MicroRNA作为原发性肝癌新标志物的应用前景*
2016-04-06董志珍姚登福
董志珍,姚登福
(南通大学附属医院诊断学教研室,江苏南通226001)
MicroRNA作为原发性肝癌新标志物的应用前景*
董志珍,姚登福
(南通大学附属医院诊断学教研室,江苏南通226001)
原发性肝癌(primary liver cancer,PLC)发病率高,进展快,早期发现难,易产生多药耐药(MDR)和复发转移,治疗难度大,且预后差,仍是严重威胁人类健康的常见恶性肿瘤,亟待探索新的诊治方法[1-2]。体内广泛存在由20~25个核苷酸组成的非编码微小RNA(microRNAs,miRNAs),在转录后水平调节基因表达,参与胚胎发育、细胞增殖与分化、凋亡等生命过程,在新血管生成、干细胞分化、浸润及转移等过程中发挥重要作用。循环血中稳定的miRNAs,不易被RNA酶消化,不受高或低pH、反复冻融和长期储存等影响,提示有可能成为新的肝癌标志物,近年倍受临床关注[3-4],在超过1 000多种miRNAs中,发现其中部分miRNAs有助于肝癌筛查或诊断、监测、治疗和预后评估。本文述评了肝癌miRNAs的诊断价值。
1 肝miRNAs经靶mRNA发挥作用
体内的miRNAs是一类内源性的具有调控功能的miRNAs,由较长的初级转录物(pri-miRNAs)经核酸酶(Drosha酶,Dicer酶)剪切加工而产生,随后组装进RNA诱导的沉默复合体(RNA-induced silencing complex,RISC),以碱基互配方式识别靶mRNA,按互补程度不同指导沉默复合体降解或阻遏靶mRNA翻译。肝miRNAs参与多种调节途径包括发育、病毒防御、造血过程、器官形成、细胞增殖和凋亡、脂肪代谢等[5-6]。始于内胚层上皮细胞的肝细胞,在不同发育阶段miRNAs表达异同,在胚胎肝miRs-18a、miRs-92a、miRs-409-3p、miRs-451和miRs-483-3p高表达;成熟肝miRs-22、miRs-23b、miRs-99a、miRs-125b、miRs-192和let-7a、let-7b及let-7c高表达。
广泛存在非编码miRNAs,在转录后水平调节基因表达,参与胚胎发育、细胞增殖、细胞分化、细胞凋亡等维持机体正常生理功能。miRNAs可调控肝细胞再生如miRs-21、miRs-127和miRs-26a,在肝再生早期miR-21明显上调,其靶基因如Btg2、RhoB和Peli1。另如miR-34a靶向Met和Inhbb基因和miR-221靶向CDK抑制剂p27和p57基因。在其它脏器很难检出的miR-122,是肝特异miRNA占肝miRNAs总量的70%,可作用于肝转录调节因子FoxA1和HNF4α,间接促进胚胎干细胞分化为成熟肝细胞。在肝细胞分化中,miRNAs可调节胆管形成,如miRs-30a、miRs-30c是胆管特异性miRNAs。经生物信息学分析,肝miRNAs涉及细胞增殖与分化,与靶mRNA共同作用,发挥生物学作用[7-8]。
2 肝miRNAs的促癌与抑癌功能
肝癌组织存在miRNA异常表达,以miRNA芯片技术对癌、癌周围组织及慢性肝炎组织,鉴定出差异表达miRNAs有30个,发现与分化相关的miRNAs有miR-92、miR-20和miR-18等,且表达水平与分化程度呈负相关[9-10]。miRNA异常表达促进肝癌的发生、发展,在癌组织有10种miRNAs表达明显上调,有16种miRNAs表达明显下调包括iR-199a-3p/miR-199b-3p等,可发生在肝细胞恶性转化过程中,即可见于慢性肝炎、肝硬化患者的肝组织[6-11]。肝miRNAs有多种上,可分为两类:致癌性miRNAs和抑癌性miRNAs。
2.1 致癌性miRNAs与功能
肝癌组织中明显上调、高表达的miRNAs,在肝癌发展过程中出现,其功能类似于癌基因的作用[12-22](表1)。以实时定量PCR(qRT-PCR)法分析,发现在肝癌早期阶段miR-155、miR-221、miR-222和miR-21表达上调,其中miR-155异常表达促进癌细胞增殖[17]。在癌组织中miR-224、miR-34a和miR-362-5p表达显著上调,与肝癌发展密切相关。应用siRNA抑制肝癌细胞miR-362-5p表达,可显著降低细胞的增殖、克隆形成、侵袭和迁移及裸鼠肝癌生长和转移,通过靶基因CYLD激活NF-κB信号通路促进肝癌进展;肝癌组织和细胞株中miR-543表达升高,通过靶基因PAQR3促进肝癌细胞增殖及侵袭,发挥癌基因作用;miR-494通过直接靶向调控TET1(Ten eleven translocation 1)基因,使多种侵袭抑制miRNAs基因组DNA去甲基化,使基因沉默致肝癌血管浸润[23]。
肝癌组织miR-106a表达较癌旁组织明显升高,其启动子甲基化状态与其表达呈负相关,作用靶基因为TP53INP1和CDKN1A;与低表达miR-106a肝癌细胞株相比,高表达miR-106a细胞株更具侵袭性、更快细胞周期进程及更强的凋亡抵抗[24];癌组织miR-520g表达上调,促进癌细胞侵袭、迁移和上皮间质转化(EMT),直接作用的靶点是Smad7。肝癌组织中miR-181a表达明显上调,TGF-β可促进miR-181a上调,诱导细胞发生EMT样变化[25]。癌组织miR-106b过度表达,与肿瘤分级显著相关,可通过Rho GTP酶、RhoA和RhoC来促进细胞迁移,促进转移。

表1 肝癌时上调的miRNAs及其生物学功能
2.2 抑癌性miRNAs与功能
肝癌组织另一类miRNA称为“抑癌性miRNA”,在正常肝组织中高表达、癌组织低表达(表2)如miR-122、miR-126和miR-375等[26-36]。肝癌组织miR-122、miR-125a/b、miR-26、miR-199和miR-375下调,在肝癌进展中发挥作用。肝癌组织miR-122下调致染色体不稳定性,而解除细胞周期G1期相关蛋白调控,间接地经细胞周期调节蛋白P53依赖途径发挥作用,使PPZA磷酸酶去磷酸化,激活Mdm-2致p53失活,抑制癌细胞增殖,促进凋亡,还可抑制血管内皮细胞分化,抑制血管生成。在非酒精性脂肪性肝病中miR-122基因沉默是个体早期事件,可作为评估患者发生癌变风险的理想指标。肝癌miRNA-21、miRNA-222和miRNA-145,经下游靶基因PTEN、p27和MAP3K发挥抑制作用[37]。

表2 肝癌时表达下调miRNAs及其生物学功能
肝癌组织及细胞株中miR-744明显下调,恢复其表达能降低癌细胞的增殖及引起细胞周期G1期阻滞,可通过靶向调控c-myc基因发挥抑制功能[38]。肝癌组织miR-148b表达显著低于对照肝组织,与肝癌血管浸润及TNM分期显著相关。多种肝癌细胞株如HepG2、MHCC97H、Hep3B及MHCC97L等miR-26b表达下降与肝癌分级显著相关,而恢复miR-26b表达能抑制肝癌细胞株侵袭及迁移力,伴随上皮标志物E-cadherin表达降低、间质标志物Vimentin表达增高及USP9X和Smad4受抑,miR-26b能经USP9X、Smad4和TGF-β信号通路抑制肝癌细胞EMT。众多miRNAs中,肝癌组织miR-125b表达下调,与细胞分化程度明显相关,可抑制肝癌细胞的EMT;主要通过Smad2和Smad4抑制EMT,改善肝癌细胞的化疗耐药等。肝癌miR-31可调节细胞周期蛋白(如HDAC2、CDK2)及EMT蛋白(如N-cadherin、E-cadherin和Vimentin等)表达,发挥抑癌功能[39]。
3 miRNAs表达与肝癌致病因素关系
肝癌发生与HBV及HCV感染、慢性酒精摄入、非酒精性脂肪性肝病及亚硝胺类物质、黄曲霉毒素及有害化学物质等诸多致病因素有关。以往不被重视的酒精性肝病、脂肪性肝病等慢性肝病发病率也急剧上升,成人酒精性脂肪性肝病患病率为4.5%,非酒精性脂肪性肝病(NAFLD)的患病率达15%,直追西方发达国家。如果不进行干预治疗,非酒精性脂肪性肝炎(NASH)也可发展为肝纤维化、肝硬化甚至肝癌[40]。综合有关肝癌的miRNAs表达的研究报道,很少见到一致性结果。探索的关键是发现肝癌特异miRNAs和肿瘤特异miRNAs。依据Columbia大学医学中心的报道,首先发现miRNAs表达与肝癌的致病因素相关(表3)。
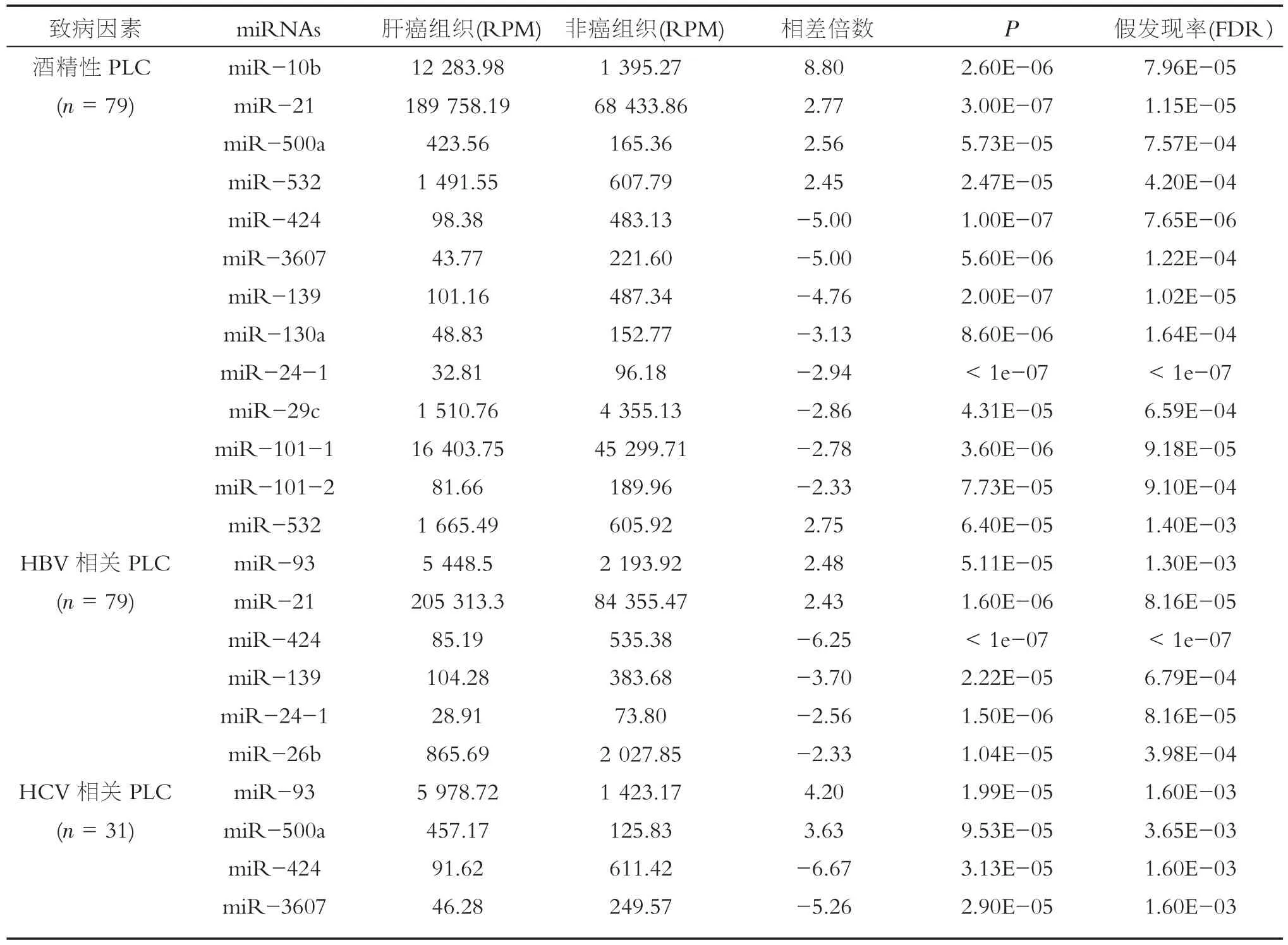
表3 肝癌致病因素与特异miRNAs异常关系[41]
对癌症基因组图谱(TCGA)和肝癌数据库中9种实体瘤的临床、流行病学和miRNA表达谱资料综合比较分析,发现肝癌与癌周组织33种miRNAs差异表达(2倍以上),其中绝多数(28 miRNAs)呈下调表达。在酒精性相关的79例肝癌组织中,发现有12种miRNAs改变明显,其中显著上调4种,显著下调8种;在HBV感染相关的79例肝癌组织中,其有7种miRNAs明显改变,其中miR-532,miR-93和miR-21呈明显上调表达,miR-424,miR-139, miR-24-1和miR-26b明显下调;在HCV相关的31例肝癌中,miR-93和miR-500a明显表达,而miR-424和miR-3607显著下调表达,提示有12种,7种和4种miRNAs分别对酒精性摄入、HBV和HCV感染具特异性并显著相关[41]。
4 miRNAs与肝癌诊断
PLC恶性程度高、病情进展快,发病隐匿,多数患者在诊断时即已出现肝内或肝外转移,失去了根治性手术治疗机会,且放、化疗效果也不理想。理想的肝癌标志物需要有较高的特异性,能将肝癌与肝硬化、肝炎、肝脏再生结节等区别开来:同时还需要较高的敏感性,早期诊断肝癌,且易检测、可重复、侵入少特点[42]。肝miRNA含量稳定,尽管血RNA酶会影响miRNA,但血miRNA表达稳定,可能是保护miRNA附加结构如膜性物质,包裹miRNA分子避免与RNA酶接触,或修饰miRNA以免于降解。肝癌患者血miR-122、miR-222和miR-223显著上调,miR-122可作为在HBV相关肝癌的潜在标志物;肝癌患者癌组织和血miR-21增高,改变比AFP早,可反映肝癌发生;以qRT-PCR法定量肝癌、慢性肝炎血miR-143和mi R-215表达水平,可作为肝癌诊断的潜在标志物[43]。

表4 人48配对肝癌与非癌组织中miRNAs的差异表达[41]
临床常用的肝癌标志物如外周血AFP、肝癌特异AFP(HS-AFP或AFP-L3)、异常凝血酶原、磷脂酰肌醇蛋白聚糖-3(GPC-3)[44]、肝癌特异γ-谷氨酰转移酶(HS-GGT)[45]、高尔基体蛋白酶-73、转化生长因子-β1、肝细胞生长因子、表皮生长因子受体和肿瘤特异生长因子等,它们单独或联合使用均有助于肝癌诊断,然而除HS-GGT和GPC-3外,良性肝病均有不同程度阳性率。肝癌、慢性肝病及健康人群血miRNAs如miR-106b、miR-10b及miR-181a均有表达,三者可鉴别良、恶性肝病,但单一miRNA与常规肝癌标志间尚未见优势,且不及Wnt3a的诊断与鉴别价值。miRNA筛选或诊断肝癌需大规模临床研究,以验证其诊断的特异价值。
5 miRNAs诊断肝癌的临床评价
肝miRNAs为重要的生物调节剂,在转录后水平上调控编码蛋白基因的表达。据估计约1/3人类基因直接或间接受其支配,并影响到肝细胞发生恶性转化的多种信号通路。有关肝癌组织及血miRNAs表达谱的研究,大多分析miRNAs异常与肝癌的临床病理学特征,并未涉及miRNAs表达与肝癌致病因素间的相互关系。在HBV相关肝癌和慢性肝病患者血中都见miR-155-5p、miR-24-3p、miR-490-3p、miR-210-3p和miR-335-5p表达,但miR-24-3p明显异常,且与肝癌患者血管浸润相关,可诊断和鉴别肝癌,ROC曲线下面积是0.636,AFP为0.627,两者联检可提高至0.834,可显著增加诊断准确度。另miR-125和miR-233可用于HBV阳性肝癌患者的早期诊断。纵观已报道miRNAs诊断肝癌的结果间差异很大。面临的最大挑战是miRNAs如何区别肝癌与其它实体肿瘤,尚未见肝癌特异miRNAs报道,临床大规模应用受限[14,16]。
令人高兴是新的肝癌特异miRNAs和肿瘤共有miRNAs被发现。在48对肝癌与癌周自身配对组织中miRNAs丰度的比较分析,发现miRNAs上调表达仅5种(miR-10b,miR-183,miR-182,miR-452和miR-21),下调表达居多为28种,有33种miRNAs可准确判别肝癌与非癌组织(表4)。
以上述33种miRNAs对癌与非癌的分类准确度较高,灵敏度为91.7%,特异性为100%,阳性预测值为100%,阴性预测值为92.3%,判别准确率95.8%和错判率为4.1%;以33种miRNAs用于302例非配对肝癌组织具同样效果,灵敏度为99.0%,特异性为97.9%,阳性预测值为99.7%,阴性预测值为94.0%,判别准确率为98.5%和错判率仅为1.5%。至于外周血中33种miRNAs的表达如何及其临床价值尚未见报道[41]。
6 展望
综上所述,肝癌相关miRNAs研究已取得较大进展,已为其诊断、治疗和预后等引入了新思路。因肝癌发生机制极其复杂,肝细胞恶性转化中涉及miRNA谱异常,除miRNA作用机制外,还需临床大规模研究包括不同病因、性别、年龄及进展阶段肝癌患者miRNAs动态表达,以筛选肝癌特异性miRNAs。miRNAs临床应用尚存亟待解决问题[46]:①内参miRNAs,以不同内参或非人源性miRNAs作为外参照,致各研究数据间兼容性和可比性差;②血miRNAs检测虽创伤小、可重复优势,需统一样本如血清或血浆;③许多因素可致结果偏倚,应流程标准化确保准确;④临床普及和提高对miRNAs的认识;⑤文献报道资料中miRNAs表达差异较大;⑥已筛选出识别肝癌与非癌的miRNAs谱,循环血中表达如何及其该成果转化亟待解决问题。但有理由相信,miRNAs应用在肝癌预防、临床诊断和治疗中具有前景[47,48]。
1.Wang L,Yue Y,Wang X,et al.Function and clinical potential of microRNAs in hepatocellular carcinoma[J].Oncol Lett,2015,10(6):3345-3353.
2.Reichl P,Mikulits W.Accuracy of novel diagnostic biomarkers for hepatocellular carcinoma:An update for clinicians[J].Oncol Rep,2016,36(2):613-625.
3.Fiorino S,Bacchi-Reggiani ML,Visani M,et al.MicroRNAs as possible biomarkers for diagnosis and prognosis of hepatitis B-and C-related-hepatocellular carcinoma [J].World J Gastroenterol,2016,22(15):3907-3936.
4.Vychytilova-Faltejskova P,Pesta M,Radova L,et al. Genome-wide microRNA expression profiling in primary tumors and matched liver metastasis of patients with colorectal cancer[J].Cancer Genomics Proteomics,2016,13 (4):311-316.
5.Yan IK,Wang X,Asmann YW,et al.Circulating extracellular RNA markers of liver regeneration[J].PLoS One,2016,11(7):0155888.
6.Gu JJ,Yao M,Yao DB,et al.Nonalcoholic lipid accumulation and hepatocyte malignant transformation[J].J Clin Transl Hepatol,2016,4(2):123-130.
7.Xiao Y,Tian Q,He J,et al.MiR-503 inhibits hepatocellular carcinoma cell growth via inhibition of insulin-like growth factor 1 receptor[J].Onco Targets Ther,2016,9: 3535-3544.
8.Kanda M,Sugimoto H,Kodera Y.Genetic and epigenetic aspects of initiation and progression of hepatocellular carcinoma[J].World J Gastroenterol,2015,21(37):10584-10597.
9.Kim GW,Lee SH,Cho H,et al.Hepatitis C virus core protein promotes miR-122 destabilization by inhibiting GLD-2[J].PLoS Pathog,2016,2(7):1005714.
10.Yen CS,Su ZR,Lee YP,et al.miR-106b promotes cancer progression in hepatitis B virus-associated hepatocellular carcinoma[J].World J Gastroenterol,2016,22(22): 5183-5192.
11.Zhang YC,Xu Z,Zhang TF,et al.Circulating microRNAs as diagnostic and prognostic tools for hepatocellular carcinoma[J].World J Gastroenterol,2015,21(34):9853-9862.
12.Lyra-González I,Flores-Fong LE,González-García I,et al.MicroRNAs dysregulation in hepatocellular carcinoma: Insights in genomic medicine[J].World J Hepatol,2015,7 (11):1530-1540.
13.Zhang Y,Wei W,Cheng N,et al.Hepatitis C virus-induced up-regulation of microRNA-155 promotes hepatocarcinogenesis by activating Wnt signaling[J].Hepatology,2012,56(5):1631-1640.
14.Liu WH,Yeh SH,Lu CC,et al.MiRNAs in hepatocellular carcinoma expression,promoting proliferation of hepatocellular carcinoma cells[J].Gastroenterology,2009, 136(2):683-693.
15.Yan Y,Luo YC,Wan HY,et al.MicroRNA-10a is involved in the metastatic process by regulating Eph tyrosine kinase receptor A4-mediated epithelial mesenchymal transition and adhesion in hepatoma cells[J].Hepatology,2013, 57(2):667-677.
16.Zhang LY,Liu M,Li X,et al.miR-490-3p modulates cell growth and epithelial to mesenchymal transition of hepatocellular carcinoma cells by targeting endoplasmic reticulum-Golgi intermediate compartment protein 3 (ERGIC3)[J].J Biol Chem,2013,288(6):4035-4047.
17.El Tayebi HM,Hosny KA,Esmat G,et al.miR-615-5p is restrictedly expressed in cirrhotic and cancerous liver tissues and its overexpression alleviates the tumorigenic effects in hepato-cellular carcinoma[J].FEBS Lett,2012, 586(19):3309-3316.
18.Tomimaru Y,Eguchi H,Nagano H,et al.MicroRNA-21 induces resistance to the antitumour effect of interferon-α/5-fluorouracil in hepatocellular carcinoma cells[J]. Br J Cancer,2010,103(10):1617-1626.
19.Xu N,Shen C,Luo Y,et al.Upregulated miR-130a increases drug resistance by regulating RUNX3 and Wnt signaling in cisplatin-treated HCC cell[J].Biochem Biophys Res Commun,2012,425(2):468-472.
20.Li J,Fu H,Xu C,et al.miR-183 inhibits TGF-beta1-induced apoptosis by downregulation of PDCD4 expression in human hepatocellular carcinoma cells[J].BMC Cancer,2010,10(1):354.
21.Yang W,Sun T,Cao J,et al.Downregulation of miR-210 expression inhibits proliferation,induces apoptosis and enhances radiosensitivity in hypoxic human hepatoma cells in vitro[J].Exp Cell Res,2012,318(8):944-954.
22.Fornari F,Milazzo M,Chieco P,et al.In hepatocellular carcinoma miR-519d is up-regulated by p53 and DNA hypomethylationandtargetsCDKN1A/p21,PTEN, AKT3 and TIMP2[J].J Pathol,2012,227(3):275-285.
23.Chuang KH,Whitney-Miller CL,Chu CY,et al.MicroRNA-494 is a master epigenetic regulator of multiple invasion-suppressor microRNAs by targeting ten eleven translocation 1 in invasive human hepatocellular carcinoma tumors[J].Hepatology,2015,62(2):466-480.
24.Hong ZS,Hong HY,Liu J,et al.miR-106a is downregulated in peripheral blood mononuclear cells of chronic hepatitis B and associated with enhanced levels of interleukin-8[J].Mediators Inflamm,2015,2015:629862.
25.Tan JYL,Habib NA,Chuah YW,et al.Identification of cellular targets of microRNA-181a in HepG2 cells:a new approach for functional analysis of microRNAs[J].PLoS One,2015,10(4):0123167.
26.Di Fazio P,Montalbano R,Neureiter D,et al.Downregulation of HMGA2 by the pan-deacetylase inhibitorpanobinostat is dependent on hsa-let-7b expression in liver cancer cell lines[J].Exp Cell Res,2012,318(15): 1832-1843.
27.Au SL,Wong CC,Lee JM,et al.Enhancer of zeste homolog 2 epigenetically silences multiple tumor suppressor microRNAs to promote liver cancer metastasis[J].Hepatology,2012,56(2):622-631.
28.Vecchio FD,Gallo F,Marco AD,et al.Bioinformatics approach to predict target genes for dysregulated microRNAs in hepatocellular carcinoma:study on a chemically-induced HCC mouse model[J].BMC Bioinformatics,2015, 16(1):408.
29.Fornari F,Milazzo M,Chieco P,et al.MiR-199a-3p regulates mTOR and c-Met to influence the doxorubicin sensitivity of human hepatocarcinoma cells[J].Cancer Res, 2010,70(12):5184-5193.
30.Hopcraft SE,Azarm KD,Israelow B,et al.Viral determinants of miR-122-independent hepatitis C virus replication[J].mSphere,2015,1(1):9-15.
31.Guo Y,Li S,Qu J,et al.MiR-34a inhibits lymphatic metastasis potential of mouse hepatoma cells[J].Mol Cell Biochem,2011,354(1):275-282.
32.Shen Q,Cicinnati VR,Zhang X,et al.Role of microRNA-199a-5p and discoidin domain receptor 1 in human hepatocellular carcinoma invasion[J].Mol Cancer,2010,9 (1):227.
33.Chang Y,Yan W,He X,et al.miR-375 inhibits autophagy and reduces viability of hepato-cellular carcinoma cells under hypoxic conditions[J].Gastroenterology, 2012,143(1):177-187.
34.Kim JK,Noh JH,Jung KH,et al.Sirtuin7 oncogenic potential in human hepatocellular carcinoma and its regulation by the tumor suppressors MiR-125a-5p and MiR-125b[J].Hepatology,2013,57(3):1055-1067.
35.Shih TC,Tien YJ,Wen CJ,et al.MicroRNA-214 downregulation contributes to tumor angiogenesis by inducing secretion of the hepatoma-derived growth factor in human hepatoma[J].J Hepatol,2012,57(3):584-591.
36.Wei X,Xiang T,Ren G,et al.miR-101 is down-regulated by the hepatitis B virus x protein and induces aberrant DNA methylation by targeting DNA methyltransferase 3A[J].Cell Signal,2013,25(2):439-446.
37.He XD,Li JJ,Guo WD,et al.Targeting the microRNA-21/AP1 axis by 5-fluorouracil and pirarubicin in human hepatocellular carcinoma[J].Oncotarget,2015,6(4):2302-2314.
38.Lin F,Ding RL,Zheng S,et al.Decrease expression of microRNA-744 promotes cell proliferation by targeting c-Myc in human hepatocellular carcinoma[J].Cancer Cell Int,2014,14:58.
39.Kim HK,Lee KS,Bae HJ,et al.MicroRNA-31 functions as a tumor suppressor by regulating cell cycle and epithelial-mesenchymal transition regulatory proteins in liver cancer[J].Oncotarget,2015,6(10):8089-8102.
40.Gu JJ,Yao M,Yao DB,et al.Nonalcoholic lipid accumulation and hepatocyte malignant transformation[J].J Clin Transl Hepatol,2016,4(2):123-130.
41.Shen J,Siegel AB,Remotti H,et al.Identifying microRNA panels specifically associated with hepatocellular carcinoma and its different etiologies[J].Hepatoma Res,2016, 2(6):151-162.
42.Della Corte C,Triolo M,Iavarone M,et al.Early diagnosis of liver cancer:an appraisal of international recommendations and future perspectives[J].Liver Int,2016,36(2): 166-176.
43.Wang L,Yao M,Dong ZZ,et al.Circulating specific biomarkers in diagnosis of hepato-cellular carcinoma and its metastasis monitoring[J].Tumor Biol,2014,35(1):9-20.
44.Yao M,Yao DF,Bian YZ,et al.Oncofetal antigen glypican-3 as a promising early diagnostic marker for hepatocellular carcinoma[J].Hepatobiliary Pancreat Dis Int,2011, 10(3):289-294.
45.Yao D,Jiang D,Huang Z,et al.Abnormal expression of hepatoma specific gamma-glutamyl transferase and alteration of gamma-glutamyl transferase gene methylation status in patients with hepatocellular carcinoma[J].Cancer, 2000,88(4):761-769.
46.Mao B,Wang G.MicroRNAs involved with hepatocellular carcinoma[J].Oncol Rep,2015,34(6):2811-2820.
47.Yao M,Wang L,Yao Y,et al.Biomarker-based microRNA therapeutic strategies for hepato-cellular carcinoma[J]. J Clin Transl Hepatol,2014,2(4):253-258.
48.Huang JT,Liu SM,Ma H,et al.Systematic review and Meta-analysis:circulating miRNAs for diagnosis of hepatocellular carcinoma[J].J Cell Physiol,2016,231(2):328-335.
(2016-7-30收稿)
R446.11
A
10.3969/j.issn.1000-2669.2016.05.002
*国家国际科技合作项目(2013DFA32150),江苏省“六大人才高峰”项目(2014-YY-028)
姚登福,男,教授,博士生导师。E-mail:yaodf@ahnmc.com
