Elevated thyroid stimulating hormone levels are associated with metabolic syndrome in a Chinese community-based population of euthyroid people aged 40 years and older
2016-04-18BojinXuHuiYangZhixiaoWangTaoYangHongweiGuoPeiChengWeiHeMinSunHuanhuanChenYuDuan
Bojin Xu,Hui Yang,Zhixiao Wang,Tao Yang,Hongwei Guo,Pei Cheng,Wei He,Min Sun, Huanhuan Chen,Yu Duan
Department of Endocrinology,The First Aff i liated Hospital,Nanjing Medical University,Nanjing,Jiangsu 210029,China.
Elevated thyroid stimulating hormone levels are associated with metabolic syndrome in a Chinese community-based population of euthyroid people aged 40 years and older
Bojin XuΔ,Hui YangΔ,Zhixiao Wang,Tao Yang,Hongwei Guo,Pei Cheng,Wei He,Min Sun, Huanhuan Chen,Yu Duan✉
Department of Endocrinology,The First Aff i liated Hospital,Nanjing Medical University,Nanjing,Jiangsu 210029,China.
This study investigated whether high-normal thyrotropin(TSH)levels are associated with metabolic syndrome in euthyroid Chinese people≥40 years old.Clinical and metabolic factors were assessed in 2,356 subjects(40–77 years old)with TSH levels in the normal range(0.35–5.00 mU/L).Using 2.50 mU/L as the cut-off point of TSH level within the normal range,we divided subjects into the high-TSH(2.50–5.00 mU/L;n=1,064)and low-TSH(0.35–2.50 mU/L;n=1,292)group.The results showed that the mean levels of body mass index(BMI),total cholesterol(TC), low density lipoprotein cholesterol(LDL-C),and fasting plasma glucose(FPG)were higher in the high-TSH group and TSH levels were signif i cantly positively correlated with BMI,LDL-C,TC,and FPG.The prevalence of central obesity,hypertriglyceridemia,low high density lipoprotein cholesterol(HDL-C),and high FPG(>5.60 mmol/L)was signif i cantly higher in females and subjects with high-TSH levels.Metabolic syndrome was also more prevalent in the high-TSH group.People over the age of 40 years with high-normal TSH levels had a 1.2-fold increased risk of metabolic syndrome,compared with those with low-normal TSH levels,after adjusting for age and gender.In conclusion,high normal TSH is a risk factor for metabolic syndrome in people≥40 years old.
thyroid stimulating hormone,euthyroid,metabolic syndrome,central obesity,dyslipidemia
Introduction
Metabolic syndrome(MS)is a constellation of metabolic abnormalities,including central obesity, glucose intolerance,hypertension,and dyslipidemia. The incidence of MS continues to increase and is becoming a major public health concern worldwide[1]. Mounting evidence suggests that MS is associated with an increased risk of cardiovascular disease(CVD)[2], type 2 diabetes[3]and all-cause mortality[4].
The hypothalamic-pituitary-thyroid(HPT)axis orchestrates a variety of metabolic processes,including thermogenesis and energy expenditure,growth promotion and lipid metabolism,which affect energy balance[5–6].Thyroid dysfunction presents a risk of CVD due to disruptions in blood pressure and lipid metabolism[7–8];this is valid for both overt and subclinical hypothyroidism[9–10].However,whether high-normal thyroid-stimulating hormone(TSH)levels are associated with metabolic derangement remainscontroversial.Studies have reported an association between subclinical hypothyroidism and MS in postmenopausal women[11]and young women of reproductive age[12];however,few studies have examined this association with subjects that include elderly men.
In 2003,the National Academy of Clinical Biochemistry(NACB)recommended lowering the upper reference limit of TSH to 2.50 mU/L based on the fi ndings of a large-scale epidemiological survey which revealed that≥95%of normal individuals have TSH levels<2.50 mU/L and those with higher TSH levels were likely to have various thyroid disorders[13–14].In this study,we used 2.50 mU/L as the cut-off point of TSH level within normal range to examine the association between high normal TSH levels and MS in a Chinese community-based population aged≥40 years in Nanjing,Jiangsu Province,China.
Subjects and methods
Subjects
The study was part of the baseline survey from the REACTION study,which investigated the association of diabetes and cancer,and was conducted among 259,657 adults aged 40 years and older in 25 communities across mainland China from 2011 to 2012[15–16].The sampling survey method was used to collect the baseline thyroid function data of 3,000 subjects from 10,000 subjects aged 40 years and older, who participated in the REACTION study in the Gulou district of Nanjing,Jiangsu Province.After exclusion of 644 individuals with abnormal thyroid function, 2,356 subjects were included in the f i nal analysis.Local ethics committees(the First Aff i liated Hospital of Nanjing Medical University,No.2011-SR-071) approved the study protocol.Written informed consent was obtained from each participant before data collection.
Data collection
The sociodemographic characteristics of all study participants were assessed by a questionnaire.Lifestyle factors,such as smoking status,alcohol intake and exercise,were also assessed.Waist circumference was measured in centimeters using the minimum circumference between the lower rib margin and the iliac crest in the standing position.Blood pressure was measured three times in all patients with at least a 5-minute interval between measurements.The average value was used to def i ne systolic blood pressure(SBP)and diastolic blood pressure(DBP).Levels of fasting plasma glucose(FPG),TSH,total cholesterol(TC), triglyceride(TG),high-density lipoprotein cholesterol (HDL-C),and low-density lipoprotein cholesterol (LDL-C)were measured after at least 12 hours of fasting in all subjects.The reference range of TSH was 0.35–5.00 mU/L.Based on the recommended cut-offvalue of TSH from the NACB guidelines[14],subjects were divided into the high-TSH(2.50–5.00 mU/L)and low-TSH(0.35–2.50 mU/L)groups.
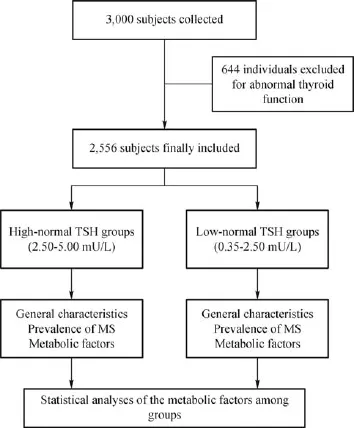
Fig.1 Flowchart of the progress of the cross-sectional study
Def i nitions
Euthyroidism was def i ned as FT3,FT4 and TSH levels within normal reference ranges(3.50–6.50 pmol/L,8.50–22.50 pmol/L,and 0.35–5.00 mU/L, respectively),without taking any thyroid medication. Body mass index(BMI)was def i ned as body weight in kilograms divided by the square of body height in meters.The presence of MS was ascertained according to the following criteria suggested by the International Diabetes Federation[17]:(I)central obesity(def i ned as waist circumference,males≥90 cm and females≥80 cm;when BMI is≥30 kg/m2,central obesity can be assumed with no need for waist circumference measurement);(II)triglycerides levels≥150 mg/dL or specif i c treatment for this lipid abnormality;(III)low HDL-C:HDL-C≤40 mg/dL(0.9 mmol/L)in males and≤50 mg/dL(1.1 mmol/L)in females;(IV)SBP/ DBP≥130/85 mmHg or treatment for previously diagnosed hypertension;(V)high FPG:FPG≥ 5.60 mmol/L.When central obesity plus two of the four above criteria were met,a diagnosis of MS was made.
Statistical analyses
Data analysis was performed using SPSS version 18.0 software(SPSS Inc.,Chicago,IL,USA).All data were expressed as mean±standard deviation(SD)or as percentages,where appropriate.Statistical comparisons were performed using independent-samples t tests for data with a normal distribution and χ2tests for data with a skewed distribution.Pearson or Spearman correlation was used to determine the relationship between TSH and other parameters.Logistic regression analysis was performed to evaluate the odds ratio that predicts the presence of MS in two groups.For all analyses,a probability(P)value<0.05 was considered statistically signif i cant.
Results
Baseline characteristics of the study subjects
Of the 2,356 participants(mean age,58.19±8.16 years;range,40-87 years)included for analysis,1,064 (45.16%)were allocated to the high-TSH group and 1,292(54.84%)to the low-TSH group(Fig.1).The general characteristics of the two groups are presented in Table 1.As shownin Table 1,gender distribution was different between the two groups(P<0.05),and the mean levels of BMI,TC,LDL-C,and FPG were all signif i cantly higher in the high-TSH group than those in the low-TSH group(24.95±3.47 vs.24.59±3.13, 5.10±1.00 vs.4.98±0.99,2.94±0.81 vs.2.86±0.77, and 6.41±1.76 vs.6.27±1.59,respectively;all P<0.05).Among all of the subjects,TSH levels were signif i cantly positively correlated with BMI,LDL-C, TC,and FPG(all P<0.05).

Table 1 Physical and metabolic characteristics of the study participants(mean±SD)

Table 2 Prevalence of metabolic syndrome and its components in different subgroups
Prevalence of MS
Table 2 shows the prevalence of MS and its components.The prevalence of MS was signif i cantly higher in the high-TSH group than in the low-TSH group(42.9%vs.38.5%,P=0.035).Central obesity (65.5%vs.57.8%,P<0.001),hypertriglyceridemia (37.8%vs.32.6%,P=0.009),and high FPG(>5.6 mmol/L)(64.9%vs.59.6%,P=0.008)were signif icantly more frequent in the high-TSH group than in the low-TSH group,respectively.Moreover,the prevalence of central obesity,hypertriglyceridemia,and high FPG (>5.60 mmol/L)in the female subgroup were also signif i cantly higher in the high-TSH group than in the low-TSH group(all P<0.05).
Multivariable risk assessment
The association of TSH level with MS and its components was assessed using binary logistical regression analysis adjusted for age and gender. Metabolic syndrome,central obesity,hypertension, high FPG,low HDL-C and high TG were applied as dependent variables and TSH as an independent variable(Table 3).The odds ratio(95%conf i dence intervals)that predicts the presence of MS,central obesity,high FPG,low HDL-C,and hypertriglyceridemia were 1.21(1.02–1.43),1.23(1.03–1.46),1.26 (1.06–1.49),1.31(1.03–1.67),and 1.31(1.10–1.55), respectively(all P<0.05)in the high-TSH group compared with the low-TSH group.
Discussion
In this study,we observed that TSH levels at the upper range of reference value were correlated with various MS parameters in 2,356 euthyroid persons aged≥40 years.The prevalence of MS was signif icantly greater among subjects in the high-TSH group (2.50–5.00 mU/L)than in the low-TSH group(0.35–2.50 mU/L).Regarding the components of MS,central obesity,high FPG,and hypertriglyceridemia were signi fi cantly frequent in the high-TSH group.These fi ndings are consistent with a previous study of young women[12].Our results indicate that high-normal TSH levels also act as a predictor of MS.

Table 3 Association of thyroid stimulating hormone as an independent variable with metabolic parameters by logistic regression analyses adjusted for age and gender.
In the present study,the mean BMI value and prevalence of central obesity were greater in the high-TSH group than in the low-TSH group,in accordance with the results of previous reports[12,18].Moreover, other studies have demonstrated a correlation between TSH and BMI.For example,Nyrnes et al.[19]found a positive and signif i cant correlation between serum TSH within the normal range and BMI both in a crosssectional and longitudinal study,possibly because elevated TSH levels promote weight gain,resulting in obesity.Hypothyroidism results in decreased basal metabolic rate,and together they affect lipid and carbohydrate metabolism, leading to weight gain[20–21].However,other studies have shown that the prevalence of subclinical hypothyroidism is associated with increased obesity[22];it has also been suggested that abnormalities in thyroid function may be secondary to weight excess[23].High-normal or slightly high TSH levels seem to be positively correlated with the degree of obesity[24].This phenomenon may be due to neuroendocrine dysfunction, especially by inappropriate secretion of leptin,a notable adipocyte-derived hormone,which accelerates excess TSH secretion in obesity[25–26].Leptin is produced by adipose tissue and may equilibrate the HPT axis by regulating TRH expression in the paraventricular nucleus[27–28],leading to subsequent modif i cation of hypothalamic TSH production[29–30].
Hypercholesterolemia is a well-known feature of hypothyroidism.Classically,hypothyroidism-associated dyslipidemia is characterized by elevated LDLC levels and has been described both in overt and subclinical disease[31].In this study,we found a signif i cant positive correlation between circulating TSH and TC,or LDL-C concentrations.These f i ndings are consistent with the well-known association between hypothyroidism and elevated levels of TC and LDL-C[32].However,the mechanism by which thyroid hormones modulate the blood lipid prof i le is multifactorial.Thyroid hormones may stimulate hydroxymethylglutaryl coenzyme A(HMG CoA),the key enzyme of LDL biosynthesis,and induce its synthesis[33].Thyroid hormones also decrease LDL catabolism by reducing the number of LDL-C receptors on the membranes of liver cells[32].In hypothyroidism,there is a decrease in the activity of hepatic lipase and cholesterol 7 alpha hydroxylase[34–36].In addition,the expression of hepatic LRP1 decreased[37].These alterations may be associated with elevated circulating remnant lipoproteins.
In our study,we found no linear association between SBP or DBP and TSH level.Moreover,there was no difference in the prevalence of hypertension between the high-and low-TSH groups.This result is not consistent with that of a previous study[12],which identif i ed a positive,linear association between SBP and DBP and TSH level.Although hypothyroidism can cause hypertension,the results of the vast majority of studies did not f i nd a signif i cant association between elevated TSH levels and blood pressure[38–39].
We observed that the prevalence of abnormal fasting glucose was quite high in the high-TSH group and that TSH levels were signif i cantly positively correlated with FPG,which is consistent with the f i ndings of many other investigations[8,12].Our study had some limitations,however,as we did not measure insulin secretion status.Since,insulin resistance(IR)is the key pathophysiological component of MS,and according to the AUC(area under the curve)and HOMA-IR (homeostasis model assessment of insulin resistance), more information may be revealed about this topic.
Moreover,we found the prevalence of central obesity, hypertriglyceridemia,and high FPG(>5.60 mmol/L)in the female subgroup were also signif i cantly higher in the high-TSH group than in the low-TSH group.The age of our subjects was 40 years and older,and females in this age group were in menopausal transition or postmenopausal.Many studies have indicated that MS is highly prevalent in postmenopausal women[40–41]and our f i nding adds to the growing body of evidence implicating menopause as a predictor of MS,independent of age[40].
In conclusion,this report extends existing information regarding the association between the components of MS and thyroid function in the euthyroid state.Our data showed that people aged≥40 years with highnormal TSH levels(>2.50 mU/L)had approximately 1.2-fold increased risk of MS compared with those with TSH levels≤2.50 mU/L after adjustment for age and gender.Therefore,high-normal or mildly elevated TSH levels in the elderly is a risk factor of MS and its components,and should be considered when evaluating such patients.
Acknowledgements
This study was supported by the grants from the Chinese Society ofEndocrinology and National ClinicalResearch CenterforMetabolicDiseases (81170726).
[1] Kassi E,Pervanidou P,Kaltsas G,et al.Metabolic syndrome: def i nitions and controversies[J].BMC Med,2011,9(48):48.
[2] Scuteri A,Najjar SS,Morrell CH,et al.,and the Cardiovascular Health Study.The metabolic syndrome in older individuals: prevalence and prediction of cardiovascular events:the Cardiovascular Health Study[J].Diabetes Care,2005,28(4): 882–887.
[3]Wannamethee SG,Shaper AG,Lennon L,et al.Metabolic syndrome vs Framingham Risk Score for prediction of coronary heart disease,stroke,and type 2 diabetes mellitus[J].Arch Intern Med,2005,165(22):2644–2650.
[4] Ford ES.The metabolic syndrome and mortality from cardiovascular disease and all-causes:f i ndings from the National Health and Nutrition Examination Survey II Mortality Study[J].Atherosclerosis,2004,173(2):309–314.
[5] al-Adsani H,Hoffer LJ,Silva JE.Resting energy expenditure is sensitive to small dose changes in patients on chronic thyroid hormone replacement[J].J Clin Endocrinol Metab,1997,82 (4):1118–1125.
[6] Krotkiewski M.Thyroid hormones and treatment of obesity[J]. Int J Obes Relat Metab Disord,2000,24(Suppl 2):S116–S119.
[7] Park SB,Choi HC,Joo NS.The relation of thyroid function to components of the metabolic syndrome in Korean men and women[J].J Korean Med Sci,2011,26(4):540–545.
[8] Roos A,Bakker SJ,Links TP,et al.Thyroid function is associated with components of the metabolic syndrome in euthyroid subjects[J].J Clin Endocrinol Metab,2007,92(2): 491–496.
[9] Fommei E,Iervasi G.The role of thyroid hormone in blood pressure homeostasis:evidence from short-term hypothyroidism in humans[J].J Clin Endocrinol Metab,2002,87(5):1996–2000.
[10]Hak AE,Pols HAP,Visser TJ,et al.Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women:the Rotterdam Study [J].Ann Intern Med,2000,132(4):270–278.
[11]Park HT,Cho GJ,Ahn KH,et al.Thyroid stimulating hormone is associated with metabolic syndrome in euthyroid postmenopausal women[J].Maturitas,2009,62(3):301–305.
[12]Oh JY,Sung YA,Lee HJ.Elevated thyroid stimulating hormone levels are associated with metabolic syndrome in euthyroid young women[J].Korean J Intern Med,2013,28(2): 180–186.
[13]Fatourechi V,Klee GG,Grebe SK,et al.Effects of reducing the upper limit of normal TSH values[J].JAMA,2003,290(24): 3195–3196.
[14]Kratzsch J,Fiedler GM,Leichtle A,et al.New reference intervals for thyrotropin and thyroid hormones based on National Academy of Clinical Biochemistry criteria and regular ultrasonography of the thyroid[J].Clin Chem,2005,51(8): 1480–1486.
[15]Ning G,and the Reaction Study Group.Risk Evaluation of cAncers in Chinese diabeTic Individuals:a lONgitudinal (REACTION)study[J].J Diabetes,2012,4(2):172–173.
[16]Bi Y,Lu J,Wang W,et al.Cohort prof i le:risk evaluation of cancers in Chinese diabetic individuals:a longitudinal(REACTION)study[J].J Diabetes,2014,6(2):147–157.
[17]Alberti KG,Zimmet P,Shaw J,and the IDF Epidemiology Task Force Consensus Group.The metabolic syndrome—a new worldwide def i nition[J].Lancet,2005,366(9491):1059–1062.
[18]Dekelbab BH,Abou Ouf HA,Jain I.Prevalence of elevated thyroid-stimulating hormone levels in obese children and adolescents[J].Endocr Pract,2010,16(2):187–190.
[19]Nyrnes A,Jorde R,Sundsfjord J.Serum TSH is positively associated with BMI[J].Int J Obes(Lond),2006,30(1):100–105.
[20]Rotondi M,Leporati P,La Manna A,et al.Raised serum TSH levels in patients with morbid obesity:is it enough to diagnose subclinical hypothyroidism[J]?Eur J Endocrinol,2009,160(3): 403–408.
[21]Sestoft L.Metabolic aspects of the calorigenic effect of thyroid hormone in mammals[J].Clin Endocrinol(Oxf),1980,13(5): 489–506.
[22]Ruiz-Tovar J,Boix E,Galindo I,et al.Evolution of subclinical hypothyroidism and its relation with glucose and triglycerides levels in morbidly obese patients after undergoing sleeve gastrectomy as bariatric procedure[J].Obes Surg,2014,24(5): 791–795.
[23]Biondi B.Thyroid and obesity:an intriguing relationship[J].J Clin Endocrinol Metab,2010,95(8):3614–3617.
[24]Iacobellis G,Ribaudo MC,Zappaterreno A,et al.Relationship of thyroid function with body mass index,leptin,insulin sensitivity and adiponectin in euthyroid obese women[J].Clin Endocrinol(Oxf),2005,62(4):487–491.
[25]Mantzoros CS,Ozata M,Negrao AB,et al.Synchronicity of frequently sampled thyrotropin(TSH)and leptin concentrations in healthy adults and leptin-def i cient subjects:evidence for possible partial TSH regulation by leptin in humans[J].J Clin Endocrinol Metab,2001,86(7):3284–3291.
[26]Winter WE,Signorino MR.Review:molecular thyroidology [J].Ann Clin Lab Sci,2001,31(3):221–244.
[27]Feldt-Rasmussen U.Thyroid and leptin[J].Thyroid,2007,17 (5):413–419.
[28]Menendez C,Baldelli R,Camiña JP,et al.TSH stimulates leptin secretion by a direct effect on adipocytes[J].J Endocrinol,2003,176(1):7–12.
[29]Ortiga-Carvalho TM,Oliveira KJ,Soares BA,et al.The role of leptin in the regulation of TSH secretion in the fed state:in vivo and in vitro studies[J].J Endocrinol,2002,174(1):121–125.
[30]Seoane LM,Carro E,Tovar S,et al.Regulation of in vivo TSH secretion by leptin[J].Regul Pept,2000,92(1-3):25–29.
[31]Bakker SJL,ter Maaten JC,Popp-Snijders C,et al.The relationship between thyrotropin and low density lipoprotein cholesterol is modif i ed by insulin sensitivity in healthy euthyroid subjects[J].J Clin Endocrinol Metab,2001,86(3): 1206–1211.
[32]Duntas LH.Thyroid disease and lipids[J].Thyroid,2002,12(4):287–293.
[33]Choi JW,Choi HS.The regulatory effects of thyroid hormone on the activity of 3-hydroxy-3-methylglutaryl coenzyme A reductase[J].Endocr Res,2000,26(1):1–21.
[34]Lam KS,Chan MK,Yeung RT.High-density lipoprotein cholesterol,hepatic lipase and lipoprotein lipase activities in thyroid dysfunction—effects of treatment[J].Q J Med,1986,59 (229):513–521.
[35]Brenta G,Berg G,Arias P,et al.Lipoprotein alterations,hepatic lipase activity,and insulin sensitivity in subclinical hypothyroidism:response to L-T(4)treatment[J].Thyroid,2007,17(5): 453–460.
[36]Zhou X,Han Y,Liu J,et al.Decreased protein and gene expression of hepatic cholesterol 7a-hydroxylase associated with dilated endoplasmic reticulum in chronic hypothyroid rats [J].Pathol Int,2009,59(10):729–734.
[37]Moon JH,Kim HJ,Kim HM,et al.Decreased expression of hepatic low-density lipoprotein receptor-related protein 1 in hypothyroidism:a novel mechanism of atherogenic dyslipidemia in hypothyroidism[J].Thyroid,2013,23(9):1057–1065.
[38]Duan Y,Peng W,Wang X,et al.Community-based study of the association of subclinical thyroid dysfunction with blood pressure[J].Endocrine,2009,35(2):136–142.
[39]Walsh JP,Bremner AP,Bulsara MK,et al.Subclinical thyroid dysfunction and blood pressure:a community-based study[J]. Clin Endocrinol(Oxf),2006,65(4):486–491.
[40]Ali SB,Belfki-Benali H,Aounallah-Skhiri H,et al.Menopause and metabolic syndrome in tunisian women[J].Biomed Res Int, 2014:1–7.
[41]Goyal S,Baruah M,Devi R,et al.Study on relation of metabolic syndrome with menopause[J].Indian J Clin Biochem,2013,28(1):55–60.
CLINICAL TRIAL REGISTRATION
The Journal requires investigators to register their clinical trials in a public trials registry for publication of reports of clinical trials in the Journal.Information on requirements and acceptable registries is available at www.icmje.org/faq_clinical.html.
ΔThese authors contributed equally to this work.
✉Corresponding author:Dr.Yu Duan,Department of Endocrinology, The First Aff i liated Hospital of Nanjing Medical University,300 Guangzhou Road,Nanjing,Jiangsu 210029,China.Tel/Fax:+86-25-68135120/13512504306/+86-25-68135120;Email:duanyu@-medmail.com.cn.
Received 18 July 2015,Revised 05 October 2015,Accepted 20 January 2016,Epub 20 May 2016
R589,Document code:A
The authors reported no conf l ict of interests.
©2016 by the Journal of Biomedical Research.All rights reserved.
10.7555/JBR.30.20150103
杂志排行
THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- The more the messier: centrosome amplification as a novel biomarker for personalized treatment of colorectal cancers
- Silibinin and colorectal cancer chemoprevention: a comprehensive review on mechanisms and efficacy
- Association between TSHR gene polymorphism and the risk of Graves' disease: a meta-analysis
- Assessment of atrial electromechanical interval using echocardiography after catheter ablation in patients with persistent atrial f i brillation
- Chronic intermittent hypoxia induces cardiac inf l ammation and dysfunction in a rat obstructive sleep apnea model
- Protein inhibitor of activated STAT 4(PIAS4)regulates liver fi brosis through modulating SMAD3 activity
