Chemical composition, antiproliferative, antioxidant and antibacterial activities of essential oils from aromatic plants growing in Sudan
2016-04-19SakinaYagiRandaBabikerTzvetomiraTzanovaHerveSchohn
Sakina Yagi, Randa Babiker, Tzvetomira Tzanova, Herve Schohn✉
1Department of Botany, Faculty of Science, University of Khartoum, PO Box 321, Khartoum, Sudan
2Department of Botany, Faculty of Science, University of Kordofan, Kordofan, Sudan
3Universite de Lorraine-UMR CNRS 7565-Structure et Reactivite des Systemes Moleculaires Complexes-Campus Bridoux-rue du General Delestraint-57070 Metz Cedex, France
Chemical composition, antiproliferative, antioxidant and antibacterial activities of essential oils from aromatic plants growing in Sudan
Sakina Yagi1, Randa Babiker2, Tzvetomira Tzanova3, Herve Schohn3✉
1Department of Botany, Faculty of Science, University of Khartoum, PO Box 321, Khartoum, Sudan
2Department of Botany, Faculty of Science, University of Kordofan, Kordofan, Sudan
3Universite de Lorraine-UMR CNRS 7565-Structure et Reactivite des Systemes Moleculaires Complexes-Campus Bridoux-rue du General Delestraint-57070 Metz Cedex, France
Article history:
Received 15 May 2016
Received in revised form 16 June 2016
Accepted 15 July 2016
Available online 20 August 2016
Boswellia papyrifera
Croton zambesicus
Cymbopogon schoenanthus
Cyperus rotundus
GC/MS profile
anti-proliferative activity
Objective: To explore the potential of essential oil, as therapeutic molecule source,from olibanum of Boswellia papyrifera (Burseraceae), leafy stems of Cymbopogon schoenanthus (Poaceae) and Croton zambesicus (Euphorbiaceae) and rhizome of Cyperus rotundus (Cyperaceae) found in Sudan. Respective essential oil was evaluated for antiproliferative, antibacterial and antioxidant activity. Methods: Essential oils were extracted by hydrodistillation and then analysed by gas chromatography coupled to mass spectrometry (GCMS). Anti-proliferative activity was determined against human cell lines (MCF7 and MDAMB231, HT29 and HCT116) by the thiazolyl blue tetrazolium bromide (MTT) procedure. Antioxidant activity was evaluated by diphenyl 2 pycril hydrazil (DPPH) assay. Antibacterial activity was determined against two Gram-positive and two Gram-negative bacteria by microdilution method. Results: The essential oil from olibanum of Boswellia papyrifera contained mainly alcohol and ester derivatives (46.82%) while monoterpenes (69.84%)dominated in Corton zambesicus oil. Sesquiterpenes were the most highly represented classes of terpene derivatives in Cyperus schoenanthus (71.59%) and Cyperus rotundus (44.26%). Oil of Cymbopogon schoenanthus revealed the best anti-proliferative activity against HCT116 cell line with IC50value at (19.1 ± 2.0) μg/mL. Oil of Croton zambesicus showed the best antioxidant activity [EC50(4.20 ± 0.19) mg/mL]. All oils showed good antibacterial activity against Escherichia coli, Bacillus subtilis and Staphylococcus aureus with minimum inhibitory concentration (MIC) value ranged from 16 to 250 μg/mL. Conclusions: The results suggest that the essential oils of these plants could be used as a source of natural anti-proliferative,antioxidant and antibacterial agents.
1. Introduction
In recent years, there has been great interest on the biologicalactivity of essential oils as cosmetic, pharmaceutical and food processing industries seek natural alternatives[1,2]. Essential oils, which are complex mixtures of substances, are considered as valuable natural source of bioactive molecules that can be of therapeutic benefit in the treatment of various diseases[3,4]. In Sudan, essential oils have a rich history of use as a source of food,medicine and for cosmetic applications. For example, aerial part of Cymbopogon schoenanthus (C. schoenanthus) L. Spreng (Poaceae) is used to treat gout, prostate inflammation, kidney diseases and for stomach pains[5]. Olibanum of Boswellia papyrifera (B. papyrifera)(Del.) Hochst. (Burseraceae) is widely used as incense at home and for the treatment of cough and respiratory infections[6]. Aerial part of Croton zambesicus (C. zambesicus) Mull-Arg (Euphorbiaceae) is used to treat constipation, malaria and cough[7]. Rhizome of Cyperus rotundus (C. rotundus) L (Cyperaceae) is used to treat stomach disorders, bowels irritation, dyspepsia, diarrhea, dysentery, ascites,cholera, ulcers, sores and fevers, as an anthelmintic, to cure wounds and for scorpion stings[8].
Data from literature showed that essential oils contain a large variety of substances with great potential as valuable source of bioactive molecules. They exhibited many biological effects such as antibacterial[9], antifungal[10], antiviral[11], anti-leishmanial[12],antioxidant[2] and anti-proliferative properties[13]. However, the literature survey revealed that limited works were undertaken on chemical composition and/or biological activities of aromatic plants growing in the Sudan. Thus, the aim of the present study was to determine the chemical constituents and evaluate the antiproliferative, antioxidant and antibacterial activities of the essential oil extracted from olibanum of B. papyrifera, leafy stems of C. schoenanthus and C. zambesicus and rhizome of C. rotundus grown in Sudan.
2. Materials and methods
2.1. Plant material
Plant samples were collected from West Kordofan on January 2015. Botanical identification and authentication were performed and voucher specimens (No. 13/BP for B. papyrifera, No.13/CS for C. schoenanthus, No. 13/CZ for C. zambesicus and No. 13/CR for C. rotundus) have been deposited in Botany Department Herbarium,Faculty of Science, University of Khartoum, Sudan.
2.2. Preparation of essential oils
Essential oils from all the plant species (500 g) were extracted by hydrodistillation using a Clevenger-type apparatus for two to four hours. The extracted oils were dried over anhydrous sodium sulfate and stored at 4 ℃, in amber-coloured bottles, before use.
2.3. Gas chromatography/mass spectrometry (GC-MS)analysis
Analysis of the chemical composition of the essential oils were performed by gas chromatography coupled to mass spectrometry(Model GC-MS-QP2010 Plus, Shimadzu, Japan) equipped with a Rtx-5MS capillary column (5% diphenyl-95% dimethylsilicone, 30.00 m × 0.25 mm × 0.25 m). The oven temperature was programmed from 45 ℃ for 1 min and then increased at a rate of 3 ℃·min-1to 300 ℃, and held isothermally for 5 min. Helium was used as the carrier gas (with a flow rate of 1 mL·min-1). The detection was performed in the full scan mode, with a mass range of 50-650 m/z. Electron impact ionisation was employed with collision energy of 70 eV and the mass spectrometer ion source was maintained at 240 ℃.
The volatile compounds were identified by matching mass spectra with spectra of reference compounds present in the National Institute of Standards and Technology (NIST 08) mass spectral library and by comparison of its retention index (RI) relative to C10-C24n-alkanes[14]. The relative amounts of individual components were expressed as percent peak areas relative to the total peak area.
2.4. Cell viability assay
2.4.1. Cell culture
Anti-proliferative activities of respective essential oils were evaluated with four cell lines established from human breast carcinoma samples (MCF7 and MDA-MB231) and from human colon adenocarcinoma samples (HT29 and HCT116). HCT116 and HT29 cells were cultivated in Dulbecco’s minimum essential medium (DMEM, Eurobio, Courtaboeuf, France) supplemented with 10% (v/v) fetal calf serum (Eurobio), 1% Penicillin/ streptomycin (Eurobio) and 2 mM L-glutamine (Eurobio). MCF7 and MDA-MB231 cells were grown in RPMI medium with the same additives. Cells were routinely seeded at 100 000 cells /mL and maintained weekly in a humidified atmosphere of 5% CO2at 37 ℃.
2.4.2. MTT procedure
Cell viability assay was performed using the thiazolyl blue tetrazolium bromide (MTT) procedure as described by Mosman[15]. In brief, cancer cells were seeded in 96-well plate at 10 000 cells/ well for HT29, MCF-7 and MDA-MB231 cells, at 5 000 cells/ well for HCT116 cells (Greiner-Bio-One GmbH, Friekenhanusen,Germany). Twenty four hours after seeding, 100 μL of medium containing increasing concentrations of each essential oil (final concentration range from 0.5 to 400.0 μg/mL) were added to each well for 72 h at 37 ℃. Essential oils were firstly diluted in DMSO at 50 mg (w/v)/mL or 200 mg (w/v)/mL. After incubation, the medium was discarded and 100 μL/well of MTT solution (0.5 mg/mL diluted in DMEM or RPMI medium) were added and incubated for 2 h. Water-insoluble formazan blue crystals were finally dissolved in DMSO. Each plate was read at 570 nm. IC50was calculated using GraphPad Prism (GraphPad Software, La Jolla, CA, USA). Data are expressed as IC50± SD obtained from quadruplicate determinations of two independent experiments (n = 8).
2.5. Antioxidant activity assay
The antioxidant activity of essential oils was estimated by diphenyl 2 pycrilhydrazil (DPPH) assay adapted in 96-well plate[16]. Ascorbic acid was used as an antioxidant molecule reference(concentration range from 1 to 20 μg/mL). Each sample (starting from concentration at 200 mg/mL) was diluted in DMSO (1/2 to 1/64) and tested. After 30 min incubation in the dark at room temperature, plates were read at 515 nm. Every analysis was done in triplicate. Antioxidant EC50was calculated after the establishment of inhibition curve as a function of sample concentration. For each diluted sample, inhibition of DPPH oxidation was calculated using the formula:
Inhibition (expressed in percentage) = [(1-(absorbancedilutedsample/ absorbancecontrol)] ×100.
2.6. Antibacterial activity assay
2.6.1. Microorganisms
Standard strains of microorganism, obtained from Medicinal and Aromatic Institute of Research, National Research Center,Khartoum, were used in this study. The bacterial species used were the Gram-negative Escherichia coli (E. coli) (ATCC 25922)and Pseudomonas aeruginosa (P. aeruginosa) (ATCC27853), and Gram-positive Bacillus subtilis (B. subtilis) (ATCC 6633) and Staphylococcus aureus (S. aureus) (ATCC 25923).
2.6.2. Minimum inhibitory concentration (MIC) assay
The two-fold serial microdilution method described by Eloff[17]was used to determine the MIC values for the essential oils against bacteria growth. All dilutions were prepared under aseptic conditions. A volume of 100 μL of the oil (1 mg/mL) dissolved in DMSO (5%) in duplicate was serially diluted two-fold with sterile distilled water and 100 μL of bacterial culture in MH broth, corresponding to 106CFU/mL, was added to each well. Gentamicin and amoxicillin were used as positive controls and DMSO as negative control. Plates were incubated overnight at 37 ℃. Afterwards, 40 μl of 0.2 mg/mL of p-iodonitrotetrazolium violet (INT) was added to each well to indicate microbial growth. The colourless salt of tetrazolium acts as an electron acceptor and is reduced to a red coloured formazan product by biologically active organisms. The solution in wells remains clear or shows a marked decrease in intensity of colour after incubation with INT at the concentration where bacterial growth is inhibited. Plates were further incubated at 37 ℃ for 2 h and the MIC was determined as the lowest concentration inhibiting microbial growth, indicated by a decrease in the intensity of the red colour of the formazan. The experiment was performed in triplicate.
3. Results
3.1. GC/MS profiles of essential oils
3.1.1. B. papyrifera
The yield of volatile oil of olibanum of B. papyrifera was 3% (w/ w) on dry weight basis. The oil was pale yellow with agreable perfumery odour. A total of 16 compounds were identified comprised 99.30% of the essential oil (Tables 1 and 2). The essential oil was dominated by the presence of alcohol and ester derivatives(46.82%) followed by monoterpenes (16.30%), sesquiterpenes(27.70%) diterpenes (5.08%) and fatty acids which represent 5.51% of the total oil. Octyl acetate was the most prominent compound found in highest concentration (22.69%) followed by nerolidolepoxyacetate (18.83%), 1-octanol (16.16 %), (4E)-1,5,9-trimethyl-1-vinyl-4,8-decadienyl formate (7.97 %) and l-limonene (7.11 %)respectively.

Table 1Composition of chemical compound families identified in essential oil.
3.1.2. C. zambesicus
The yield of the light yellowish essential oil of the leafy stems of C. zambesicus was 0.28% (w/w). In total, 18 components were identified representing 93.15% of the total oil composition and results are presented in Tables 1 and 3. The oil was dominated with monoterpenes(69.84%) where oxygenated monoterpenes represented by 45.45%. Predominance of hydrocarbons (10.98%) over oxygenated (4.64%)derivatives stands for the sesquiterpene fraction. The composition of the oil was marked by the presence of 1,8-cineole (27.07%) followed by cymene (13.80%), α-terpineol (6.87%) and L-linalool (5.77%).
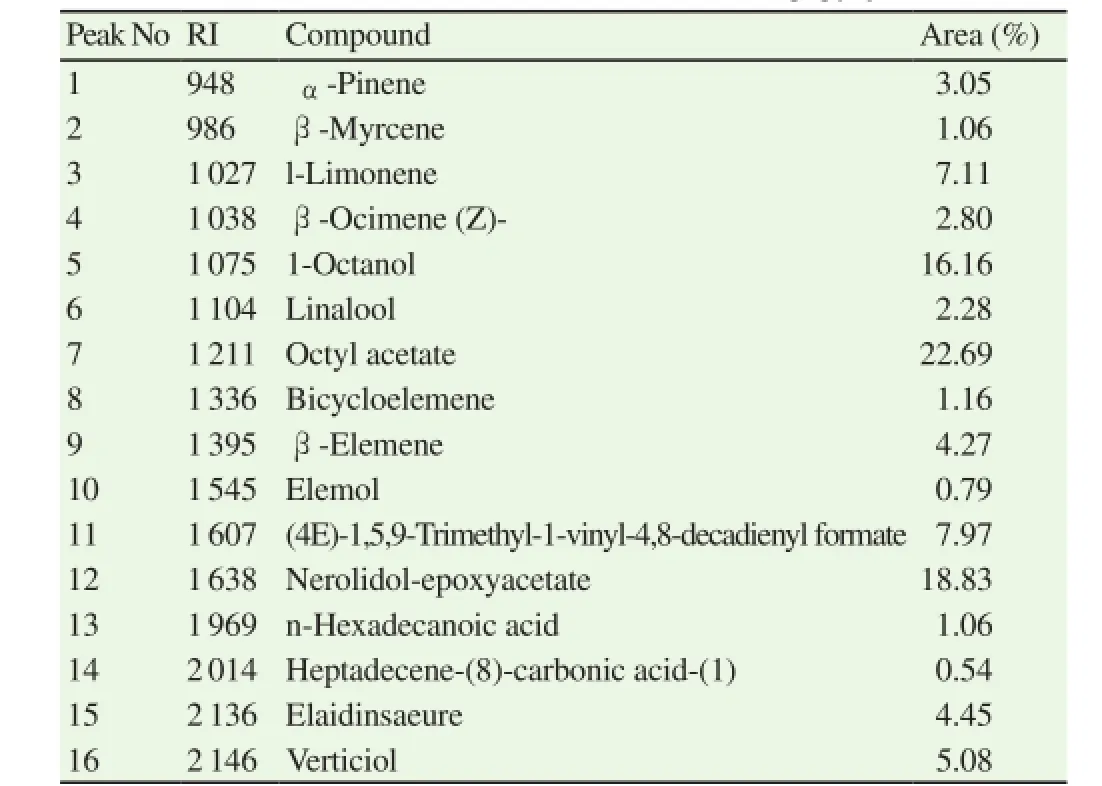
Table 2Chemical composition of essential oil extracted from B. papyrifera olibanum.

Table 3Chemical composition of essential oil extracted from C. zambesicus leafy stems.
3.1.3. C. schoenanthus
The dried leafy stems of C. schoenanthus yielded 2.1% (w/w)light yellowish oil. 49 components were identified representing 93.03% of the total oil composition (Tables 1 and 4). The proportion of sesquiterpenes (71.59%) was higher than that of monoterpenes (21.44%). The oil revealed the presence of monoterpenes (1.6%), oxygenated monoterpenes (19.84%), sesquiterpenes (11.02%), oxygenated and sesquiterpenes (60.57%) and other components as, ester and diterpene,were also detected at trace levels. The major components were the monoterpene piperitone (18.48%) followed by the sesquiterpenes elemol(18.33%), eudesm-11-en-1-ol (17.09%), α-eudesmol (10. 69%),bulnesol (7.08%) and γ-eudesmol (5.80%), respectively.
3.1.4. C. rotundus
Hydrodistillation of the dried rhizomes of C. rotundus yielded 2.6%(w/w) pale yellowish oil. A total of 58 components were identified representing 90.30 % of the total oil composition (Table 1 and 5). The oil was characterised by larger amounts of oxygenated sesquiterpenes(38.77%) and monoterperenes (14.33%). Hydrocarbons sesquiterpenes and monoterpenes represented 5.49% and 2.99% respectively. The major constituent of the oil was 2,5,9-trimethylcycloundeca-4,8-dienone (13.44%) followed alloaromadendrene oxide-(1) (8.47%),piperitone (7.37%), β-elemol (7.14%), selina-6-en-4-ol (7%) and longiverbenone (4.30%) respectively.

Table 4Chemical composition of essential oil extracted from C. schoenanthus leafy stems.

Table 5Chemical composition of essential oil extracted from C. rotundus rhizomes.
3.2. Biological activity
3.2.1. Cell viability assay
Essential oils of studied species were tested, in vitro, for their potential anti-proliferative activity against HT29, HCT116, MCF7 and MDA-MB231 cell lines (Table 6). C. schoenanthus, C. zambesicus and C. rotundus essential oils exhibited interesting anti-proliferative activity against HT29 and HCT116 cell lines with IC50values in the range of 19.9 - 28.8 μg/mL. B. papyrifera essential oil was active against HT29 cell line but with cell viability activities higher than 30 μg/mL. IC50values were estimated at 38.40-50.35 μg/mL when MCF7 cells were tested and at 41.30-53.77 μg/mL against MDAMB231 cell line.
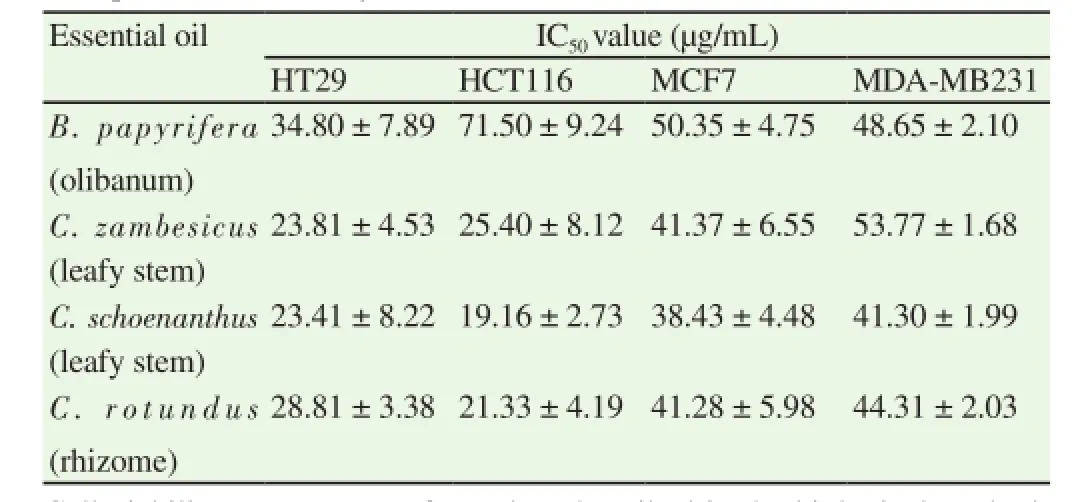
Table 6Anti-proliferative activity of essential oils.
3.2.2. Antioxidant activity
The potential antioxidant activity of the oils was determined according to the basis of scavenging activity of the stable free radical DPPH. Only essential oils of C. zambsicus and B. papyrifera possessed DPPH free radical scavenging activity with EC50estimated at (4.20 ± 0.19) and (5.90 ± 0.17) mg/mL while those C. rotundus and C. schoenanthus were inactive (Figure 1). The value EC50of ascorbic acid was (5.37 ± 0.44) μg/mL.

Figure 1. Antioxidant inhibition curve established for C. zambesicus (black circle) and B. papyrifera (white circle) essential oils.Antioxidant activity was determined as described in the biological methods section. Each point corresponds to mean ± SD of three determinations.
3.2.3. Antibacterial activity
Results of MIC for the four essential oils against E. coli, P. aeruginosa, B. subtilis and S. aureus are presented in Table 7. E. coli was inhibited by B. papyrifera, C. zambesicus and C. schoenanthus at 16 μg/mL and by C. rotundus at 32 μg/mL. P. aeruginosa was less susceptible to the oils, it was inhibited by C. rotundus and C. zambesicus at 126 and 250 μg/mL respectively. C. zambesicus and C. schoenanthus displayed antibacterial activity against B. subtilis and S. aureus with MIC value of 16 μg/mL while C. rotundus and B.papyrifera inhibited the former at 32 and 250 μg/mL respectively and the latter at 16 and 32 μg/mL respectively.

Table 7Antibacterial activity of essential oils of olibanum of B. papyrifera, leafy stems of C. schoenanthus and C. zambesicus and rhizome of C. rotundus.
4. Discussion
The chemical profile of olibanum of B. papyrifera in the present study was closer to that reported for B. papyrifera from Ethiopia[18]. Moreover, octyl acetate was the dominant compound but in lower concentration than those reported for samples collected in other countries[18-20]. Interestingly, the diterpene verticiol which represents 5.08% of the total oil has only been previously reported for Boswellia elongate collected from different locations in Yemen[21]. It could also be seen that some notable compounds such as incensole, incensyl acetate and geraniol, that were characteristics of B. papyrifera, were not identified in the present study.
Generally, hydrocarbons monoterpene presented the main fraction of most Croton species oils than their oxygenated counterparts and are marked with a high presence of β-caryophyllene and/or α/β -pinene as major constituents[22]. In this study the oil of leafy stems of C. zambesicus was dominated with oxygenated monoterpenes. The composition of the oil was marked by the presence of 1,8-cineole and cymene and the absence of borneol which was found to be in high amount in samples from Saudi Arabian and Cameroon[23,24]. Of note, 1,8-cineole was reported previously as the major compound from the essential oil of C. sakamaliensis stem from Madagascar[22]. Essential oil of leafy stems of C. schoenanthus was characterized by higher proportion of sesquiterpenes, however, samples collected from other countries like Burkina Faso[25], Togo[26], Tunisia[27],Algeria[28] and Brazil[29] showed that the proportion of monoterpenes was higher than that of sesquiterpenes. Moreover, the chemical composition of sample in this study was closer to those obtained from Burkina Faso and Togo species with piperitone (42%-61%) as the major oil compound[25,26,30-33].
The nature and proportion of compounds that constitute the essential oil of C. rotundus rhizomes in this study are not the same as those reported in previous studies; the rhizome oils of this plant from different countries showed compositional differences and four chemotypes (H-, K-, M- O-types), of the essential oils from different parts of Asia have been reported[34]. These four types besides oils of Brazilian[35], Germany[36], Hawaii[37,38], Nigerian[39]and Tunisian[40,41] species, were characterised by the presence ofα -cyperone, cyperotundone, cyperene, cyperol, cyperotundone which were not detected in the present work or by others on rhizomes samples from the Sudan[42].
It was clear that oils obtained from these aromatic plants growing in Sudan showed quantitative and qualitative differences. As reported earlier by several researchers, the plant organ that the oil was extracted from, time of harvest, geographical and environmental factors were the key factors influencing the chemical composition, quality and quantity of the plant essential oil and could probably contribute to create a unique and spectacular chemical composition[22,43].
The ranking order of the investigated plants essential oils on the basis of their anti-proliferative activity against HT29 cell line(in terms of IC50value) was C. schoenanthus> C. zambesicus> C. rotundus> B. papyrifera. Their ranking order on the basis of their antiproliferative activity against HCT116 and MCF7 cell lines was C. schoenanthus>C. rotundus>C. zambesicus>B. papyrifera. Thus, it was clear that C. schoenanthus revealed the best anti-proliferative activity. Terpenes and their derivatives were shown to possess antiproliferative and chemopreventive activities in various models[44]. For example, caryophyllene oxide, α-cadinol, α-humulene,α-pinene, β-pinene, α-phellandrene, d-limonene, linalool and trans-caryophyllene were previously reported to exhibit moderate to strong anti-proliferative effects against these or different tumor cell lines[44,45]. Moreover, although the major components reflect the biological activities of essential oils, many authors suggested that the bioactivity of an essential oil is hardly due to a single active compound, but it is rather ascribed to a synergic activity of different kinds of chemicals that may not be necessarily the most abundant[1,46].
Essential oils of C. zambesicus and B. papyrifera possessed interesting DPPH free radical scavenging activity. Generally,Brazilian Croton species; Croton zenthmeri, Croton nepetaefolius and Croton argyrophylloides exhibited good antioxidant activities[47]. Essential oil of stem bark of Croton lechleri was reported to show antioxidant property with IC50(DPPH) at (15.28 ± 0.94) mg/mL[48]. Antioxidant activity of olibanum of Boswellia apecies was previously evaluated for Boswellia socotrana, Boswellia elongate, Boswellia dioscorides where Boswellia socotrana exhibited high radical scavenging effect[21,49].
All oil samples inhibited both Gram positive and Gram negative bacteria. According to Salvat et al.[50], plant extracts with MIC’s less than/or around 500 μg/mL indicate good antibacterial activity. Thus,it could be suggested that the oils samples in this study showed good antibacterial activity against all tested bacteria except B. Papyrifera and C. Schoenanthus which were less activity against P. aeruginosa. Furthermore, oxygenated monoterpenes were reported to be responsible for the antimicrobial activity of several essential oils[51]. Our findings show for the first time that the essential oils extractedfrom C. schoenanthus, C. zambesicus and C. rotundusm with their complex mixtures of volatile substances, presented anti-proliferative property against several human cancer cell lines as demonstrated by cell viability assay. Moreover, they showed good antibacterial activity. Furthermore, essential oils of C. zambesicus and B. papyrifera possessed DPPH free radical scavenging activity. Thus, these results demonstrated the potential of these oils to exert beneficial antiproliferative and antibacterial effect and could be a natural source for antioxidant agents. Further studies are needed to isolate active compounds of these essential oils and to investigate in depth their modes of action.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
Authors would like to acknowledge Prof. Maha Kordofani (Botany Department, Faculty of Science, University of Khartoum) for the identification of the plants.
[1] Voon CH, Bhat R, Rusul G. Flower extracts and their essential oils as potential antimicrobial agents for food uses and pharmaceutical applications. Comp Rev Food Sci Food Saf 2012; 11(1): 34-55.
[2] Shaaban HAE, El-Ghorab AH, Shibamoto T. Bioactivity of essential oils and their volatile aroma components. J Essential Oil Res 2012; 24(2): 203-212.
[3] Lawal OA, Ogunwande IA. Essential oils from medicinal plants of Africa. In: Kuete V, editor. Medicinal plant research in Africa. London: Elsevier Ldt; 2013. p. 203-224.
[4] Ahmad A, Viljoen A. The in vitro antimicrobial activity of Cymbopogon essential oil (lemon grass) and its interaction with silver ions. Phytomed 2015; 22(6): 657-665.
[5] Elghazali GB, El Tohami MS, El Egami AB, Abdalla WS, Mohammed MG. Medicinal plants of the Sudan, Part 4. Medicinal plants of Northern Kordofan. In: Research Institute. National Center for Research, editor. Medicinal and aromatic plants. 1st ed. Khartoum: Omdurman Islamic University Press; 1997, p. 59.
[6] El Ghazali GE. The promising medicinal plants of the Sudan. 1st ed. Khartoum: University Press; 1997, p. 63.
[7] Anonym. Medicinal and aromatic plants of the Sudan. 1st ed. Khartoum:Arrow P. Press; 1982, p. 11.
[8] Elghazali GB, El Tohami MS, El Egami AB. Medicinal plants of the Sudan: Part 3. Medicinal plants of the White Nile Province. In: Research Institute. National Center for Research, editor. Medicinal and aromatic plants. 1st ed. Khartoum: University Press; 1994, p. 50.
[9] Chen Z, He B, Zhou J, He D, Deng J, Zeng R. Chemical compositions and antibacterial activities of essential oils extracted from Alpinia guilinensis against selected foodborne pathogens. Ind Crops Prod 2016;83: 607-613.
[10] Hossain F, Follett P, Vu KD, Harich M, Salmieri S, Lacroix M. Evidence for synergistic activity of plant-derived essential oils against fungal pathogens of food. Food Microbiol 2016; 53(Part B): 24-30.
[11] Gavanji S, Sayedipour SS, Larki B, Bakhtari A. Antiviral activity of some plant oils against herpes simplex virus type 1 in Vero cell culture. J Acute Med 2015; 5(3): 62-68.
[12] Essid R , Rahali FZ, Msaada K , Sghair I, Hammami M, Bouratbine A, et al. Antileishmanial and cytotoxic potential of essential oils from medicinal plants in Northern Tunisia. Ind Crops Prod 2015; 77: 795-802.
[13] Park SE, Shin WT, Park C, Hong SH, Kim GY, Kim SO, et al. Induction of apoptosis in MDA-MB-231 human breast carcinoma cells with an ethanol extract of Cyperus rotundus L. by activating caspases. Oncol Rep 2014; 32(6): 2461-2470.
[14] Adams RP. Identification of essential oils components by gas chromatography/quadrupole mass spectroscopy. 4th ed. Carol Stream:Allured Publishing Corporation; 2001.
[15] Mosmann T. Rapid colorimetric assay for cellular growth and survival:application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65(1-2): 55-63.
[16] Molyneux P. The use of the stable free radical diphenylpicryl-hydrazyl(DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol 2004; 26(2): 211-219.
[17] Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Medica 1998; 64(8): 711-713.
[18] Bekana D, Kebede T, Assefa M, Kassa H. Comparative phytochemical analyses of resins of Boswellia Species (B. papyrifera (Del.) Hochst., B. neglecta S. Moore, and B. rivae Engl.) from Northwestern, Southern, and Southeastern Ethiopia. ISRN Anal Chem 2014; 2014(2014): Article ID 374678.
[19] Hamm S, Bleton J, Connan J, Tchapla A. A chemical investigation by headspace SPME and GC-MS of volatile and semi-volatile terpenes in various olibanum samples. Phytochem 2005; 66(12):1499-1514.
[20] Camarda L, Dayton T, Di Stefano V, Pitonzo R, Schillaci D. Chemical composition and antimicrobial activity of some oleogum resin essential oils from Boswellia spp. (Burseraceae). Annali di Chimica 2007; 97(9):837-844.
[21] Awadh-Ali NA, Wurster M, Arnold N, Teichert A, Schmidt J, Lindequist U, et al. Chemical composition and biological activities of essential oils from the Oleogum resins of three endemic Soqotraen Boswellia species. Rec Nat Prod 2008; 2: 6-12.
[22] Radulovi N, Mananjarasoa E, Harinantenaina L, Yoshinori A. Essential oil composition of four Croton species from Madagascar and their chemotaxonomy. Biochem Syst Ecol 2006; 34(8): 648-653.
[23] Mekkawi AG. The essential oil of Croton zambesicus. Fitoterpia 1985; 56:181-183.
[24] Boyom FF, Keumedjio F, Jazet DPM, Ngadjui BT, AmvamZollo PH,MenutC, et al. Essential oils from Croton zambesicus Muell. Arg. growing in Cameroon. Flavour Fragrance J 2002; 17(3): 215-217.
[25] Onadja Y, Ouedraogo A, Samate AD. Chemical composition and physical characteristics of the essential oil of Cymbopogon schoenanthus (L.)Spreng of Burkina Faso. J Appl Sci 2007; 7(4): 503-506.
[26] Ketoh GK , Koumaglo HK, Glitho IA. Inhibition of Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) development with essential oil extracted from Cymbopogon schoenanthus L. Spreng. (Poaceae), and the wasp Dinarmus basalis (Rondani) (Hymenoptera: Pteromalidae). J Stored Prod Res 2005; 41(4): 363-371.
[27] Khadri A, Serralheiro MLM, Nogueira JMF, Neffati M, Smiti S, Araújo MEM. Antioxidant and antiacetylcholinesterase activities of essential oils from Cymbopogon schoenanthus L. Spreng. Determination of chemical composition by GC-mass spectrometry and 13C NMR. Food Chem 2008; 109(3): 630-637.
[28] de Sousa EMBD, Camara APC, Costa WA, Costa ACJ, Oliveira HNM,Galvao EL, et al. Evaluation of the extraction process of the essential oil from Cymbopogon schoenanthus with pressurized carbon dioxide. Braz Arch BiolTechn 2005; 48: 231-236.
[29] Katiki LM , Chagas ACS, Bizzo HR , Ferreira JFS, Amarante AFT. Anthelmintic activity of Cymbopogon martinii, Cymbopogon schoenanthus and Mentha piperita essential oils evaluated in four different in vitro tests. Vet Parasitol 2011; 183(2011): 103-108.
[30] Koba K, Sanda K, Raynaud C, Nenonene YA, Millet J, Chaumont JP. Activités antimicrobiennes d’huiles essentielles de trois Cymbopogonsp africains vis-àvis de germes pathogènes d’animaux de compagnie. Ann Méd Vét 2004; 148: 202-206.
[31] Ketoh GK, Koumaglo HK, Glitho IA, Huignard J. Comparative effects of Cymbopogon schoenanthus essential oil and piperitone on Callosobruchus maculatus development. Fitoterapia 2006; 77(7-8): 506-510.
[32] Yentema O, Alioune O, Dorosso SA. Chemical composition and physical characteristics of the essential oil of Cymbopogon schoenanthus (L.)Spreng of Burkina Faso. J Appl Sci 2007; 7(4): 503-506.
[33] Bossoua AD, Ahoussi E, Ruysbergh E, Adams A, Smagghe G, De Kimpe N, et al. Characterization of volatile compounds from three Cymbopogon species and Eucalyptus citriodora from Benin and their insecticidal activities against Tribolium castaneum. Ind Crops Prod 2015; 76: 306-317.
[34] Jirovetz L, Wobus A, Buchbauer G, Shafi MP, Thampi PT. Comparative analysis of the essential oil and SPME-headspace aroma compounds of Cyperus rotundus L. roots/tubers from South-India using GC, GC-MS and olfactometry. J Essent Oil-Bearing Plants 2004; 7(2): 100-106.
[35] Zoghbi MDGB, Andrade EHA, Carreira LMM, Rocha EAS. Comparison of the main components of the essential oils of ‘priprioca’: Cyperus articulates var. articulatus L., C. articulatus var. nodosus L., C. prolixus Kunth and C. rotundus L. J Essent Oil Res 2008; 20(1): 42-46.
[36] Sonwa MM , Koenig WA. Chemical study of essential oil Cyperus rotundus. Phytochem 2001; 58(5): 799-810.
[37] Komai K , Tang C. A chemotype of Cyperus rotundus in Hawaii. Phytochem 1989; 28(7): 1883-1886.
[38] Komai K, Shimizu M, Tang CT, Tsutsui H. Sesquiterpenoids of Cyperus bulbosus, Cyperus tuberosus and Cyperus rotundus. Mem Fac Agr Kinki Univ 1994; 27: 39-45.
[39] Ekundayo O, Oderinde R, Ogundeyin M , Biskup ES. Essential oil constituents of Cyperus tuberosus Rottb Rhizomes. Flav Fragr J 1991;6(4): 261-264.
[40] Kilani S, Ledauphin J, Bouhlel I, Ben Sghaier M, Boubaker J, Skandrani I, et al. Comparative study of Cyperus rotundus essential oil by a modified GC/MS analysis method. Evaluation of its antioxidant, cytotoxic and apoptotic effects. Chem Biodivers 2008; 5(5): 729-742.
[41] Kilani S, Abdelwahed A, Chraief I, Ben Ammar R, Hayder N, Hammami M, et al. Chemical composition, antibacterial and antimutagenic activities of essential oil from (Tunisian) Cyperus rotundus. J Essent Oil Res 2005;17: 695-700.
[42] Eltayeib AA, Um Ismaeel H. Extraction of Cyperus rotundus rhizomes oil,identification of chemical constituents and evaluation of antimicrobial activity of the oil in North Kordofan state. Int J Adv Res Chem Sci 2014;1(9): 18-29.
[43] Olivero-Verbel J, Nerio LS, Stashenko EE. Bioactivity against Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) of Cymbopogon citratus and Eucalyptus citriodora essential oils grown in Colombia. Pest Manage Sci 2010; 66: 664-668.
[44] Kuttan G, Pratheeshkumar P, Manu KA, Kuttan R. Inhibition of tumor progression by naturally occuringterpenoids. Pharm Biol 2011; 49(10):995-1007.
[45] Dar MY, Shaha WA, Mubashir S, Rather MA. Pinus wallichina essential oil growing in high altitude areas of Kashmir, India. Phytomed 2012; 19:1228-1233.
[46] Ramos JO, Santos CA, Santana DG, Santos DA, Alves PB, Thomazzi SM. Chemical constituents and potential antiinfla mmatory activity of the essential oil from the leaves of Croton argyrophyllus. Rev Bras Farmacogn 2013; 23(4): 644-650.
[47] Morais de SM, Catunda Junior FEA, da Silva ARA, Neto JSM, Rondina D, Cardoso JHL. Antioxidant activity of essential oils from Northeastern Brazilian Croton species. Quim Nova 2006; 29(5): 907-910.
[48] Rossi D, Guerrini A, Maietti S, Bruni R, Paganetto G, Poli F, et al. Chemical fingerprinting and bioactivity of Amazonian Ecuador Croton lechleri Müll. Arg. (Euphorbiaceae) stem bark essential oil: A new functional food ingredient? Food Chem 2011; 126(3): 837-848.
[49] Ruberto G, Barata MT. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem 2000; 69(2): 167-174.
[50] Salvat A , Antonacci L , Fortunato RH , Suarez EY, Godo HM. Antimicrobial activity in methanolic extracts of several plant species from Northern Argentina. Phytomedicine 2004; 11(2-3): 230-234.
[51] Mothana RAA , Hasson SS , Schultze W, Mowitz A , Lindequist Y. Phytochemical composition and in vitro antimicrobial and antioxidant activities of essential oils of three endemic Soqotraen Boswellia species. Food Chemistry 2011; 126(3): 1149-1154.
10.1016/j.apjtm.2016.06.009
Dr Sakina Yagi, Associate professor (phD), Botany Department - Faculty of Science, University of Khartoum, PO Box 321, Khartoum Sudan.
Tel: + 249 9 15030005
E-mail: sakinayagi@gmail.com
✉Corresponding author: Herve Schohn, Universite de Lorraine-UMR CNRS 7565-Structure et Reactivite des Systemes Moleculaires Complexes-Campus Bridoux-rue du General Delestraint-57070 Metz Cedex, France.
Tel: +33 3 87 37 84 09
E-mail: herve.schohn@uiv-lorraine.fr Fax: + 33 3 87 31 58 01
ARTICLE INFO
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- State of the art in neurocysticercosis
- Insight of ZnS nanoparticles contribution in different biological uses
- Zika: As an emergent epidemic
- Toxoplasmosis and anti-Toxoplasma effects of medicinal plant extracts-A mini-review
- Unlocking the in vitro anti-Trypanosoma cruzi activity of halophyte plants from the southern Portugal
- Development and application of quantitative detection method for nervous necrosis virus (NNV) isolated from sevenband grouper Hyporthodus septemfasciatus
