RIPK3基因转染的SH-SY5Y细胞中HIF-1α基因及其信号通路相关基因表达变化
2016-04-18张国禄程世翔徐忠伟衣泰龙廖吉连涂悦张赛
张国禄,程世翔,徐忠伟,衣泰龙,廖吉连,涂悦,张赛
(1武警后勤学院附属医院脑科医院*,天津300162;2武警后勤学院中心实验室)
RIPK3基因转染的SH-SY5Y细胞中HIF-1α基因及其信号通路相关基因表达变化
张国禄1,程世翔1,徐忠伟2,衣泰龙1,廖吉连1,涂悦1,张赛1
(1武警后勤学院附属医院脑科医院*,天津300162;2武警后勤学院中心实验室)
摘要:目的观察受体相互作用蛋白激酶3(RIPK3)基因转染的神经母细胞瘤细胞系SH-SY5Y中低氧诱导因子1α(HIF-1α) mRNA及其信号通路相关基因表达变化。方法 构建表达RIPK3基因的pCMV6-AC-GFP质粒(重组质粒),培养SH-SY5Y细胞,分为实验组及对照组,分别转染重组质粒和空载质粒。采用Western blotting法检测细胞中的RIPK3蛋白,分别于培养8、14、20、26、32、38 h后,通过MTT实验检测细胞增殖情况(OD值)。采用转录组测序技术(RNAseq)及Ingenuity Pathway Analysis(IPA)软件检测并筛选RIPK3-HIF1α下游信号通路中的关键基因。采用微滴式数字PCR(ddPCR)检测两组细胞中的HIF-1α mRNA。结果实验组细胞中RIPK3蛋白相对表达量(0.806±0.097 5)高于对照组(0.455±0.088 6),P<0.05。随培养时间延长,实验组细胞增殖受到抑制。实验组细胞中HIF-1α mRNA相对表达量(0.015 43±0.003 47)低于对照组(0.046 28±0.010 26),P<0.05。在HIF-1α为核心的相互作用关系网络中,筛选出关键分子泛素缀合酶样蛋白(UBC)、希佩尔-林道蛋白(VHL)、转录延伸因子B多肽1(TCEB1)、血管内皮生长因子A(VEGFA)。结论 RIPK3基因转染SH-SY5Y后,细胞中HIF-1α mRNA表达下调,同时HIF-1α信号通路相关基因(UBC、VHL、TCEB1、VEGFA)的表达水平受到影响。
关键词:神经母细胞瘤;受体相互作用蛋白激酶3;低氧诱导因子1α;泛素缀合酶样蛋白;希佩尔-林道蛋白;转录延伸因子B多肽;血管内皮生长因子A
受体相互作用蛋白激酶3(RIPK3)属于受体相互作用蛋白家族(RIPs)成员。作为肿瘤坏死因子(TNF)重要的下游效应分子,RIPK3广泛表达于人体各组织,并被证实在细胞增殖、凋亡过程中发挥重要作用[1~4]。低氧诱导因子1α(HIF-1α)表达于所有已知的多细胞物种[5],是组织适应缺氧环境的重要转录因子[6],在缺血再灌注、肿瘤及黏膜炎症等进程中发挥作用[7, 8]。本研究建立了稳定表达RIPK3的人神经母细胞瘤细胞模型,观察细胞中HIF-1α及其下游信号分子转录水平的变化,探索RIPK3的潜在作用及其信号通路网络。
1材料与方法
1.1实验细胞与试剂人神经母细胞瘤细胞系SH-SY5Y购自美国标准生物品收藏中心(ATCC),细胞接种于含有10 %胎牛血清(Gibicol公司)、90% F12与DMEN配比为1∶1的完全培养基(Gibicol公司)中,37 ℃、5% CO2环境下培养,取对数生长期细胞用于实验。质粒小提中量试剂盒(天根公司),Lipofectamine3000(Life公司),TRIzol(Invitrogen公司),SYBR Green试剂盒(Roche公司),β-actin抗体(Sigma公司),RIPK3抗体(Abcam公司),辣根过氧化物酶标记的羊抗兔、羊抗小鼠二抗(KPL公司),QX200 ddPCR EvaGreen Supermix(Bio-Rad公司)。
1.2表达RIPK3的pCMV6-AC-GFP质粒的获取将RIPK3(NM_006871) Human cDNA ORF Clone质粒及对应空载质粒分别转化DH5-α感受态大肠杆菌,菌液接种于含氨苄青霉素(50 μg/mL)的LB固体培养基中,37 ℃培养过夜。挑取单个菌落接种于含氨苄青霉素(50 μg/mL)的液体LB培养基中,37 ℃恒温摇床震荡16 h,提取质粒DNA,测定浓度后分装,-80 ℃保存。
1.3质粒转染及RIPK3蛋白检测SH-SY5Y细胞接种于6孔板中,分为对照组与实验组。将Lipofectamine3000试剂分别与空载质粒及RIPK3过表达质粒DNA混合构成质粒-脂质体复合体,孵育后的两种质粒-脂质体复合体分别加入对照组与实验组培养孔中孵育2~4 d。以含G418(1 000 μg/mL)的完全培养基筛选转染细胞以获取稳定转染的SH-SY5Y细胞,传代2次后G418浓度减为800 μg/mL维持培养,并冻存备用。取两组对数生长期细胞分别提取蛋白,BCA定量法测定蛋白浓度后分装置于-80 ℃保存。采用Western blotting法检测RIPK3蛋白。

1.5SH-SY5Y细胞中HIF-1α mRNA检测采用微滴式数字PCR(ddPCR)检测两组细胞中的HIF-1α mRNA。HIF-1α、GAPDH基因引物序列见表1。采用美国Bio-Rad公司生产的QX200 ddPCR系统,将由MILIQ水6 μL、cDNA 2 μL、上游引物1 μL、下游引物1 μL及ddPCR EvaGreen Supermix构成的反应体系进行微滴化处理,形成约20 000个微滴,将微滴转移到PCR管中进行扩增,完成PCR反应后90 ℃维持5 min以稳定微滴,对扩增产物进行信号采集及数据分析,获得基因绝对浓度,以目的基因浓度/GAPDH基因浓度计算目的基因相对表达量。

表1 HIF-1α、GAPDH基因引物序列
1.6HIF-1α信号通路相关基因的筛选与检测提取两组细胞总RNA,用带有多加多聚T尾标记的磁珠及试剂盒处理,以获取高纯度mRNA。将纯化的mRNA打断为短片段,以此为模板合成双链cDNA。纯化cDNA并进行末端修复、链接多聚A尾及测序接头,选择合适长度的片段进行PCR反应,获得最终的cDNA文库。对cDNA进行检测,确认符合实验要求后进行RNAseq。测序结果经Ingenuity Pathway Analysis(IPA)软件进行数据分析。筛选RIPK3-HIF-1α信号通路相关信号分子,将这些基因输入到STRING10数据库网站(http://string-db.org)中获得其相互作用网络。相关基因表达检测通过RNAseq技术完成。
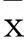
2结果
2.1两组细胞中RIPK3蛋白表达变化实验组与对照组细胞中RIPK3蛋白相对表达量分别为0.45±0.11、0.81±0.12,两组相比,P<0.05。
2.2两组细胞增殖情况比较随培养时间延长,实验组细胞增殖受到抑制,培养20、26、32、38 h时两组OD值差异有统计学意义。见表2。
表2 两组细胞增殖情况比较±s)
注:与实验组相比,*P<0.05,**P<0.01。2.3两组细胞中HIF-1α mRNA表达比较RNAseq结果显示实验组、对照组细胞中HIF-1α mRNA相对表达量分别为0.046 282 1、0.015 431 0。ddPCR结果显示,实验组、对照组细胞中HIF-1α mRNA相对表达量分别为0.004 00±0.000 347、0.001 11±0.000 163;实验组细胞中HIF-1α mRNA相对表达量低于对照组(P均<0.05),与RNAseq结果一致。
2.4HIF-1α信号通路相关基因筛选及其表达在HIF-1α为核心的相互作用关系网络中,共筛选出四种相关分子:泛素缀合酶样蛋白(UBC)、希佩尔-林道蛋白(VHL)、转录延伸因子B多肽1(TCEB1)、血管内皮生长因子A(VEGFA)。
3讨论
RIPK3作为细胞程序性坏死信号通路中的重要一环越来越引起学者的关注。作为TNF-α下游重要的信号分子,RIPK3参与天冬氨酸特异酶切的半胱氨酸蛋白酶88(Caspase-8)及核转录因子κB(NF-κB)活化等过程[9~12]。然而,RIPK3在神经系统中的作用机制尚待进一步研究。本实验以SH-SY5Y细胞为基础建立了稳定过表达RIPK3基因的神经元细胞模型[13],并通过RNAseq及IPA数据分析寻找潜在的RIPK3下游效应分子。本实验发现,SH-SY5Y细胞转染RIPK3基因后,细胞中HIF-1α的表达水平明显下调。
HIF-1α是一种重要的氧敏感转录因子。HIF的一个重要作用是维持氧供需平衡,以减少活性氧簇(ROS)的生成[14]。在氧充足情况下,HIF-1α与VHL结合,后者能聚集泛素连接酶标记HIF-1α而使HIF-1α降解;UBC在此泛素化的过程中发挥重要作用,将泛素从泛素相关蛋白(UBA)的巯基位点转移至蛋白底物上,进而进入泛素依赖性蛋白水解途径[15]。有报道[16, 17]指出,HIF-1α可直接或间接调节血管内皮细胞中2%以上的基因。最近研究[18]显示,HIF-1α在成人体内能通过诸多信号通路促进组织再生,其机制包括从转录水平激活修复基因及其受体,如VEGF、胎盘生长因子(PLGF)、血小板衍生因子(PDGF)、血管生成素1(ANGPT1)[19];通过调节促血管生成因子及其受体如1-磷酸神经鞘氨醇(S1P)[20]、趋化因子受体4(CXCR4),从而促进内皮祖细胞在缺氧部位聚集[21];通过调控参与细胞周期和DNA复制过程的基因从而促进内皮细胞增殖、分化[22]。本研究中,HIF-1α及其信号通路相关分子构成一个复杂的信号网络。有学者[23]发现,海马神经元损伤后,RIPK3表达明显上调且集中分布于细胞膜,这种效应独立于RIPK1的调控。本研究结果显示,转染RIPK3基因可对SH-SY5Y细胞中HIF-1α及其信号通路相关分子表达水平造成影响,推测RIPK3在神经系统损伤修复过程中发挥十分重要的作用,通过控制RIPK3的活性有望促进神经组织再生与修复。
由于客观因素限制,我们仅从表观对RIPK3过表达引起的HIF-1α及其信号通路相关分子表达变化进行观察,且仅对HIF-1α进行验证,未能进一步研究RIPK3与HIF-1α具体的相互作用关系,这仍需通过后续实验进一步验证。
参考文献:
[1] Yu PW, Huang BC, Shen M, et al. Identification of RIP3, a RIP-like kinase that activates apoptosis and NFkappaB[J]. Curr Biol, 1999,9(10):539-542.
[2] Vanden Berghe T, Linkermann A., Jouan-Lanhouet S, et al. Regulated necrosis: the expanding network of non-apoptotic cell death pathways[J]. Nat Rev Mol Cell Biol, 2014,15(2):135-147.
[3] Kikuchi M, Kuroki S, Kayama M, et al. Protease activity of procaspase-8 is essential for cell survival by inhibiting both apoptotic and nonapoptotic cell death dependent on receptor-interacting protein kinase 1 (RIP1) and RIP3[J]. J Biol Chem, 2012,287(49):41165-41173.
[4] Moriwaki K, Chan FK. Necrosis-dependent and independent signaling of the RIP kinases in inflammation[J]. Cytokine Growth Factor Rev, 2014,25(2):167-174.
[5] Loenarz C, Coleman ML, Boleininger A, et al. The hypoxia-inducible transcription factor pathway regulates oxygen sensing in the simplest animal, Trichoplax adhaerens[J]. EMBO Rep, 2011,12(1):63-70.
[6] Semenza GL. Hypoxia-inducible factors in physiology and medicine[J]. Cell, 2012, 148(3):399-408.
[7] Ruggieri MR Sr. Mechanisms of disease: role of purinergic signaling in the pathophysiology of bladder dysfunction[J]. Nat Clin Pract Urol, 2006, 3(4):206-215.
[8] Eckle T, Hartmann K, Bonney S, et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia[J]. Nat Med, 2012, 18(5):774-782.
[9] Belizario J, Vieira-Cordeiro L, Enns S. Necroptotic cell death signaling and execution pathway: lessons from knockout mice[J]. Mediators Inflamm, 2015, 2015:128076.
[10] Amini P, Stojkov D, Wang X, et al. NET formation can occur independently of RIPK3 and MLKL signaling[J]. Eur J Immunol, 2016,46(1):178-184.
[11] Cuda CM, Misharin AV, Khare S, et al. Conditional deletion of caspase-8 in macrophages alters macrophage activation in a RIPK-dependent manner[J]. Arthritis Res Ther, 2015,17:291.
[12] Moriwaki K, Balaji S, McQuade T, et al. The necroptosis adaptor RIPK3 promotes injury-induced cytokine expression and tissue repair[J]. Immunity, 2014, 41(4):567-578.
[13] Jia DP, Wang S, Zhang BC, et al. Paraptosis triggers mitochondrial pathway-mediated apoptosis in Alzheimer′s disease[J]. Exp Ther Med, 2015, 10(2):804-808.
[14] Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations[J]. J Clin Invest, 2013,123(9):3664-3671.
[15] Miro-Murillo M, Elorza A, Soro-Arnaiz I, et al. Acute VHL gene inactivation induces cardiac HIF-dependent erythropoietin gene expression[J]. PLoS One, 2011, 6(7):e22589.
[16] Park C, Kim TM, Malik AB. Transcriptional regulation of endothelial cell and vascular development[J]. Circ Res, 2013,112(10):1380-1400.
[17] Mistry IN, Smith PJ, Wilson DI, et al. Probing the epigenetic regulation of HIF-1alpha transcription in developing tissue[J]. Mol Biosyst, 2015,11(10):2780-2785.
[18] Pan XY, Zhang ZH, Wu LX, et al. Effect of HIF-1a/VEGF signaling pathway on plasma progesterone and ovarian prostaglandin F(2)a secretion during luteal development of pseudopregnant rats[J]. Genet Mol Res, 2015, 14(3):8796-8809.
[19] Zou D, Zhang Z, He J, et al. Blood vessel formation in the tissue-engineered bone with the constitutively active form of HIF-1alpha mediated BMSCs[J]. Biomaterials, 2012,33(7):2097-2108.
[20] Kalhori V, Kemppainen K, Asghar MY, et al. Sphingosine-1-Phosphate as a Regulator of Hypoxia-Induced Factor-1alpha in Thyroid Follicular Carcinoma Cells[J]. PLoS One, 2013,8(6):e66189.
[21] Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1[J]. Nat Med, 2004,10(8):858-864.
[22] Nauta TD, van Hinsbergh VW, Koolwijk P. Hypoxic signaling during tissue repair and regenerative medicine[J]. Int J Mol Sci, 2014,15(11):19791-19815.
[23] Yin B, Xu Y, Wei RL, et al. Inhibition of receptor-interacting protein 3 upregulation and nuclear translocation involved in Necrostatin-1 protection against hippocampal neuronal programmed necrosis induced by ischemia/reperfusion injury[J]. Brain Res, 2015,1609:63-71.
Expression changes of HIF-1α and its signaling pathway related gene in SH-SY5Y cells transfected by RIPK3 gene
ZHANGGuolu1,CHENGShixiang,XUZhongwei,YITailong,LIAOJilian,TUYue,ZHANGSai
(1Neurology&NeurosurgeryHospitalAffiliatedtoLogisticalCollegeofChineseArmedPoliceForces,Tianjin300162,China)
Abstract:ObjectiveTo observe the expression changes of hypoxia-inducible factor 1α (HIF-1α) and its signaling pathway related gene in the neuroblastoma cell line SH-SY5Y transfected by receptor interacting serine/threonine kinase 3 (RIPK3) gene. MethodsThe pCMV6-AC-GFP plasmid expressing RIPK3 gene (recombinant plasmid) was constructed. SH-SY5Y cells were cultured and then were transfected with the recombinant plasmid and empty vector plasmid as the experimental group and control group respectively. Expression of RIPK3 protein was detected by Western blotting, and the OD value was detected by MTT assay at 8, 14, 20, 26, 32 and 38 h. RNA transcriptome sequencing (RNAseq) and Ingenuity Pathway Analysis (IPA) was used to detect and screen the key genes in the downstream signaling pathway of RIPK3-HIF-1α. Droplet Digital PCR (ddPCR) was used to detect the HIF-1α mRNA in the two groups. ResultsThe RIPK3 expression in the experimental group (0.806±0.097 5) was higher than that of the control group (0.455±0.088 6), P<0.05. The proliferation of SH-SY5Y was inhibited as the cell incubation time was prolonged. The expression of HIF-1α mRNA in the experimental group (0.01543±0.00347) was strongly down-regulated as compared with that of the control group (0.04628±0.01026) (P<0.05). In the interaction network of HIF-1α which was taken as the core, we screened the key molecules such as ubiquitin-conjugating enzyme (UBC), von Hippel-Lindau (VHL), transcription elongation factor B polypeptide 1 (TCEB1) and vascular endothelial growth factor A (VEGFA). ConclusionAfter the SY5Y cells were trasfected by RIPK3, the HIF-1α mRNA expression was down-regulated in SH, and the expression levels of HIF-1α signaling pathway-related genes (UBC, VHL, TCEB1 and VEGFA) were affected.
Key words:neuroblastoma; receptor interacting serine/threonine kinase 3; hypoxia-inducible factor 1α; ubiquitin-conjugating enzyme-like protein; Von Hippel-Lindau protein; transcription elongation factor B polypeptide 1; vascular endothelial growth factor A
(收稿日期:2015-09-28)
中图分类号:R34
文献标志码:A
文章编号:1002-266X(2016)07-0013-04
doi:10.3969/j.issn.1002-266X.2016.07.005
通信作者简介:张赛(1956-),男,医学博士、教授、主任医师,主要研究方向为神经创伤及危重症。E-mail: zhangsai718@vip.126.com
作者简介:第一张国禄(1990-),男,硕士研究生在读,从事神经外科专业的研究。E-mail: zhangguolu.wjyxy@163.com
基金项目:国家自然科学基金项目(31200809);武警部队后勤科研项目(WJHQ2012-20)。
*暨天津市神经创伤修复重点实验室、武警部队脑创伤与神经疾病研究所。
