血小板抑制剂有关,当二者合用时,总体的血小板抑制水平并未在二者的基础上成倍增加。另外,近期1项研究提示腺苷与替罗非班合用与单用替罗非班相比,用药后48h患者的血小板聚集率并无差异,但是冠状动脉血流得到改善[18]。由此我们推断,腺苷作用的发挥可能受替罗非班影响。因此,当替格瑞洛与替罗非班合用时,由腺苷引发的呼吸困难可能也会受替罗非班合并用药的影响。我们的研究还发现,合并使用他汀类药物及质子泵抑制剂均与替格瑞洛呼吸困难的发生具有独立相关性。前期研究证实,替格瑞洛呼吸困难的发生率呈剂量依赖性,这表明替格瑞洛或其代谢物的血浆浓度可能会影响替格瑞洛呼吸困难的发生[13,15]。体外实验表明,替格瑞洛既是CYP3A4的底物,又是CYP3A4较弱的抑制剂[19],而他汀类药物与质子泵抑制剂均是CYP3A4的反应底物[20,21],这表明替格瑞洛与其他CYP3A4底物之间具有潜在的药物相互作用。因此,他汀类药物及质子泵抑制剂与替格瑞洛之间可能存在药物相互作用,替格瑞洛的血浆水平因此降低,从而使替格瑞洛呼吸困难的发生风险降低。

表1 服用替格瑞洛冠心病患者的基线信息
CAD: coronary artery disease; MI: myocardial infarction; CABG: coronary artery bypass grafting; PCI: percutaneous coronary intervention; ACS: acute coronary syndrome; SCAD: stable coronary artery disease; LVEF: left ventricular ejection fraction

表2 与替格瑞洛呼吸困难相关的影响因素分析

表3 随访6个月期间持续服用替格瑞洛患者缺血和出血终点事件的比较
CAD: coronary artery disease
呼吸困难往往提示患者临床预后不佳[22],尽管如此,本研究并未发现替格瑞洛呼吸困难与未发生者在缺血和出血终点事件上的差异,该结果与前期一项基于稳定型冠心病患者的研究结果相一致[23],但前期一项基于ACS患者的研究结果发现替格瑞洛呼吸困难会影响患者的预后[15]。以上临床转归的差异考虑与纳入人群的CAD严重程度、样本量、终点事件的定义及随访时间的不同有关。由于本研究入选患者来自单中心,病例数较少,随访时间短,因此,与替格瑞洛呼吸困难相关的临床转归有待进一步多中心、大规模的临床研究证实。
[1] Amsterdam EA, Wenger NK, Brindis RG,. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines[J]. Circulation, 2014, 130(25): 2354−2394.
[2] Authors/Task Force Members, Roffi M, Patrono C,. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC)[J]. Eur Heart J, 2015, DOI 10.1093/eurheartj/ehv320. [Epub ahead of print]
[3] Wallentin L, Becker RC, Budaj A,. Ticagrelorclopidogrel in patients with acute coronary syndromes[J]. N Engl J Med, 2009, 361(11): 1045−1057.
[4] Society of Cardiology, Chinese Medical Association. The consensus of antiplatelet therapy in China[J]. Chin J Cardiol, 2013, 41(3): 183−194. [中华医学会心血管病分会. 抗血小板治疗中国专家共识[J]. 中华心血管病杂志, 2013, 41(3): 183−194.]
[5] Wittfeldt A, Emanuelsson H, Brandrup-Wognsen G,. Ticagrelor enhances adenosine-induced coronary vasodilatory responses in humans[J]. J Am Coll Cardiol, 2013, 61(7): 723−727.
[6] Nylander S, Femia EA, Scavone M,. Ticagrelor inhibits human platelet aggregationadenosine in addition to P2Y12 antagonism[J]. J Thromb Haemost, 2013, 11(10): 1867−1876.
[7] Storey RF, Becker RC, Harrington RA,. Pulmonary function in patients with acute coronary syndrome treated with ticagrelor or clopidogrel (from the Platelet Inhibition and Patient Outcomes [PLATO] pulmonary function substudy)[J]. Am J Cardiol, 2011, 108(11): 1542−1546.
[8] Shao CL, Yan HB, Qiao SB,. Investigation on ticagrelor-related dyspnea[J]. Chin J Intervent Cardiol, 2015, 23(2): 85−88. [邵春丽, 颜红兵, 乔树宾, 等. 替格瑞洛相关呼吸困难的调查分析[J]. 中国介入心脏病学杂志, 2015, 23(2): 85−88.]
[9] Li W, Zhang Y, Du DY,. Ticagrelor induced dyspnea: thirty-eight cases report[J]. Clin Pharm, 2015, 9(2): 40−41. [李 巍, 张 莹, 杜大勇, 等. 替格瑞洛致呼吸困难38例分析[J]. 临床药学, 2015, 9(2): 40−41.]
[10] Parodi G, Storey RF. Dyspnea management in acute coronary syndrome patients treated with ticagrelor[J]. Eur Heart J Acute Cardiovasc Care, 2015, 4(6): 555−560.
[11] Mega JL, Braunwald E, Mohanavelu S,. Rivaroxabanplacebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomised, double-blind, phase Ⅱ trial[J]. Lancet, 2009, 374(9683): 29−38.
[12] Sabatine MS, Antman EM, Widimsky P,. Otamixaban for the treatment of patients with non-ST-elevation acute coronary syndromes (SEPIA-ACS1 TIMI 42): a randomised, double-blind, active-controlled, phase 2 trial[J]. Lancet, 2009, 374(9692): 787−795.
[13] Cannon CP, Husted S, Harrington RA,. Safety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidogrel, in patients with non-ST-segment elevation acute coronary syndrome: primary results of the DISPERSE-2 trial[J]. J Am Coll Cardiol, 2007, 50(19): 1844−1851.
[14] Gurbel PA, Bliden KP, Butler K,. Response to ticagrelor in clopidogrel nonresponders and responders and effect of switching therapies: the RESPOND study[J]. Circulation, 2010, 121(10): 1188−1199.
[15] Storey RF, Becker RC, Harrington RA,. Characterization of dyspnea in PLATO study patients treated with ticagrelor or clopidogrel and its association with clinical outcomes[J]. Eur Heart J, 2011, 32(23): 2945−2953.
[16] Ostrowska M, Adamski P, Kozinski M,. Off-target effects of glycoprotein Ⅱb/Ⅲa receptor inhibitors[J]. Cardiol J, 2014, 21(5): 458−464.
[17] Liu Y, Liu H, Hao Z,. Efficacy and safety of different doses of tirofiban combined with ticagrelor on diabetic patients with AMI receiving in emergency percutaneous coronary intervention (PCI)[J]. Int J Clin Exp Med, 2015, 8(7): 11360−11369.
[18] Zhao DH, Fan Q, Liu JH,. Safety and efficacy of tirofiban combined with adenosine during interventional therapy in patients with acute non-ST elevation myocardial infarction[J]. J Xinxiang Med Coll, 2015, 32(4): 332−335. [赵东晖, 范 谦, 柳景华, 等. 替罗非班联合腺苷应用在急性非ST段抬高型心肌梗死患者介入治疗中的有效性和安全性[J]. 新乡医学院学报, 2015, 32(4): 332−335.]
[19] Zhou D, Andersson TB, Grimm SW.evaluation of potential drug-drug interactions with ticagrelor: cytochrome P450 reaction phenotyping, inhibition, induction, and differential kinetics[J]. Drug Metab Dispos, 2011, 39(4): 703−710.
[20] Goodman SG, Clare R, Pieper KS,. Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: insights from the platelet inhibition and patient outcomes trial[J]. Circulation, 2012, 125(8): 978−986.
[21] Tantry US, Jeong YH, Gurbel PA. The clopidogrel-statin interaction[J]. Circ J, 2014, 78(3): 592−594.
[22] Pelter MM, Riegel B, McKinley S,. Are there symptom differences in patients with coronary artery disease presenting to the ED ultimately diagnosed with or without ACS[J]. Am J Emerg Med, 2012, 30(9): 1822−1828.
[23] Storey RF, Bliden KP, Patil SB,. Incidence of dyspnea and assessment of cardiac and pulmonary function in patients with stable coronary artery disease receiving ticagrelor, clopidogrel, or placebo in the ONSET/OFFSET study[J]. J Am Coll Cardiol, 2010, 56(3): 185−193.
(编辑: 周宇红)
Clinical analysis of ticagrelor-related dyspnea in coronary artery disease patients
WANG Xu-Yun, XI Shao-Zhi, LIU Jia, JING Jing, CHEN Yun-Dai, YIN Tong*
(Department of Cardiology, Chinese PLA General Hospital, Beijing 100853, China)
To determine the influencing factors associated with ticagrelor-related dyspnea in the patients with coronary artery diseases (CAD), and investigate the impact of the side effect on compliance and clinical outcomes of ticagrelor treatment.Consecutive CAD patients treated by ticagrelor in our department from January 2014 to February 2015 were recruited in this study. The in- and out-hospital incidence of ticagrelor-related dyspnea was recorded in 6-month follow-up, and the influencing factors for the side effect were analyzed by multivariate regression analysis. The influence of ticagrelor-related dyspnea on the occurrence of ischemic and bleeding events was observed during the 6 months’ follow-up.There were 647 CAD patients recruited in this study, and ticagrelor-related dyspnea was found in 85 patients (13.14%). The side effect was observed in 43 patients within 1 week after ticagrelor treatment, 35 patients within 1 month, and 7 patients after 1 month. Cessation of its administration was in 15 patients due to ticagrelor-related dyspnea. Concomitant therapy with tirofiban was associated with higher risk of ticagrelor-related dyspnea (OR=2.45, 95%CI: 1.41−4.27,=0.001), while concomitant therapy with statins (OR=0.20, 95%CI: 0.04−0.92,=0.04) and proton pump inhibitors (PPI; OR=0.56, 95%CI: 0.32−0.97,=0.04) were with lower risk of the effect. For the patients (=353) continuously taking ticagrelor in the follow-up period, no significant difference was seen in the occurrences of ischemic and bleeding events between those with ticagrelor-related dyspnea and those without.The side effect of ticagrelor-related dyspnea usually occurs within 1 month after ticagrelor treatment in CAD patients. Co-medication may be the main relevant factor influencing the occurrence. Ticagrelor-related dyspnea impacts the compliance of ticagrelor, but may have no effect on the clinical prognosis.
ticagrelor; ticagrelor-related dyspnea; influencing factor; compliance; prognosis
(7152129)(2012FC-TSYS-3043).
R541.1; R595.3; R972.9
A
10.11915/j.issn.1671-5403.2016.02.027
2015−12−04;
2015−12−21
北京市自然科学基金面上项目(7152129);解放军总医院临床扶持基金(2012FC-TSYS-3043)
尹 彤, E-mail: yintong2000@yahoo.com
