In vitro anti-hydatic and immunomodulatory effects of ginger and [6]-gingerol
2016-04-19ManelAmriChafiaTouilBoukoffa
Manel Amri, Chafia Touil-Boukoffa
University of Sciences and Technology Houari Boumediene, Faculty of Biological Science, Laboratory of Cellular and Molecular Biology, Team‘Cytokines and NO Synthases’, Algiers 16111, Algeria
In vitro anti-hydatic and immunomodulatory effects of ginger and [6]-gingerol
Manel Amri, Chafia Touil-Boukoffa✉
University of Sciences and Technology Houari Boumediene, Faculty of Biological Science, Laboratory of Cellular and Molecular Biology, Team‘Cytokines and NO Synthases’, Algiers 16111, Algeria
ARTICLE INFO
Article history:
Received 15 May 2016
Received in revised form 16 June 2016
Accepted 15 July 2016
Available online 20 August 2016
Ginger
[6]-gingerol
Echinococcosis
Cytotoxic activity
Nitric oxide
Objective: To study in vitro anti-hydatic and immunomodulatory effects of ginger and [6]-gingerol as an alternative therapy for Cystic echinococcosis. Methods: Effect of a commonly used herbal product and ginger (Zingiber officinale) towards protoscoleces (PSC) and cyst wall in vitro was studied. The effect of [6]-gingerol, and the pungent constituent of ginger, was also evaluated on PSC culture. Furthermore, the activity of both extracts in association with interferon-gamma (IFN-γ) on PSC co-cultured with mononuclear cells of hydatic patients was evaluated. The nitric oxide (NO) production was measured in each co-culture. Results: Ginger exhibited a concentration- and time-dependent cytotoxic effect against PSC and cyst wall. Interestingly, ginger was more effective than the [6]-gingerol. Moreover, additional parasitic effect between extracts and IFN-γ are also observed in co-cultures. Furthermore, both extracts attenuated the NO production elicited by this infection or by the IFN-γ. Conclusions: Ginger has an important anti-hydatic effect in vitro. This effect is amplified in the presence of IFN-γ. Moreover, this herbal product may protect against host’s cell death by reducing the high levels of NO. Ginger may act, at least, through the [6]-gingerol. All our data suggest the promising use of ginger in the treatment of Echinococcus granulosus infection.
1. Introduction
Cystic echinococcosis is a zoonotic disease in humans and sheep. This infection is caused by the larval stage of Echinococcus granulosus (E. granulosus). Man is being infected after ingestion of disseminated eggs in water, plants or on over the definitive hosts(dogs). Each egg, containing an oncosphere, gives an unilocular cyst especially in the liver and lungs. Surrounded by a thin wall: cystwall (CW), the cyst is filled with fluid containing brood capsules and protoscoleces (PSC).
The development of hydatic cyst for a long period induces many complications like organ malfunction and can lead to death[1]. The preferred therapy is cyst removal by surgery. However, the protoscoleces dissemination can occur during surgery leading to a high frequency of relapse. Chemotherapy with benzimidazole carbamate derivatives, amphotericin B and praziquantel is also used. This is the only option in many inoperable cases like cyst in brain or multiple cysts and in the immune-depressed host. Nevertheless,these drugs are not efficient in some cases and leads to liver toxicity and other side effects[2]. All these data prompted us to find new drugs for therapy.
The natural products are attracting attention as new therapeutic drugs against a number of diseases. Among their effects we found antiparasitic and antioxidant activities[3]. One of commonly used herbal product in traditional medicine all over the world is ginger(Zingiber officinale)[4,5]. There are few studies about the direct effectof this rhizome or other natural products against the helminthes. Some of these studies are related to Echinococcus granulosus and Echinococcus multilocularis using ginseng derivates or other herbs or natural products[6]. However, as far as we know, there are no studies about the effect of ginger on Echinococcus granulosus metacestode in culture or coculture with mononuclear cells (PBMC) of hydatic patients. In this sense, we assessed here the effect of crude aqueous extract of dried ginger on Echinococcus granulosus CW and PSC in culture. Furthermore, we evaluated the activity of this extract in association with IFN-γ on PSC viability and nitric oxide (NO)production in coculture and culture performed with PBMC of hydatic patients and healthy donors. The effects of ginger were also compared with those of [6]-gingerol, one of the active components of ginger.
2. Materials and methods
2.1. Ginger and [6]-gingerol
The dried rhizome of Zingiber officinale Roscoe (family Zingiberaceae), was procured from a local market in Algiers, Algeria and then has been identified and authenticated by a botanist. The bioactive compound [6]-gingerol was obtained from Calbiochem(San Diego, CA).
2.2. Preparation of ginger crude aqueous extract and [6]-gingerol
The crude aqueous extract was prepared by dissolving 1mg of the dried extract of ginger in 1ml of RPMI-1640 medium supplemented with 5% of heat-inactivated FCS (fetal calf serum, sigma) and antibiotics. Tubes were then mixed and centrifuged. The aqueous extract was then filtred through 0.22 μm (Millipore) and stored at -20 ℃until use.
The [6]-gingerol was diluted from the stock solution (dis solved in DMSO) with the culture medium (RPMI 1640) at the maximum final concentration of DMSO at 0.1%.
2.3. Patients
The peripheral blood samples were obtained from twenty Algerian patients carrying hydatic cyst in lungs (n=20) at Department of Surgery, Mustapha Bacha Hospital, Algiers, Algeria. The blood samples were obtained before (no more than 1 week) and after (24-72) h surgery. All patients having other infection or inflammatory chronic diseases were excluded from this study. Blood samples from healthy donors (n=10, from the same region of Algeria) were included in this study as healthy controls. All samples were used immediately after collection for PBMC preparation and culture.
All participants (patients and healthy donors) gave informed consent for this study, which was in accordance with the guidelines of the ethic committee of the Algerian agency of research and development in health (ATRSS). All procedures were performed in accordance with Helsinki declaration of 2008.
2.4. Preparation of PSC and CW
The hydatid cysts (n=5) were obtained after surgery in lungs and livers at Mustapha Bacha hospital, Algiers, Algeria. Cysts were washed several times with sterile PBS, pH 7.4 supplemented with antibiotics.
CW was prepared as previously described[7]. Briefly, cyst wall,obtained after hydatid fluid removal, was washed several times with sterile PBS. This membrane was then cut into small pieces (1 cm×1 cm) and washed again. CW was finally maintained in RPMI-1640 medium supplemented with 5 % of heat-inactivated FCS and antibiotics until culture.
PSC were prepared as previously described[8,9]. Briefly, free PSC were obtained by centrifugation of hydatid fluid followed by washing in sterile PBS. The viability of PSC was determined prior the cultures by microscopic examination using 0.1% eosin as a vital stain. While viable PSC still unstained, dead PSC become stained. The appropriate percentage of viability was 95% or more. PSC were then maintained in RPMI-1640 medium supplemented with 5% of heat-inactivated FCS until culture and coculture.
2.5. CW and PSC culture and treatment
Two pieces of CW and 2×103viable PSC were placed in each well of 24-well plastic trays. They were cultured in RPMI1640 medium supplemented with antibiotics and 10% heat-inactivated FCS and treated with different concentrations of ginger (100 μg/mL for CW and 1, 10 and 100 μg/mL for PSC). PSC cultures were also treated with [6]-gingerol (100 μg/mL). The untreated cultures were used as a negative control. PSC treated with 0.1% DMSO vehicle without[6]-gingerol were also considered as a control. Cultures were then incubated at 37 ℃ in a humid atmosphere and 5% CO2for 72 h for CW and 24 h and 48 h for PSC.
Cultures were observed under microscope before and after incubation. Percentages of viable proto scoleces were determined by microscopic examination using 0.1% eosin as a vital stain.
2.6. PBMC culture and coculture with PSC
PBMC were prepared from blood of hydatic patients and healthy donors as previously described[8]. Briefly, PBMC isolated by density gradient were washed three times with sterile PBS. Cell viabilitywas checked using 0.2% trypan blue dye. The appropriate percent of viable cells was always > 98%. Viable of 106cells/mL were then resuspended in RPMI-1640 medium supplemented with antibiotics and 10% of FCS.
Cells were immediately cocultured with PSC and stimulated with 100 μg/mL of ginger or [6]-gingerol alone or with IFN-γ (100 U/ mL). The unstimulated cocultures were used as negative control. The cocultures treated with 0.1% DMSO vehicle without [6]-gingerol were also considered as a control. Cells were also stimulated with ginger extract (1, 10 and 100 μg/mL) and [6]-gingerol (100 μg/mL). The effects of ginger and [6]-gingerol on nitrites production was demonstrated using L-NMMA (0.5 mM), (Sigma) and human recombinant IFN-γ (100 U/mL)[8] (HuIFN-γ is a gift from Dr. J. Wietzerbin, INSERM U365- ‘Interférons et cytokines’- Institut Curie, INSERM U932- ‘Immunité et cancer’- Institut Curie, Paris,France).
Cocultures and cultures were then incubated at 37 ℃ in humid atmosphere and 5% CO2. After 20 h, percentages of viable proto scoleces were determined in coculture as described previously in section 2.4. Moreover, nitrites production was evaluated in coculture and culture supernatants.
2.7. Nitrites measurement
Nitric oxide production was evaluated by measuring nitrites concentration (NO2-) in each supernatant by Griess method as previously described[10]. Briefly, 100 μL of supernatant were incubated for 5 min with 0.05% naphthyldiamine dihydrochloride and 0.5% sulfanilamide prepared in 5% HCl. The OD was measured by spectrophotometer at 543 nm. The data were expressed as μM of nitrites per 106cells.
2.8. Statistical analysis
The results obtained were expressed as mean±SD. Data analysis was performed using the Minitab 16. The comparison between different groups was performed using Student’s t-test. Differences were considered to be statistically significant at P<0.05.
3. Results
3.1. Cytotoxic effect of ginger on cyst wall
The effect of ginger on CW was examined in vitro. In this sense,morphological change in CW was evaluated by microscopic examination in the absence and presence of crude aqueous extract of dried ginger (100 μg/mL) for 72 h.
Our results showed that ginger effect correlated with exposure times. In fact, between 12 to 24 h, the germinal layer (GL) becomes darker and small areas of degeneration are observed after 36 h of exposure. After 48 h, GL loses completely its integrity followed by the laminated layer (LL). CW loses its integrity after 60 to 72 h (Figure 1).

Figure 1. Progressive morphological alterations of CW after treatment with 100 μg/mL of ginger for 72 h showing by light microscopy.A: CW culture at t=0. B: CW culture after 72 h without ginger. C, D, E, F, G and H: CW culture with ginger (100 μg/mL) after 12 h, 24 h, 36 h, 48 h, 60 h and 72 h, respectively. Discontinued arrow: Altered germinal layer (GL);Arrow: degeneration of GL. Arrowhead: loss of layers in the laminated layer(LL). (Ob×05).
3.2. Cytotoxic effect of ginger and [6]-gingerol on protoscoleces
In the PSC culture, our results show that ginger exhibited a dosedependent cytotoxic effect on PSC (Figure 2).
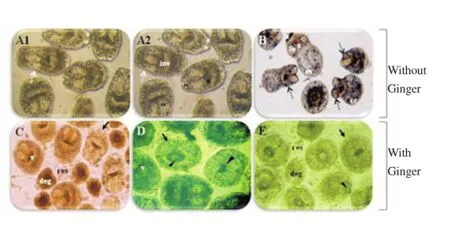
Figure 2. Protoscolicidal effect of ginger showing by light microscopy.PSC were cultured and treated with ginger for 24 h, at 37 C, in 5% CO2. A:PSC culture at t = 0 (97% of viability). B: PSC culture after 24 h. C, D and E:PSC culture after 24 h with Ginger at 1, 10 and 100 μg/mL, respectively. cc:calcareous corpuscles; deg: degeneration; ev: evaginated PSC; H: hooks; inv:invaginated PSC; R: rostellum; res: PSC residues; su: suckers; v: viable PSC. Arrow: Altered tegument; Arrowhead: loss of hooks. Black discontinued arrow: evagination and invagination of the head; White discontinued arrow:tegument shift. (×108).
Remarkably, ginger induced a strong morphological change with loss of the membrane integrity, the order of hooks, and the homogeneity of the calcareous corpuscles. Moreover, contractile movements of membrane and suckers disappear gradually followed by complete PSC degeneration and death. In contrast, no appreciable change was detected in the PSC viability of untreated culture (Figure 3).

Figure 3. Morphological alterations of protoscoleces after treatment with ginger showing by light microscopy.
To confirm these observations, we also evaluated the PSC viability using 0.1% eosin as a vital stain (Figure 3). The figure 4a shows the percentage of viable PSC after ginger exposure for 24 h and 48 h of culture. We notice that scolicidal effect of ginger is strongly related to concentration and exposure times. Interestingly, concentrations of 1 μg/mL and 100 μg/mL of ginger kill respectively 51.80% and 89.72% (P<0.000 1) of parasite after 24 h of culture (Figure 4). Ginger exhibited also a time-dependent protoscolocidal effect after 24h and 48h with respective maximum reduction of 89.72% and 100% (P<0.000 1) in PSC viability for 100 μg/mL (Figure 4A).
In the presence of 100 μg/mL [6]-gingerol, time-dependently reduced cell viability was also observed (Figure 4B). Interestingly,[6]-gingerol was less effective than ginger (P<0.000 1). For note,PSC treated with vehicle alone remained viable throughout the incubation period (24 and 48 h with respectively P = 0.897 and P = 0.371).

Figure 4. Killing of E. granulosus protoscoleces after exposure to ginger (A)and [6]-gingerol (B) for 24 h and 48 h of culture.Each histogram represents the mean percentage of viable protoscoleces from five different experiments. Differences between different groups are indicated(△△P<0.01,△△△P<0.001,△△△△P<0.000 1; NS: no significance). Triangles(△) and NS above single bars indicate differences with the negative control(untreated cocultures) for 24 h and 48 h. Asteriks above horizontal brackets indicate difference between 24 h and 48 h.
3.3. Scolicidal effect of ginger and [6]-gingerol in mononuclear cells- protoscoleces cocultures
In the second part of our study, we investigate the scolicidal effect of ginger and [6]-gingerol on PSC cocultured with mononuclear cells (PBMC) in presence or absence of IFN-γ. For note, we have previously highlighted the role of this cytokine in host defense and parasite killing[8].
Our results show that 100 μg/mL of ginger and [6]-gingerol reduces protoscoleces viability in vitro from 61.94%±2.72% to respectively 39.82%±0.57% (P<0.000 1) and 46.63%±1.67% (P<0.000 1) (for PBMC from patients before sugery). Interestingly, synergistic scolicidal effects between these two extracts and IFN-γ are also observed in PSC-PBMC co-cultures. In fact, incubation with extracts and IFN-γ induced the most damage to the protoscoleces as indicated by viability loss in comparison with co-cultures treated with IFN-γ or extracts alone (Figure 5A).
The same results are reported for co-cultures performed with PBMC from patients before and after surgery and healthy donors.However, incubation of PBMC from healthy donors with ginger or[6]-gingerol induced less damage as manifested by the high levels of PSC viability.
3.4. Inhibitory effect of ginger and [6]-gingerol on NO production by mononuclear cells
In our previous study, we have reported that the scolocidal effect of IFN-γ is associated with an increase in NO production[8]. On the basis of these results, we investigated here if ginger and [6]-gingerol act by the same mechanism. During our experiment, loss of viability in the presence of ginger and [6]-gingerol is associated with a decrease in nitrites levels. This effect contrasts with the increase observed in the presence of IFN-γ[8]. Interestingly, ginger and [6]-gingerol also attenuate the increase in NO production elicited by IFN-γ (Figure 5B).

Figure 5. Killing of E. granulosus protoscoleces.(A) in coculture with mononuclear cells (PBMC) treated with ginger or [6]-gingerol (100 μg/mL) alone or with human recombinant IFN-γ (100 U/mL)for 20 h. Correlation with nitrites production (B). Each histogram represents the mean percentage of viable protoscoleces from different experiments(n=20 and n=10 for patients and controls respectively). Differences between different groups are indicated (△△P<0.01,△△△P<0.001,△△△△P<0.000 1; NS: no significance). Triangles (△) and NS above single bars indicate differences with the negative control (untreated cocultures). Asteriks above horizontal brackets indicate difference between IFN-γ alone or with ginger or [6]-gingerol. Gin: ginger, veh: vehicle, 6-ging: [6]-gingerol.
The same results are also observed for culture with a dosedependent inhibitory effect of ginger on nitrites production. However, the inhibition observed using 1 μg/mL of ginger is statistically not significant for all cultures (Figure 6).
To confirm the effects of ginger on NO production, L-NMMA (NO Synthase inhibitor) and IFN-γ are used in PBMC culture. While L-NMMA decreases nitrites production, IFN-γ has an opposite effect. Remarkably, ginger and [6]-gingerol decrease the levels on nitrites when used alone or with IFN-γ (Figure 6).

Figure 6. Nitric oxide production in PBMC cultures treated with ginger (1,10 and 100 μg/mL) for 20 h.Human recombinant IFN-γ (100 U/mL) and L-NMMA (0.5 mM) were also used to confirm the effect of ginger on NO production. Each histogram represents the mean nitrite levels from different experiments (n=20 and n=10 for patients and controls respectively). Differences between different groups are indicated (△△P<0.01,△△△P<0.001,△△△△P<0.000 1; NS: no significance). Triangles (△) and NS above single bars indicate differences with the negative control (untreated cultures). Asteriks above horizontal brackets indicate difference between IFN-γ alone or with ginger. Gin 1, 10 and 100 (concentrations of ginger in μg/mL).
4. Discussion
Nowadays, several studies have been done to find new natural compounds that treat parasitic diseases. However, hydatidosis is among the most neglected parasitic infections, and development of new therapy and drugs received very little attention. In this study, the role of ginger (Zingiber officinale) against Echinococcus granulosus larvae has been investigated. Interestingly, we found that ginger exhibited dose- and time-dependent larvicidal effect against Echinococcus granulosus metacestode and protoscoleces.
Our results are in the same line with previous studies showing that ginger has an anthelmintic activity against some parasitic speciesboth in vitro and in vivo. In their report, Iqbal et al. reported that methanol extracts of ginger killed all the Haemonchus contortus worms within two hours post exposure[11]. These findings agree with other works revealing that ginger extract affects the viability of Schistosoma mansoni adult pairs[12] and Ascaridia galli[13], both in vitro and in vivo in mice.
Our present results are also in harmony with previous in vivo studies conducted by Datta and Sukul showing a significant anthelmintic efficacy of ginger alcoholic extract in dogs, naturally infected with Dirofilaria immitis[14]. Later, Iqbal et al. demonstrated that crude powder and crude aqueous extract of dried ginger exhibit anthelmintic activity in sheep naturally infected with different species of gastrointestinal nematodes including: Haemonchus contortus, Trichostrongylus colubriformis, Trichostrongylus axei,Oesophagostomum columbianum, Strongyloides papillosus and Trichuris ovis[15]. More recently, Mostafa et al. and Aly and Mantawy reported that the crude aqueous extract of ginger exhibited antischistosomal activity in mice as showed by the reduction in worm and egg burden in the hepatic tissue and faeces of infected mice[16,17].
Our data show that in vitro treatment with ginger extract induces significant alterations in cyst wall and PSC leading to parasite death. In vivo, such damage to the cyst wall destroys the defense system of the parasite. Thus, the PSC could easily be attacked by the protective host immune responses. We have found that ginger induces a loss of spontaneous movement of PSC and damage to the hooks in a time- and dose-dependent manner. These effects cause loss of PSC ability to adhere to the host tissues. Moreover, the observed tegumental alterations could be a committed mechanism by ginger to destroy the parasite. Our observations are more or less similar to the alterations observed after treatment with genistein as observed by Naguleswaran et al.[6]. The alterations in the surface architecture of E. granulosus metacestode were used by several investigators as a deleterious indicator for the evaluation of anti-hydatic activities of different drugs. Moreover, the reduction observed in PSC viability was considered by several authors as a strong evidence of the efficiency of anti-hydatic drugs[6,18,19].
Interestingly, ginger acts synergistically with IFN-γ to kill PSC. However, the immunomodulatory effect of ginger on host immune response has not been evaluated during E. granulosus infection. Based on our previous work showing a significant induction of nitric oxide (NO) production after in vitro treatment with IFN-γ[8], we wished to determine the effect of ginger on NO production. This present work demonstrates, for the first time, that ginger reduces NO production by host cells in response to E. granulosus infection in vitro.
It is known that pro-inflammatory cytokines (likes IFN-γ and TNF-α)simulate iNOS expression and activity in macrophages[20,21]. Thus, when the production of these cytokines is inhibited, the generation of NO is likely to be reduced. Several studies have shown that ginger inhibited the expression of genes encoding proinflammatory cytokines by various cells[22,23]. These findings are consistent with in vivo study showing that ginger extract inhibit the production of NO, TNF-α and IL-12 in mice infected with Shistosoma mansoni[17].
Although our previous studies have demonstrated that IFN-γ and iNOS pathways are involved in protective immunity against E. granulosus[8,9,24]. Our present study indicates that IFN-γ kills the parasite even although NO production was reduced by ginger. Thus,our data suggest the commitment of another mechanism by which IFN-γ kills the protoscoleces in vitro.
In summary, our study indicates for the first time that ginger possesses cestocidal activity against E. granulosus However; further studies are required to clarify the mechanisms involved in this effect. Sanderson et al. and Iqbal et al. reported that ginger kills Shistosoma mansoni and gastrointestinal nematode in vivo through its binding to parasite beta-tubulin and inhibition of glucose uptake[12,15]. Another study attributed the significant reduction in tissue egg after ginger treatment to the disturbance in worm fecundity[25]. Nevertheless,the bioactivity of ginger may be due to its constituents. In fact,phytochemical reports show that ginger contains over 400 different compounds. The major constituents are zingerone, paradol, gingerols and shogaols. According to several literature reviews, [6]-gingerol is the most abundant active constituent of whole ginger. It has various pharmacological and microbiological proprieties, including antiparasitic, anti-tumorigenic, anti-inflammatory and anti-oxidant activities. Interestingly, in many study, researchers used the [6]-gingerol as a marker substance of ginger[4,26,27].
These data prompted us to investigate the effect of [6]-gingerol on E. granulosus PSC and NO production. In this study, we have found that [6]-gingerol significantly kills PSC in vitro both in culture and coculture. Moreover, [6]-gingerol reduces NO production by host mononuclear cells. These results confirm our hypothesis about the implication of [6]-gingerol in the cytotoxic and regulatory effects of ginger respectively on PSC and NO production. Various studies reported that [6]-gingerol possess a variety of pharmacological effects like anthelmintic and anti-inflammatory activities[4,26,27]. Our results are in the line with those reported by Goto et al. indicating a significant anti-nematodal activity of [6]-gingerol against Anisakis larvae in vitro[28]. In the same way, Adewunmi et al. demonstrated that gingerol completely abolished the infectivity of Shistosoma mansoni miracidia and cercariae in Biomphalaria glabrata snailand mice, respectively[29]. [6]-gingerol was also found to kill Angiostrongylus cantonensis larvae or to reduce their spontaneous movements[25]. Our findings are in agreement with a recent in vivo study in mice assigning a significant anthelmintic activity to [6]-gingerol against Schistosoma mansoni[17].
According to Lee et al., [6]-gingerol reduces iNOS and TNF-expression by blocking NF-κB and PKC signaling pathway in lipopolysaccharide-stimulated mouse macrophages[30]. It is well known that PKC activates NF-κB[31] which in turn regulates at the transcriptional level the iNOS expression[32]. Results reported by another team suggested that [6]-gingerol inhibits NO production and iNOS expression in activated J774.1 mouse macrophages[33]. In the same way, Tripathi et al. also demonstrated that [6]-gingerol inhibits the production of TNF-α, IL-1β, and IL-12 in murine peritoneal macrophages[34].
It is well known that inflammatory mediators (like NO) play a crucial role in chronic inflammation, oxidative stress, and fibrosis that affect tissue architecture and impair organ function[35,36]. Thus, we can conclude here, that ginger extract and [6]-gingerol could minimize the deleterious effects of this parasite on the vital functions of infected organs[1] through its immunomodulatory effect on the iNOS pathway. This active component of ginger may have a direct effect on the vitality and viability of PSC and NO production. However, ginger had a higher efficacy than that of [6]-gingerol. Another component of ginger such as the [6]-shogaol may act in synergy with [6]-gingerol[17,25]. Further studies to explore the effect of these components on E. granulosus metacestode appear to be useful.
This study is the first to determine the larvicidal activity of ginger and [6]-gingerol on E. granulosus metacestode and protoscoleces. Interestingly, we found that ginger and its main component have also an immunomodulatory effect that did not adversely affect their larvicidal activity. Further investigations for the mechanisms underlying ginger effects are necessary. Above results might contribute to the use of ginger as new alternative therapy. However,in vivo studies on an animal model should be done before using ginger or ginger derivatives for treatment of human hydatidosis.
Acknowledgements
This work was supported by National Project in Health (PNRSanté-2011-2014).
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Wu XW, Peng XY, Zhang SJ, Niu JH , Sun H , Xi Y. Formation mechanisms of the fibrous capsule around hepatic and splenic hydatid cyst. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2004;22: 1-4.
[2] Brunetti E, Kern P, Vuitton DA. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 2010; 114(1): 1-16.
[3] Parveen A, Parveen B, Parveen R, Ahmad S. Challenges and guidelines for clinical trial of herbal drugs. J Pharm Bioallied Sci 2015; 7(4): 329-333.
[4] Ali BH, Blunden G , Tanira MO, Nemmar A. Some phytochemical,pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem Toxicol 2008; 46: 409-420.
[5] Kumar G, Karthik L, Bhaskara Rao KV. A review on pharmacological and phytochemical properties of Zingiber officinale Roscoe (Zingiberaceae). J Pharmacy Res 2011; 4(9): 2963-2966.
[6] Naguleswaran A, Spicher M, Vonlaufen N, Ortega Mora LM, Torgerson P, Gottstein B, et al. In vitro metacestodicidal activities of genistein and other isoflavones against Echinococcus multilocularis and Echinococcus granulosus. Antimicrob Agents Chemother 2006; 50(11): 3770-3778.
[7] Zeghir-Bouteldja R, Amri M, Aitaissa S, Bouaziz S, Mezioug D, Touil-Boukoffa C. In vitro study of nitric oxide metabolites effects on human hydatid of Echinococcus granulosus. J Parasitol Res 2009; 2009: 624919.
[8] Amri M, Aissa SA, Belguendouz H, Mezioug D, Touil-Boukoffa C. In vitro antihydatic action of IFN-gamma is dependent on the nitric oxide pathway. J Interferon Cytokine Res 2007; 27: 781-787.
[9] Amri M, Touil-Boukoffa C. A protective effect of the laminated layer on Echinococcus granulosus survival dependent on upregulation of host arginase. Acta Trop 2015; 149: 186-194.
[10] Touil-Boukoffa C, Bauvois B, Sanceau J, Hamrioui B, Wietzerbin J. Production of nitric oxide (NO) in human hydatidosis. Relationship between nitrite production and interferon-gamma levels. Biochimie 1998;80: 739-744.
[11] Iqbal Z, Nadeem QK , Khan MN, Akhtar MS , Waraich FN. In vitro anthelmintic activity of Allium sativum, Zingiber officinale, Curcurbita mexicana and Ficus religiosa. Int J Agri Biol 2001; 3(4): 454-457.
[12] Sanderson L , Bartlett A, Whitfield PJ. In vitro and in vivo studies on the bioactivity of a ginger (Zingiber officinale) extract towards adult schistosomes and their egg production. J Helminthol 2002; 76: 241-247.
[13] Bazh EKA, El-Bahy NM. In vitro and in vivo screening of anthelmintic activity of ginger and curcumin on Ascaridia galli. Parasitol Res 2013;112: 3679-3686.
[14] Datta A, Sukul NC. Antifilarial effect of Zingiber officinale on Dirofilariaimmitis. J Helminthol 1987; 61(3): 268-270.
[15] Iqbal Z , Lateef M , Akhtar MS , Ghayur MN, Gilani AH. In vivo anthelmintic activity of ginger against gastrointestinal nematodes of sheep. J Ethnopharmacol 2006; 106: 285-287.
[16] Mostafa OMS, Eid RA, Adly MA. Antischistosomal activity of ginger(Zingiber officinale) against Schistosoma mansoni harbored in C57 mice. Parasitol Res 2011; 109: 395-403.
[17] Aly HF, Mantawy MM. Efficiency of ginger (Zingbar officinale) against Schistosoma mansoni infection during host-parasite association. Parasitol Int 2013; 62: 380-389.
[18] Verma VC, Gangwar M , Yashpal M, Nath G. Anticestodal activity of Endophytic Pestalotiopsis sp. on protoscoleces of hydatid cyst Echinococcus granulosus. BioMed Res Int 2013; 2013: 308515.
[19] Pensel PE , Maggiore MA, Gende LB, Eguaras MJ, Denegri MG ,Elissondo MC. Efficacy of essential oils of Thymus vulgaris and Origanum vulgare on Echinococcus granulosus. Interdiscipl Perspect Infect Dis 2014; 2014: 1-12.
[20] Rath M, Müller I, Kropf P, Closs EI, Munder M. Metabolism via arginase or nitric Oxide synthase: two competing arginine pathways in macrophages. Front Immunol 2014; 5: 532.
[21] Bogdan C. Nitric oxide synthase in innate and adaptive immunity: an update. Trends Immunol 2015; 36(3): 161-178.
[22] Hong SS, Oh JS. Phenylpropanoid ester from Zingiber officinale and their inhibitory effects on the production of nitric oxide. Arch Pharm Res 2012;35(2): 315-320.
[23] Chantaranothai C, Palaga T, Karnchanatat A, Sangvanich P. Inhibition of nitric oxide production in the macrophage-like RAW 264.7 cell line by protein from the rhizomes of Zingiberaceae plants. Prep Biochem Biotechnol 2013; 43(1):60-78.
[24] Amri M, Mezioug D, Touil-Boukoffa C. Involvement of IL-10 and IL-4 inevasion strategies of Echinococcus granulosus to host immune response. Eur Cytokine Netw 2009; 20: 63-68.
[25] Lin RJ, Chen CY, Chung LY, Yen CM. Larvicidal activities of ginger(Zingiber officinale) against Angiostrongylus cantonensis. Acta Trop 2010;115(1-2): 69-76.
[26] Rahmani AH, Al shabrmi FM, Aly SM. Active ingredients of ginger as potential candidates in the prevention and treatment of diseases via modulation of biological activities. Int J Physiol Pathophysiol Pharmacol 2014; 6(2):125-136.
[27] Wang S, Zhang C, Yang G, Yang Y. Biological properties of 6-gingerol: a brief review. Nat Prod Commun 2014; 9(7): 1027-1030.
[28] Goto C, Kasuya S, Koga K, Ohtomo H, Kagei N. Lethal efficacy of extract from Zingiber officinale (traditional Chinese medicine) or [6]-shogaol and [6]-gingerol in Anisakis larvae in vitro. Parasitol Res 1990;76(8): 653-656.
[29] Adewunmi CO , Oguntimein BO , Furu P. Molluscicidal and antischistosomal activities of Zingiber officinale. Planta Med 1990; 56(4):374-376.
[30] Lee TY, Lee KC, Chen SY, Chang HH. 6-Gingerol inhibits ROS and iNOS through the suppression of PKC-α and NF-κB pathways in lipopolysaccharide-stimulated mouse macrophages. Biochem Bioph Res Co 2009; 382(1): 134-139.
[31] Gao X, Ikuta K, Tajima M, Sairenji TL. 12-O-tetradecanoylphorbol-13-acetate induces Epstein-Barr virus reactivation via NF-kappaB and AP-1 as regulated by protein kinase C and mitogen-activated protein kinase. Virology 2001; 286: 91-99.
[32] Simon PS, Sharman SK, Lu C, Yang D, Paschall AV, Tulachan SS, et al. The NF-κB p65 and p50 homodimer cooperate with IRF8 to activate iNOS transcription. BMC Cancer 2015; 15: 770.
[33] Ippoushi K, Azuma K, Ito H, Horie H, Higashio H. [6]-Gingerol inhibits nitric oxide synthesis in activated J774.1 mouse macrophages and prevents peroxynitrite-induced oxidation and nitration reactions. Life Sci 2003; 73: 3427-3437.
[34] Tripathi S , Maier KG, Bruch D , Kittur DS. Effect of 6-gingerol on pro-inflammatory cytokine production and costimulatory molecule expression in murine peritoneal macrophages. J Sur Res 2007; 138: 209-213.
[35] Beschin A, De Baetselier P, Van Ginderachter JA. Contribution of myeloid cell subsets to liver fibrosis in parasite infection. J Pathol 2013;229(2): 186-197.
[36] Iwakiri Y. Nitric oxide in liver fibrosis: The role of inducible nitric oxide synthase. Clin Mol Hepatol 2015; 21(4): 319-325.
10.1016/j.apjtm.2016.06.013
Manel Amri, University of Sciences and Technology Houari Boumediene, Faculty of Biological Science, Laboratory of Cellular and Molecular Biology, Team ‘Cytokines and NO Synthases’, PB 32 El-Alia, Algiers 16111, Algeria. Tel:213550531365
E-mail:manelamri@yahoo.fr
✉Corresponding author: Chafia Touil-Boukoffa, University of Sciences and Technology Houari Boumediene, Faculty of Biological Science, Laboratory of Cellular and Molecular Biology, Team ‘Cytokines and NO Synthases’, PB 32 El-Alia, Algiers 16111, Algeria.
Tel: 00213550819857
E-mail: touilboukoffa@yahoo.fr
Foundation project: This work was supported by National Project in Health (PNRSanté-2011-2014).
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- State of the art in neurocysticercosis
- Chemical composition, antiproliferative, antioxidant and antibacterial activities of essential oils from aromatic plants growing in Sudan
- Zika: As an emergent epidemic
- Toxoplasmosis and anti-Toxoplasma effects of medicinal plant extracts-A mini-review
- Unlocking the in vitro anti-Trypanosoma cruzi activity of halophyte plants from the southern Portugal
- Development and application of quantitative detection method for nervous necrosis virus (NNV) isolated from sevenband grouper Hyporthodus septemfasciatus
