有机溶质在双烷烃咪唑醋酸盐离子液体中无限稀释活度系数的测定
2016-03-30丁珊,魏立纲,王艳涛,王琳琳
丁 珊, 魏 立 纲, 王 艳 涛, 王 琳 琳
( 大连工业大学 轻工与化学工程学院, 辽宁 大连 116034 )
有机溶质在双烷烃咪唑醋酸盐离子液体中无限稀释活度系数的测定
丁 珊,魏 立 纲,王 艳 涛,王 琳 琳
( 大连工业大学 轻工与化学工程学院, 辽宁 大连116034 )
摘要:用反气相色谱法测定了11种探针溶质323.15~353.15 K内在双烷烃咪唑醋酸盐离子液体([R1R2IM]OAc)中的无限稀释活度系数。实验结果显示,无限稀释活度系数表现出明显的非理想性,这主要是由于溶质与溶剂之间的相互作用存在强弱差异。温度对无限稀释活度系数有明显影响,温度升高,无限稀释活度系数增大。在一定温度范围内,探针溶质的无限稀释活度系数对数随温度变化的线性拟合结果较好,可以对无限稀释活度系数对数进行适度的内插和外推。离子液体中的阳离子烷基链长度影响无限稀释活度系数。
关键词:反相气相色谱法;离子液体;无限稀释活度系数

0引言
作为一种新型环境友好溶剂,离子液体具有优良的溶解性、高热力学及化学稳定性、结构可设计等优点。离子液体的应用研究受到国内外研究者的广泛关注。研究离子液体物理化学性质、建立基础数据库,对其在化工过程中应用有指导作用。

图1双烷烃咪唑醋酸盐离子液体[R1R2IM]OAc的结构
Fig.1StructuresofdialkylimidazoliumacetateILs[R1R2IM]OAc
1实验
1.1材料与仪器
材料:采用离子液体为双烷烃咪唑醋酸盐 ([R1R2IM]OAc),纯度>98%,中科院兰州化物所。有机化合物:正戊烷、正辛烷、正壬烷、正癸烷、2-丁酮、2-戊酮、甲苯、1,2-二氯乙烷、甲醇、乙醇、三氯甲烷、吡啶、丙酮,均为色谱纯,阿拉丁试剂(上海)有限公司。
仪器:TECHCOMP7890 Ⅱ型气相色谱仪,TCD检测器;色谱柱(1 000mm×2mm),大连中汇达科技有限公司。
1.2实验方法
1.2.1固定相的涂布
将ILs用丙酮溶解,向溶液中加入80~100目担体,混合均匀。常温下待溶剂挥发后,用抽吸法装入不锈钢U形色谱柱管353.15K老化6h。
1.2.2保留时间的测定
氢气为载气,用皂膜流量计校准气体流量,体积流量为20mL/min。汽化室和检测器的温度均为523.15K,柱箱温度:323.15、333.15、343.15、353.15K。用正戊烷标定死时间,将各探针分子在同样的条件下重复测量3次,进样量为0.1μL。
2结果与讨论
2.1理论基础
采用Everett[5]和Cruickshank等[6]提出的无限稀释活度系数公式计算:

(1)
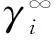
净保留体积VN遵循标准色谱方程[7]:
(2)
式中,t′R为探针分子的净保留时间,Tcol和Tr分别为柱温和室温,U0为载气体积流量,pw和po分别为水在室温时的饱和蒸汽压和色谱柱出口压力。式(1)、式(2)中的压力梯度校正因子J可表达为
(3)
式中,pi和po分别为色谱柱的进口压力和出口压力。
溶质的第二维里系数B11和溶质与载气的混合维里系数B12采用McGlashan和Potter公式[8]计算:
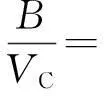
(4)
式中,TC和VC分别为探针分子的临界温度和临界体积,n为溶剂分子中碳原子的数目。
2.2溶质在ILs中的无限稀释活度系数


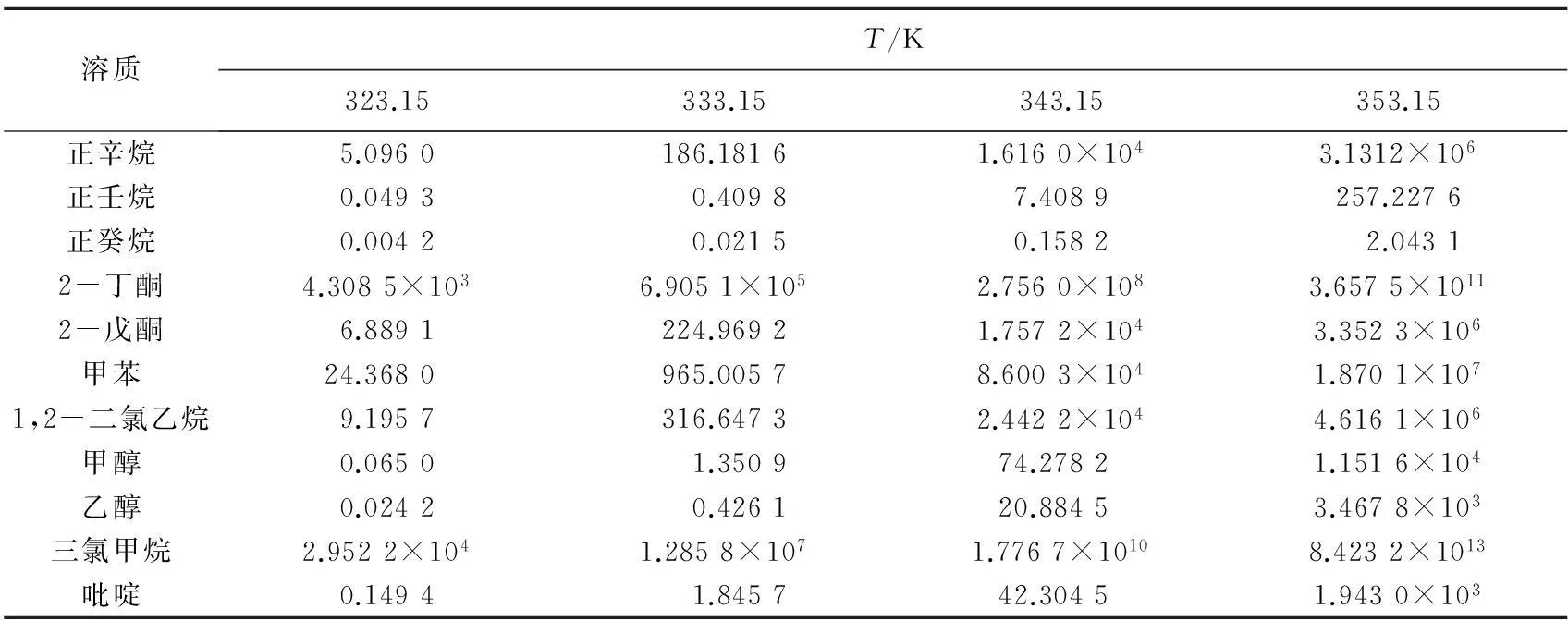
表1 不同温度下11种溶质在[EMIM]OAc中的无限稀释活度系数

(5)
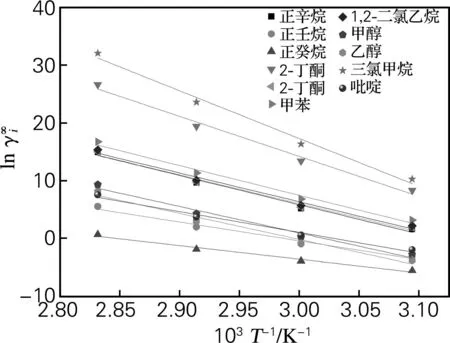
图2 11种溶质的随103T-1的变化
2.2.2溶质种类对的影响
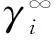


表2 无限稀释活度系数的线性回归相关参数
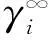



表3 温度323.15 K下溶质在离子液体[R1R2IM]OAc中的无限稀释活度系数
3结论
参考文献:
[1] SCHREIBER L B, ECKERT C A. Use of infinite dilution activity coefficients with wilson’s equation[J]. Industrial and Engineering Chemistry Process Design and Development, 1971, 10(4): 572-576.
[2] CHIAPPE C, PIERACCINI D. Ionic liquids: solvent properties and organic reactivity[J]. Journal of Physical Organic Chemistry, 2005, 18(4): 275-297.
[3] 葛明兰,熊杰明,王利生.有机化合物在离子液体中的无限稀释活度系数理论推测[J].中国科学,2009,5(10):1419-1423.
[4] THOMAS E R, ECKERT C A. Prediction of limiting activity coefficients by a modified separation of cohesive energy density model and unifac[J]. Industrial and Engineering Chemistry Process Design and Development, 1984, 23(1): 194-209.
[5] EVERETT D H. Effect of gas imperfection on gas-liquid chromatography measurements: a refined method for determining activity coefficients and second virial coefficients[J]. Transactions of Faraday Society, 1966, 61(512): 1637-1645.
[6] CRUICKSHANK A J B, WINDSOR M L, YOUNG C L. Use of gas-liquid chromatography to determine activity coefficients and second virial coefficients of mixtures. Ⅰ. theory and verification of data analysis[J]. Proceedings of the Royal Society, 1966, 295(1442): 259-270.
[7] YOO B, AFZAL W, PRAUSNITZ J M. Solubility parameters for nine ionic liquids[J]. Industrial and Engineering Chemistry Research, 2012, 51(29): 9913-9917.
[8] MCGLASHAN M L, POTTER D J B. An apparatus for the measurement of the second virial coefficients of vapours; the second viral coefficients of some n-alkanes and of some mixtures of n-alkanes[J]. Proceedings of the Royal Society, 1962, 267(1331): 478-500.
[9] 邓丽霜,王强,张正方,等.反气相色谱法测定离子液体1-己基-3-甲基咪唑三氟甲磺酸盐的热力学参数[J].中国科学,2014,32(2):169-173.
[10] 刘洪勤.无限稀释活度系数的应用与测定[J].化学工业与工程,1995,12(1):14-20.
Measurement of infinite dilution activity coefficients for organic solutes in dialkylimidazolium acetate ionic liquids
DINGShan,WEILigang,WANGYantao,WANGLinlin
( School of Light Industry and Chemical Engineering, Dalian Polytechnic University, Dalian 116034, China )
Abstract:Inverse gas chromatography (IGC) was used to measure the infinite diluted activity coefficients of eleven solutes dialkylimidazolium acetate ILs ([R1R2IM]OAc) at 323.15 to 353.15 K. A non-ideal behavior of the infinite diluted activity coefficients was observed, which mainly came from the interaction between solute and solvent. Temperature had an obvious influence on the infinite diluted activity coefficients, and the value was increased with the increasing temperature. Linear fitting results for the infinite diluted activity coefficients of logarithmic were obtained at the temperature ranging from 323.15 to 353.15 K, which indicated that a moderate amount of interpolation and extrapolation for the infinite diluted activity coefficients of logarithmic could achieve. The alkyl chain length of cation for ILs affected the infinite diluted activity coefficients.
Key words:inverse gas chromatography (IGC); ionic liquids; infinite dilution activity coefficient
作者简介:丁 珊(1989-),女,硕士研究生;通信作者:魏立纲(1972-),男,副教授.
基金项目:国家自然科学基金资助项目(21106011,21276034);辽宁省教育厅科学研究一般项目(L2012195).
收稿日期:2014-09-29.
中图分类号:O642.1
文献标志码:A
文章编号:1674-1404(2016)01-0029-04
