Postoperative sedation by intranasal dexmedetomidine in patients with hypertensive cerebral hemorrhage: study protocol for a randomized parallel-cohort controlled trial
2016-04-09ChaoliangTangJunLi
Chao-liang Tang*, Jun Li
Department of Anesthesiology, Southern District of Anhui Provincial Hospital, Hefei, Anhui Province, China
Background
Cerebral hemorrhage may occur as a result of non-traumatic spontaneous bleeding in the brain. Its causes are diverse,but the most common is rupture of cerebral blood vessels due to hypertensive arteriolosclerosis (Zhang and Ying,2004). Approximately one third of patients with hypertension will have a cerebral hemorrhage, and about 95% of patients with cerebral hemorrhage suffer from hypertension(Billet et al., 1974; Kurata et al., 1993). There is evidence that prompt intervention can improve clinical outcomes for patients with cerebral hemorrhage (Feldstein, 2014).Although early surgical evacuation of cerebral hematoma can reduce compression and destruction of adjacent brain tissue and address secondary brain injury, early postoperative secondary hemorrhage may cause further neuronal injury and substantially impair outcomes (Zhao et al., 2005).Clinicians must be mindful of the factors that increase the risk of adverse patient outcomes in the postoperative management of cerebral hemorrhage. In patients with cerebral hemorrhage, the sympatho-adreno-medullary system and hypothalamo-hypophyseal-adrenal axis are activated,leading to the paracrine or juxtacrine release of excitatory neurotransmitters by the adrenal medulla (Liu et al., 2010;Xu et al., 2012). The actions of excitatory amino acids at their receptors lead to stress-induced injury of hippocampal function and structure (Collingridge et al., 1983; Deng et al., 2013). Postoperative stress can also increase blood glucose concentration in patients with cerebral hemorrhage, resulting in lactic acidemia, further injury to brain cells, and cell membrane ion pump function impairment;ultimately causing cell swelling and even death (Ribo et al., 2007; Huang et al., 2013). If hemodynamic and pulmonary pathophysiologic changes occur simultaneously,pulmonary dysfunction will occur, leading to hypoxemia,metabolic acidosis and disturbance of the microcirculation.This pathophysiologic disturbance contributes to a vicious cycle of further cerebral edema and intracranial hypertension, the final result of which is likely to be a minimally conscious state or death (Robertson et al., 1999). Surgical stress can also impair immunity, leading to infection, sepsis and further complications (Wu and Zheng, 2000).
Cerebral hemorrhage is often accompanied by conscious disturbance and restlessness regardless of the size or location of the hemorrhage, and sedation is frequently necessary(Molina et al., 2004; Evron et al., 2007). Dexmedetomidine(Dex) is a novel, highly-selective α2-adrenergic agonist that exhibits dose-dependent sedative, anxiolytic and analgesic effects (Yuen et al., 2007; Chrysostomou and Schmitt, 2008;Takimoto et al., 2011; Wujtewicz et al., 2013; Nonaka et al.,2015; Tang et al., 2015b). There is evidence that Dex is ideal for sedating patients with traumatic brain injury undergoing mechanical ventilation (Hao et al., 2013). There have been no reports of the sedative effects of Dex in patients who are not mechanically ventilated. Intranasal Dex reportedly provide more stable breathing and circulation, produce fewer adverse events than intravenous administration, and are considered to be a safer and more effective method of providing anesthesia (Iirola et al., 2011; Han et al., 2014).The abundance and permeability of capillaries and lymphatic vessels in the subcutaneous layer of the nasal mucosa means that the bioavailability of drugs administered by the intranasal route is high, and peak blood concentrations are achieved rapidly (Villa et al., 2012; Tang et al., 2015b).We will undertake a study of intranasal Dex administered immediately after craniotomy in patients with hypertensive cerebral hemorrhage. Patients who are anesthetized, sedated or who have been administered neuromuscular blocking drugs are not able to describe the intensity of pain or distress that they are experiencing; however, pain-related behaviors and physiologic indices, including blood pressure, heart rate and respiratory frequency can also reflect pain intensity(Chawla and Kochar, 1999; Tousignant-Laflamme et al.,2005; Saccò et al., 2013). Therefore, we used blood pressure and heart rate as surrogate outcomes of the sedative effects of Dex.
Methods/design
Study design
A randomized parallel-cohort controlled trial.
Study setting
The trial will be undertaken at the Department of Anesthesiology, Southern District of Anhui Provincial Hospital, China.
Ethics approval and informed consent
Patients’ legal representatives will be invited to give informed consent, as patients will have reduced levels of consciousness and will not be able to provide consent for participation. On recovery of consciousness, the benefits and risks of participation will be explained to each patient, and written informed consent will be obtained. The trial has been approved by Clinical Research Ethics Committee of Anhui Provincial Hospital,China (permission No. 2015, ethics No. 07). The trial will be conducted in accordance with the Declaration of Helsinki.
Study participants
Patients with hypertensive cerebral hemorrhage who receive treatment in the Department of Neurosurgery,South Branch of Anhui Provincial Hospital, China will be enrolled according to the following criteria.
Inclusion criteria
● Patients with hypertensive cerebral hemorrhage confirmed by computed tomography and/or magnetic resonance imaging
● Patients who have undergone craniotomy for evacuation of intracranial hematoma
● Age 18–70 years
● American Society of Anesthesiologists (ASA) physical status I–IV (Fitz-Henry, 2011)
● Patients with hypertension, defined as a systolic blood pressure ≥140 mmHg and/or diastolic blood pressure≥ 90 mmHg
Exclusion criteria
● Patients with abnormal blood coagulation
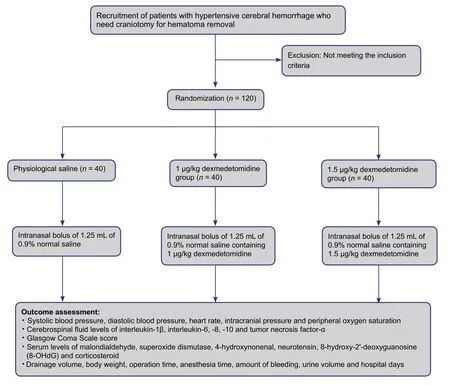
Figure 1: Flow chart of the trial protocol.
● Patients with known hypersensitivity to the study drug
● Severe hypertension (defined as systolic blood pressure ≥180 mmHg and/or diastolic blood pressure ≥ 110 mmHg with cardiovascular disease)
● Hepatic and/or renal insufficiency
● Sedative drug administered within 2 days of surgery
● Sinus bradycardia
● Requirement for postoperative mechanical ventilation
Randomization procedure and intervention measures
Patients will be allocated to groups using a computer-based simple randomization method, then randomly coded and provided with a corresponding emergency envelope. Blind codes will be provided to the drug administrator. If any error or disclosure with regard to randomization occurs, a new randomization sequence will be generated starting from the problematic serial number and applied to subsequent patients. Patients, outcome assessors, and statisticians will be blinded to information regarding grouping. Once severe adverse events occur, the researcher in charge will open the group assignment envelope, and the date and reasons for unblinding will be recorded in the medical records.
Patients will be randomly divided into groups administered 0.9% normal saline (inactive placebo), or 1 μg/kg or 1.5 μg/kg Dex, with 40 patients per group. After craniotomy for hematoma removal, all patients will be immediately transferred to the post-anesthesia care unit and monitored by nurse anesthetists. Study drng, either undiluted Dex 1 or 1.5 μg/kg (Dex group) or the equal volume of 0.9% normal saline (placebo group), was administered to each naris asdrops. Thirty minutes later, patients will undergo CT imaging of the brain and then be transferred to the neurosurgical intensive care unit.
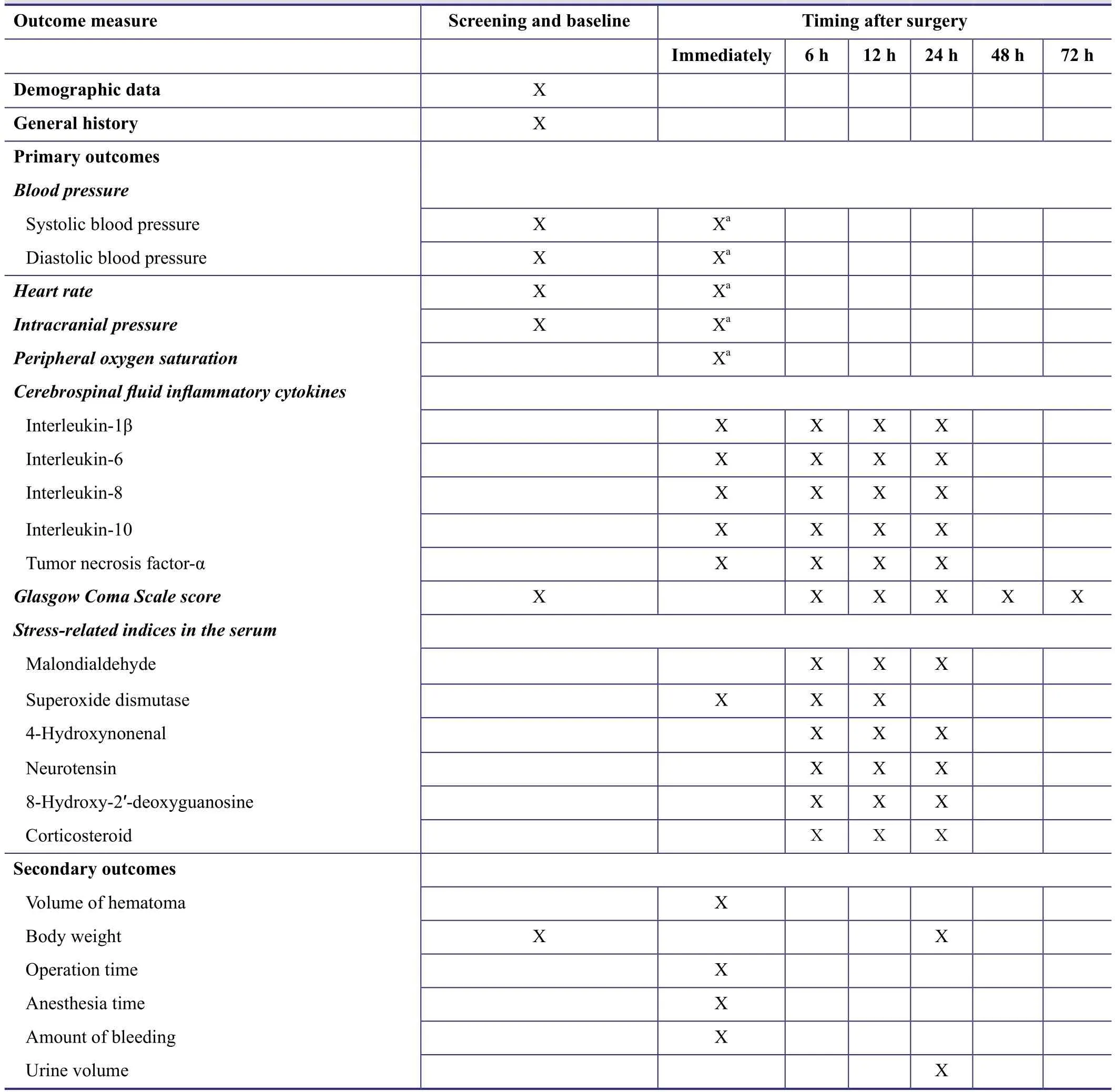
Table 1: Timing of outcome assessment
A trial protocol flow chart is shown in Figure 1.
Outcome measures
Primary outcomes will be: (1) systolic blood pressure,diastolic blood pressure, heart rate, intracranial pressure and peripheral oxygen saturation; (2) cerebrospinal fluid concentrations of interleukin-1β, -6, -8, -10 and tumor necrosis factor-α; (3) Glasgow Coma Scale score (Teasdale and Jennett, 1974); and (4) serum concentrations of malondialdehyde, superoxide dismutase, 4-hydroxynonenal, neurotensin, 8-hydroxy-2′-deoxyguanosine and corticosteroid. Secondary outcome measures will be: volume of hematoma drained; body weight; duration of surgery;duration of anesthesia; blood loss; urine output and length of hospital stay. All outcome measures will be recorded by the administrators of the neurosurgical intensive care unit who are blinded to the trial protocol and patient grouping.The timing of outcome assessment is shown in Table 1.
Data management and statistical analysis
The researchers will ensure the accuracy of all data entered and recorded in the case-report forms. After confirmation,the correct information will be input into the database and statistical analysis will be undertaken by the researcher in charge. All data will be analyzed using SPSS software version 12.0 (SPSS, Chicago, IL, USA). Measurement data will be expressed as the mean ± standard deviation. The independent samples t-test will be used to compare normally distributed data between groups, and independent samples non-parametric tests will be used for non-normally distributed data. The chi-squared test will be used to compare categorical data between groups. A P value < 0.05 will be considered statistically significant.
Data preservation
Data collected in the trial will be preserved and managed in accordance with the guidance of Good Clinical Practice.All clinical data will be preserved for 5 years after completion of the trial.
Trial quality assurance and control
All researchers will receive uniform training in the trial methodology, and the same group of assessors will record study parameters and outcomes, and assess the therapeutic effects of the study drug to avoid measurement bias. Factors that might influence the therapeutic effect, such as comorbid disease, disease duration and sex, will be analyzed.
DISCUSSION
Adequate sedation provides anxiolysis, reduces the stress response to surgery and critical illness, and reduces cerebral oxygen consumption and intracranial pressure, providing neuroprotection against secondary brain injury, and reducing the risk of further intracranial hemorrhage caused by elevated blood pressure (Villa et al., 2012). Sedatives also reportedly alleviate the stress response by reducing catecholamine and neuropeptide release (Goldstein et al., 1982). Inadequate sedation is likely to result in substantial fluctuations in blood pressure, heart rate and respiratory frequency, increased oxygen consumption, impaired cerebral perfusion and increased risk of secondary intracranial hemorrhage(Lim et al., 2015). Adequate sedation is therefore beneficial and necessary for patients with cerebral hemorrhage.Excessive sedation may make conscious level difficult to evaluate, mask the manifestations of underlying disease,and increase the risk of death. An ideal sedative should have the following features: (1) patients can be roused in response to voice while being sedated; (2) have analgesic and anxiolytic properties; (3) not impair respiratory drive or hemodynamic function; (4) not have cumulative effects;and (5) have few adverse effects such as nausea, vomiting or constipation (Yuen et al., 2007). Dex exhibits sedative and analgesic effects (Yuen et al., 2007; Chrysostomou and Schmitt, 2008; Takimoto et al., 2011; Wujtewicz et al., 2013;Nonaka et al., 2015), has little influence on hemodynamic or respiratory function (Nishida et al., 2002; Guler et al., 2005;Chen et al., 2014), and patients remain easy to rouse (Yuen et al., 2008). Therefore, Dex can be used for sedation when patients are not mechanically ventilated, and also alleviate inflammation and oxidative stress in the acute period and improve recovery of neurological function after surgery(Tang et al., 2015a).
Results from this randomized parallel-cohort controlled trial will provide objective evidence for the safe and effective use of intranasal Dex for sedation in patients with hypertensive cerebral hemorrhage. Design of the trial will ensure patient safety and compliance, and the straightforward protocol will facilitate its completion.
Trial status
Recruitment of participants is ongoing.
Conflicts of interest
None declared.
Author contributions
CLT and JL conceived and designed experimental procedures, contributed to article writing, and approved the final version of this article.
Plagiarism check
This paper was screened twice using CrossCheck to verify originality before publication.
Peer review
This paper was double-blinded and stringently reviewed by international expert reviewers.
Billet R, Rascol A, Salamon G (1974) Cerebral hemorrhage and arterial hypertension. Neurochirurgie 20:283-332.
Chawla PS, Kochar MS (1999) Effect of pain and nonsteroidal analgesics on blood pressure. WMJ 98:22-25, 29.
Chen KZ, Ye M, Hu CB, Shen X (2014) Dexmedetomidine vs remifentanil intravenous anaesthesia and spontaneous ventilation for airway foreign body removal in children. Br J Anaesth 112:892-897.
Chrysostomou C, Schmitt CG (2008) Dexmedetomidine: sedation,analgesia and beyond. Expert Opin Drug Metab Toxicol 4:619-627.
Collingridge GL, Kehl SJ, McLennan H (1983) Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol 334:33-46.
Deng Y, Wang M, Wang W, Ma C, He N (2013) Comparison and effects of acute lamotrigine treatment on extracellular excitatory amino acids in the hippocampus of PTZ-kindled epileptic and PTZ-induced status epilepticus rats. Neurochem Res 38:504-511.
Evron S, Ezri T, Rigini N, Gomel A, Szmuk P, Sadan O, Kohelet D (2007) The outcome of preterm neonates with intraventricular hemorrhage delivered with intravenous meperidine or epidural analgesia. J Anesth 21:90-93.
Feldstein CA (2014) Early treatment of hypertension in acute ischemic and intracerebral hemorrhagic stroke: Progress achieved,challenges, and perspectives. J Am Soc Hypertens 8:192-202.
Fitz-Henry J (2011) The ASA classification and peri-operative risk.Ann R Coll Surg Engl 93:185-187.
Goldstein DS, Dlonne R, Sweet J, Gracely R, Brewer BH, Gregg R, Keiser HR (1982) Circulatory, plasma catecholamine, cortisol,lipid, and psychological responses to a real-life stress (third molar extractions): effects of diazepam sedation and of inclusion of epinephrine with the local anesthetic. Psychosom Med 44:259-272.
Guler G, Akin A, Tosun Z, Eskitascoglu E, Mizrak A, Boyaci A(2005) Single-dose dexmedetomidine attenuates airway and circulatory reflexes during extubation. Acta Anaesthesiol Scand 49:1088-1091.
Hao J, Luo JS, Weng Q, He Y, Liu J, Yang MH, Bian GY, Liu T(2013) Effects of dexmedetomidine on sedation and β-endorphin in traumatic brain injury: a comparative study with propofol.Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 25:373-376.
Huang J, Liu B, Yang C, Chen H, Eunice D, Yuan Z (2013) Acute hyperglycemia worsens ischemic stroke-induced brain damage via high mobility group box-1 in rats. Brain Res 1535:148-155.
Kurata A, Miyasaka Y, Kitahara T, Kan S, Takagi H (1993) Subcortical cerebral hemorrhage with reference to vascular malformations and hypertension as causes of hemorrhage. Neurosurgery 32:505-511.
Lim YC, Kim CH, Kim YB, Joo JY, Shin YS, Chung J (2015) Incidence and risk factors for rebleeding during cerebral angiography for ruptured intracranial aneurysms. Yonsei Med J 56:403-409.
Liu H, Zhang G, Bie X, Liu M, Yang J, Wan H, Zhang Y (2010) Effect of polydatin on dynamic changes of excitatory amino acids in cerebrospinal fluid of cerebral hemorrhage rats. Zhongguo Zhong Yao Za Zhi 35:3038-3042.
Molina PE, Zambell KL, Zhang P, Vande Stouwe C, Carnal J (2004)Hemodynamic and immune consequences of opiate analgesia after trauma/hemorrhage. Shock 21:526-534.
Nishida T, Nishimura M, Kagawa K, Hayashi Y, Mashimo T (2002)The effects of dexmedetomidine on the ventilatory response to hypercapnia in rabbits. Intensive Care Med 28:969-975.
Nonaka T, Inamori M, Miyashita T, Harada S, Inoh Y, Kanoshima K, Matsuura M, Higurashi T, Ohkubo H, Iida H, Endo H, Kusakabe A, Maeda S, Gotoh T, Nakajima A (2015) Feasibility of deep sedation with a combination of propofol and dexmedetomidine hydrochloride for esophageal endoscopic submucosal dissection.Dig Endosc doi: 10.1111/den.12559.
Ribo M, Molina CA, Delgado P, Rubiera M, Delgado-Mederos R,Rovira A, Munuera J, Alvarez-Sabin J (2007) Hyperglycemia during ischemia rapidly accelerates brain damage in stroke patients treated with tPA. J Cereb Blood Flow Metab 27:1616-1622.
Robertson CS, Valadka AB, Hannay HJ, Contant CF, Gopinath SP,Cormio M, Uzura M, Grossman RG (1999) Prevention of secondary ischemic insults after severe head injury. Crit Care Med 27:2086-2095.
Saccò M, Meschi M, Regolisti G, Detrenis S, Bianchi L, Bertorelli M, Pioli S, Magnano A, Spagnoli F, Giuri PG, Fiaccadori E, Caiazza A (2013) The relationship between blood pressure and pain.J Clin Hypertens 15:600-605.
Takimoto K, Ueda T, Shimamoto F, Kojima Y, Fujinaga Y, Kashiwa A, Yamauchi H, Matsuyama K, Toyonaga T, Yoshikawa T (2011)Sedation with dexmedetomidine hydrochloride during endoscopic submucosal dissection of gastric cancer. Digestive Endoscopy 23:176-181.
Tang C, Chai X, Kang F, Huang X, Hou T, Tang F, Li J (2015a) I-gel laryngeal mask airway combined with tracheal intubation attenuate systemic stress response in patients undergoing posterior fossa surgery. Mediators Inflamm 2015:965925.
Tang C, Huang X, Kang F, Chai X, Wang S, Yin G, Wang H, Li J(2015b) Intranasal dexmedetomidine on stress hormones, inflammatory markers, and postoperative analgesia after functional endoscopic sinus surgery. Mediators Inflamm 2015:939431.
Teasdale G, Jennett B (1974) Assessment of coma and impaired consciousness. A practical scale. Lancet 2:81-84.
Tousignant-Laflamme Y, Rainville P, Marchand S (2005) Establishing a link between heart rate and pain in healthy subjects: a gender effect. J Pain 6:341-347.
Villa F, Iacca C, Molinari AF, Giussani C, Aletti G, Pesenti A, Citerio G (2012) Inhalation versus endovenous sedation in subarachnoid hemorrhage patients: effects on regional cerebral blood flow.Crit Care Med 40:2797-2804.
Wu FM, Zheng YL (2000) Effects of stress on the brain function.Zhongguo Shenjing Kexue Zazhi 16:279-281,289.
Wujtewicz M, Maciejewski D, Misiołek H, Fijałkowska A,Gaszyński T, Knapik P, Lango R (2013) Use of dexmedetomidine in the adult intensive care unit. Anaesthesiol Intensive Ther 45:235-240.
Xu X, Zhang J, Chen X, Liu J, Lu H, Yang P, Xiao X, Zhao L, Jiao Q, Zhao B, Zheng P, Liu Y (2012) The increased expression of metabotropic glutamate receptor 5 in subventricular zone neural progenitor cells and enhanced neurogenesis in a rat model of intracerebral hemorrhage. Neuroscience 202:474-483.
Yuen VM, Hui TW, Irwin MG, Yuen MK (2008) A comparison of intranasal dexmedetomidine and oral midazolam for premedication in pediatric anesthesia: a double-blinded randomized controlled trial. Anesth Analg 106:1715-1721.
Yuen VM, Irwin MG, Hui TW, Yuen MK, Lee LH (2007) A doubleblind, crossover assessment of the sedative and analgesic effects of intranasal dexmedetomidine. Anesth Analg 105:374-380.
Zhang SM, Ying XP (2004) Progress on the study of cerebral hemorrhage. Zhonghua Yi Xue Za Zhi 84:2023-2025.
Zhao JZ, Zhou DB, Zhou LF, Wang RZ, Wang DJ, Wang S, Yuan G, Kang S, Zhao YL, Ji N, Ye X (2005) The efficacy of three different approaches in treatment of hypertensive intracerebral hemorrhage: a multi-center single-blind study of 2464 patients.Zhonghua Yixue Zazhi 85:2238-2242.
杂志排行
Asia Pacific Journal of Clinical Trials:Nervous System Diseases的其它文章
- Correlating single nucleotide polymorphisms in vitamin D metabolism-related genes to autism susceptibility and vitamin D treatment: study protocol of a non-randomized parallelcohort controlled trial
- Effects of ultra-low frequency transcranial magnetic stimulation on motor function and intelligence of children with spastic cerebral palsy: study protocol for a randomized parallel-cohort controlled trial
- Use of low-dose dexmedetomidine in general anesthesia improves postoperative recovery of patients with supratentorial tumors: study protocol for a randomized controlled trial
- Intensive versus nonintensive insulin therapy for hyperglycemia after traumatic brain injury: study protocol for a randomized controlled trial
- The optimal time window for the use and dosage of nimodipine for acute massive cerebral infarction: study protocol for a randomized controlled trial
