近距离治疗联合外放射治疗及内分泌治疗对局部高危前列腺癌的疗效
2016-03-30严维刚李汉忠纪志刚周智恩麦智鹏
陈 健,严维刚,李汉忠,纪志刚,周 毅,周智恩,麦智鹏
中国医学科学院 北京协和医学院 北京协和医院泌尿外科,北京100730
近距离治疗联合外放射治疗及内分泌治疗对局部高危前列腺癌的疗效
陈 健,严维刚,李汉忠,纪志刚,周 毅,周智恩,麦智鹏
中国医学科学院 北京协和医学院 北京协和医院泌尿外科,北京100730
目的 研究近距离治疗联合外放射治疗及内分泌治疗对局部高危前列腺癌的疗效。方法 2003年12月至2007年12月北京协和医院泌尿外科收治前列腺癌近距离治疗患者132例,其中局部高危患者97例,局部中、低危患者35例。通过门诊随访,监测患者术后血清前列腺特异性抗原 (prostate specific antigen,PSA)水平,记录患者出现生化复发、进展至去势抵抗性前列腺癌 (castration-resistant prostate cancer,CRPC)或肿瘤远处转移、死亡等事件,了解患者无生化复发生存率 (biochemical progression-free survival,bPFS)、疾病特异性生存率 (cause-specific survival,CSS)及总体生存率 (overall survival,OS)。结果 132例患者bPFS、CSS、OS分别为83.3%、91.7%、84.8%,局部高危患者为81.4%、88.7%、81.4%,局部低、中危患者为88.6%、100%、94.3%。局部高危患者bPFS及OS与局部低、中危患者相比差异无统计学意义 (P=0.433,0.098);而局部低、中危患者CSS明显高于局部高危患者 (P=0.037)。按不同Gleason评分、TNM临床分期、术前PSA水平分别分组,各组患者间bPFS差异均无统计学意义 (P=0.084,0.537,0.850)。结论 对于局部高危前列腺癌患者,近距离治疗联合外放射治疗或内分泌治疗可有效控制PSA水平并延缓生化复发。
局部高危前列腺癌;近距离治疗;无生化复发生存率
Med J PUMCH,2016,7(2):104-109
对前列腺癌进行危险因素等级分析,有助于指导治疗和评估预后。美国国家综合癌症网络 (National Comprehensive Cancer Network,NCCN)根据血清前列腺特异性抗原 (prostate specific antigen,PSA)水平、Gleason评分和TNM临床分期将前列腺癌分为低、中、高危三个等级[1]。对于局部低、中危前列腺癌 (T1~T2b),近距离治疗与前列腺癌根治性切除、前列腺癌外放射治疗效果相当,均能达到根治水平[2-8]。而局部高危前列腺癌,甚至包括Gleason评分为5分的前列腺癌,单纯近距离治疗效果欠佳[9-11]。本研究探讨近距离治疗联合外放射治疗及内分泌治疗对局部高危前列腺癌的疗效。
对象和方法
对象
2003年12月至2007年12月北京协和医院泌尿外科收治前列腺癌近距离治疗患者132例,其中局部高危患者97例,局部中、低危患者35例。患者术前均经会阴前列腺穿刺活检病理证实,并行B超、胸腹盆CT或磁共振成像、全身骨扫描证实肿瘤无远处转移及包膜外侵犯。
仪器设备
前列腺癌近距离治疗系统 (美国CMS公司)包括实时图像捕获、自动粒子纺织、三维剂量编辑和成像的内植入治疗计划软件 (3.2版本),AccuSeed定位仪和定位架,西门子SONOLINE Adara SLC数字化增强型超声,Mick 200粒子植入器,Premier静电计和便携式放射量调查计,标准影像3000活度仪。
治疗方法
患者治疗前3~5 d先经直肠超声行预计划,明确125I粒子数及粒子分布情况,预订粒子。常规进行粒子活度检测,粒子活度偏差<5%。手术采用联合阻滞麻醉。粒子分布计划按照西雅图模式,即修正均匀布源法则,处方剂量为144 Gy,以Mick枪将粒子推入前列腺内。
参考美国近距离治疗协会 (American Brachytherapy Society,ABS)推荐[12],低危前列腺癌患者仅行单纯近距离治疗;对中危患者选择近距离治疗联合6个月内分泌治疗,并根据术后PSA水平变化,决定是否追加外放射治疗;而对高危患者,则在近距离治疗基础上联合6个月以上内分泌治疗,并根据术后PSA水平变化,决定是否联合外放射治疗。117例患者(88.6%)接受最大限度雄激素阻断内分泌治疗,包括黄体生成素释放激素类似物及雄激素受体拮抗剂。25例患者 (18.9%)除接受内分泌治疗外,还接受外放射治疗,照射剂量为45~50 Gy/5周,每日照射剂量为1.8~2.0 Gy,每周5次。
随访方法
术后患者前3个月每月复查PSA,之后每3个月复查1次,2年后每6个月复查1次,5年后每12个月复查1次,以观察记录PSA变化规律,反映治疗疗效。对随访过程中出现PSA升高、骨痛等症状的患者复查胸腹盆CT及全身骨扫描,明确是否出现局部或远处转移。
生化复发标准参照 PSA在最低值基础上升高0.40 μg/L或以上[13]。患者出现肿瘤远处转移或进展为去势抵抗性前列腺癌 (castration-resistant prostate cancer,CRPC)即认为治疗失败。随访终点包括生化复发、远处转移或CRPC、死亡,计算患者无生化复发生存率 (biochemical progression-free survival,bPFS)、疾病特异性生存率 (cause-specific survival,CSS)和总体生存率 (overall survival,OS)。
统计学处理
采用SPSS 19.0软件处理数据。正态分布资料结果用均数±标准差表示。分别使用Kaplan-Meier法及Log-rank检验绘制整体样本及各亚组的生存曲线并进行比较;使用卡方检验比较高危组与低、中危组的bPFS、CSS及OS差异;使用Cox回归分析评估影响bPFS、CSS和OS的风险因素。以P<0.05为差异有统计学意义。
结果
一般临床资料
132例患者平均年龄为 (73.0±6.2)岁 (48~84岁),治疗前PSA为 (20.23±13.55)μg/L(0.36~56.40 μg/L),前列腺体积为 (31.3±12.6)ml(12.5~71.7 ml),穿刺病理Gleason评分为 (7.02±1.20)分(4~9分),穿刺针数阳性比例为 (57±29.5)% (9.1% ~100%),D90为 (135.4±14.4)Gy(88~162 Gy)。患者疾病严重程度、合并症及治疗等临床资料见表1。

表1 132例前列腺癌患者临床资料
随访情况
132例患者平均随访时间 (79.3±23.1)个月 (9~114个月)。至随访结束,20例患者死亡,其中12例死于前列腺癌,另8例分别因直肠癌、胃癌、胆管细胞癌、胰腺癌、肺癌、心肌梗死、肺部感染及慢性肾功能衰竭死亡。
预后及生存分析结果
132例患者bPFS为83.3%,CSS为91.7%,OS为84.8%(图1)。局部高危患者和局部低、中危患者间 bPFS及 OS相比较差异无统计学意义 (P= 0.433,0.098),而局部低、中危患者CSS明显高于局部高危患者 (P=0.037) (表2)。按Gleason评分分组,Gleason≤6、7及≥8三组的 bPFS分别为82.6%、75.6%、92.7% (P=0.084);按临床分期分组,T1~T2a、T2b及T2c~T3三组的bPFS分别为89.3%、82.6%、81.5% (P=0.537);按术前PSA水平分组,PSA<10 μg/L、10~19.9 μg/L及≥20 μg/L三组的bPFS分别为85.7%、81.8%、83.7% (P=0.850)(图2)。
预后及生存影响因素
患者前列腺穿刺阳性比例 (P=0.001)、T分期(P=0.010)、近距离治疗是否联合内分泌治疗 (P= 0.029)及内分泌治疗的时间 (P=0.009)影响患者bPFS。而患者 CSS仅与前列腺穿刺阳性比例 (P= 0.021)相关。患者OS与手术时年龄 (P=0.032)、前列腺穿刺阳性比例 (P=0.013)、是否联合内分泌治疗 (P=0.003)及内分泌治疗的时间 (P=0.043)有显著相关性 (表3)。
讨论
2004年,Stock等[14]首次报道对于局部高危前列腺癌,近距离治疗联合外放射治疗及内分泌治疗在控制PSA水平、提高bPFS方面效果良好。此后,越来越多的研究显示,前列腺癌近距离治疗联合外放射治疗,或联合内分泌治疗,10~15年的 bPFS可达61% ~90%[15-21]。与既往高危前列腺癌采用单纯前列腺外放射治疗或前列腺癌根治性切除的研究相比,在控制PSA水平及延长无生化复发生存上更显优势[22-29]。Bittner等[30]统计131例前列腺癌近距离治疗联合外放射治疗或内分泌治疗患者资料,随访9年的bPFS、CSS及OS分别为91.0%、86.5%、87.3%;随访12年的bPFS、CSS及OS分别为87.3%、70.5%、60.5%。
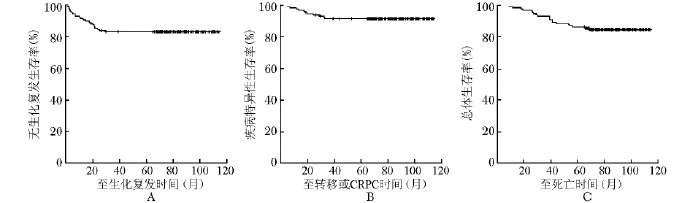
图1 132例前列腺癌患者的无生化复发生存率 (A)、疾病特异性生存率 (B)和总体生存率 (C) CRPC:去势抵抗性前列腺癌
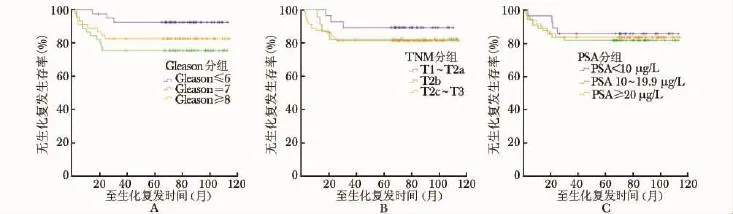
图2 按Gleason评分 (A)、TNM分期 (B)及手术前PSA水平 (C)分组患者的无生化复发生存率PSA:同表1

表2 局部低、中危组与局部高危前列腺癌患者预后及生存比较
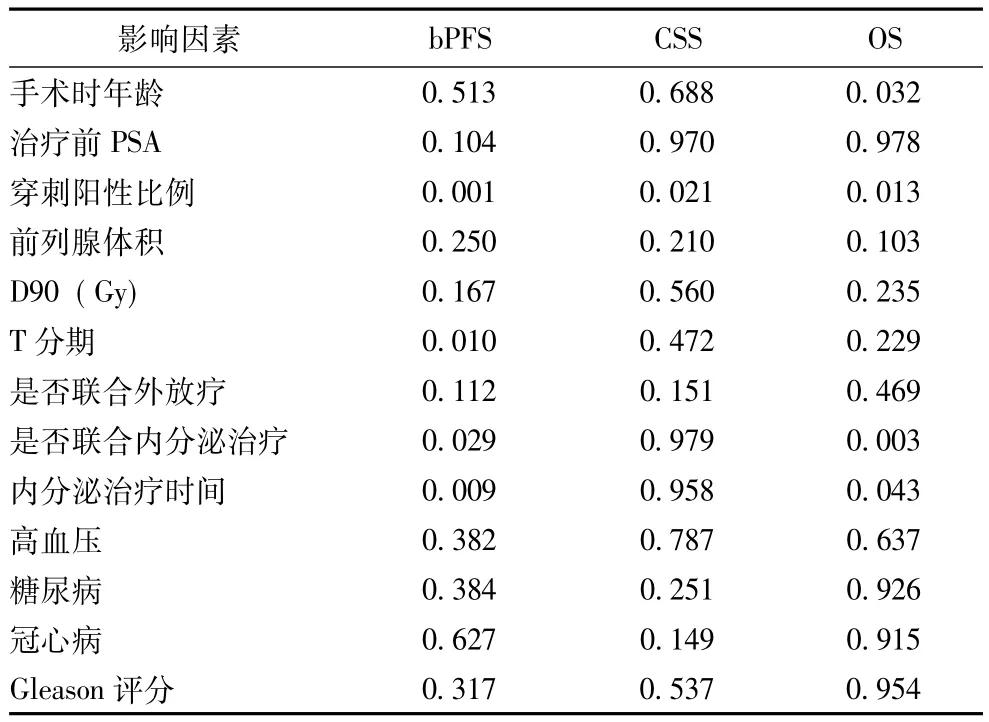
表3 前列腺癌预后及生存相关因素分析P值
但各研究中高危患者比例的差异及前列腺癌恶性程度的不同,也可能导致治疗结果有较大差别。在RTOG 94-13研究中,患者平均术前PSA水平为22 μg/L,Gleason评分≥7分者比例及TNM分期为T2c~T3者比例均为70%[31-32],说明该研究患者肿瘤恶性程度普遍较高。而大部分局部高危前列腺癌近距离治疗联合外放射治疗或内分泌治疗的研究中,患者肿瘤恶性程度偏低,这也可能导致此类研究治疗效果优于既往单纯外放射治疗或根治切除治疗。
本研究132例患者,以ABS推荐[12]为原则,对低危前列腺癌患者行单纯近距离治疗,对中、高危前列腺癌患者行近距离治疗联合内分泌治疗和外放射治疗。具体治疗方案的选择综合考虑了多方面因素,包括患者对内分泌治疗的耐受性及不良反应,对外放射治疗后下尿路症状的耐受程度,以及患者的经济状况。最终132例患者bPFS为83.3%,CSS为91.7%,OS为84.8%。治疗效果也优于单纯外放射治疗或前列腺癌根治性切除术[22-29]。本研究患者平均术前PSA水平为20.23 μg/L,T2c~T3患者比例为61.4%,均低于RTOG 94-13研究;而Gleason评分≥7分者比例为68.9%,与RTOG 94-13研究持平。说明本研究患者的肿瘤恶性程度也较RTOG 94-13研究低,可能是导致治疗结果较好的原因之一。
本研究显示高危组患者与低、中危组患者在bPFS上差异无统计学意义。而高危组前列腺癌患者在出现生化复发以后,进展为CRPC或出现远处转移的可能性较低、中危组患者大。但两组的OS亦无明显差别。这说明局部高危前列腺癌患者选择近距离治疗联合外放射治疗或联合内分泌治疗,可取得良好的效果。而对患者按Gleason评分、TNM分期和术前PSA水平分别分组,各组间的bPFS差异均无统计学意义。该结果进一步说明,存在前列腺癌高危因素任意一项,选择近距离治疗联合外放射治疗或内分泌治疗,疗效与低、中危患者相当。
综上,近距离治疗联合外放射治疗及内分泌治疗,对于局部高危前列腺癌患者,可有效控制PSA水平及延缓生化复发。其bPFS、CSS与OS与单纯外放射治疗及前列腺癌根治性切除相比,效果相当甚至更佳。并且在bPFS与OS方面,疗效与低、中危前列腺癌患者无明显差别。
[1]National Comprehensive Cancer Network.NCCN Clinical Practice Guidelines in Oncology[S/OL].[2011-03-07].http:// www.nccn.org/professionals/physician_gls/about.asp.
[2]Sylvester J,Grimm P,Blasko J,et al.15-Year biochemical relapse free survival in clinical T1-T3 prostate cancer following combined external beam radio therapy and brachytherapy;Seattle experience[J].Int J Radiat Oncol Biol Phys,2007,67: 57-64.
[3]Taira A,Merrick G,Butler W,et al.Long-term outcome for clinically localized prostate cancer treated with permanent interstitial brachytherapy[J].Int J Radiat Oncol Biol Phys,2011,79:1336-1342.
[4]Critz F,Levinson K.10-Year disease-free survival rates after simultaneous irradiation for prostate cancer with a focus on calculation methodology[J].J Urol,2004,172:2232-2238.
[5]Potters L,Morgenstern C,Calugaru E,et al.12-Year outcomes following permanent prostate brachytherapy in patients with clinically localized prostate cancer[J].J Urol,2005,173:1562-1566.
[6]Dattoli M,Wallner K,True L,et al.Long-term outcomes after treatment with brachytherapy and supplemental conformal radiation for prostate cancer patients having intermediate and highrisk features[J].Cancer,2007,110:551-555.
[7]Stock RG,Cesaretti JA,Hall SJ,et al.Outcomes for patients with high-grade prostate cancer treated with a combination of brachytherapy,external beam radio therapy and hormonal therapy[J].BJU Int,2009,104:1631-1636.
[8]Carpenter TJ,Forsythe K,Kao J,et al.Outcomes for patients with extraprostatic prostate cancer treated with trimodality therapy,including brachytherapy,external beam radiotherapy,and hormone therapy[J].Brachytherapy,2011,10:261-268.
[9]Nanda A,Chen MH,Renshaw AA,et al.Gleason pattern 5 prostate cancer:Further stratification of patients with high-risk disease and implications for future randomized trials[J].Int J Radiat Oncol Biol Phys,2009,74:1419-1423.
[10]Sabolch A,Feng FY,Daignault-Newton S,et al.Gleason pattern 5 is the greatest risk factor for clinical failure and death from prostate cancer after dose-escalated radiation therapy and hormonal ablation[J].Int J Radiat Oncol Biol Phys,2011,81:351-360.
[11]Stenmark MH,Blas K,Halverson S,et al.Continued benefit to androgen deprivation therapy for prostate cancer patients treated with dose-escalated radiation therapy across multiple definitions of high-risk disease[J].Int J Radiat Oncol Biol Phys,2011,81:335-344.
[12]Brian D,Eric H,Robert L,et al.American Brachytherapy Society consensus guidelines for transrectal ultrasound-guided permanent prostate brachytherapy[J].Brachytherapy,2012,11:6-19.
[13]Kuban DA,Levy LB,Potters L,et al.Comparison of biochemical failure definitions for permanent prostate brachytherapy[J].Int J Radiat Oncol Biol Phys,2006,65:1487-1493.
[14]Stock RG,Cahlon O,Cesaretti JA,et al.Combined modality treatment in the management of high-risk prostate cancer[J].Int J Radiat Oncol Biol Phys,2004,59:1352-1359.
[15]Sylvester J,Grimm P,Blasko J,et al.15-Year biochemical relapse free survival in clinical T1-T3 prostate cancer following combined external beam radio therapy and brachytherapy;Seattle experience[J].Int J Radiat Oncol Biol Phys,2007,67: 57-64.
[16]Taira A,Merrick G,Butler W,et al.Long-term outcome for clinically localized prostate cancer treated with permanent interstitial brachytherapy[J].Int J Radiat Oncol Biol Phys,2011,79:1336-1342.
[17]Critz F,Levinson K.10-Year disease-free survival rates after simultaneous irradiation for prostate cancer with a focus on cal-culation methodology[J].J Urol,2004,172:2232-2238.
[18]Potters L,Morgenstern C,Calugaru E,et al.12-Year outcomes following permanent prostate brachytherapy in patients with clinically localized prostate cancer[J].J Urol,2005,173:1562-1566.
[19]Dattoli M,Wallner K,True L,et al.Long-term outcomes after treatment with brachytherapy and supplemental conformal radiation for prostate cancer patients having intermediate and highrisk features[J].Cancer,2007,110:551-555.
[20]Stock RG,Cesaretti JA,Hall SJ,et al.Outcomes for patients with high-grade prostate cancer treated with a combination of brachytherapy,external beam radiotherapy and hormonal therapy[J].BJU Int,2009,104:1631-1636.
[21]Carpenter TJ,Forsythe K,Kao J,et al.Outcomes for patients with extraprostatic prostate cancer treated with trimodality therapy,including brachytherapy,external beam radiotherapy,and hormone therapy[J].Brachytherapy,2011,10:261-268.
[22]Bastian P,Gonzalgo M,Aronson W,et al.Clinical and pathologic outcome after prostatectomy for prostate cancer patients with a preoperative Gleason sum of 8 to 10[J].Cancer,2006,107:1265-1272.
[23]Boorjian S,Karnes R,Rangel L,et al.Impact of prostate specific antigen testing on the clinical and pathological outcomes after radical prostatectomy for Gleason 8-10 cancers[J].BJU Int,2008,101:299-304.
[24]Yossepowitch O,Eggener S,Bianco F,et al.Radical prostatectomy for clinically localized,high risk prostate cancer:Critical analysis of risk assessment methods[J].J Urol,2007,178:493-499.
[25]Roehl K,Han M,Ramos C,et al.Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients:Long-term results[J].J Urol,2004,172:910-914.
[26]Kuban D,Tucker S,Dong L,et al.Long-term results of the M.D.Anderson randomized dose-escalation trial for prostate cancer[J].Int J Radiat Oncol Biol Phys,2008,70:67-74.
[27]Zelefsky M,Fuks Z,Hunt M,et al.High dose radiation delivered by intensity modulated conformal radiotherapy improves the outcome of localized prostate cancer[J].J Urol,2001,166:876-881.
[28]Bolla M,Collette L,Blank L,et al.Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer(an EORTC study):A phaseⅢ randomised controlled trial[J].Lancet,2002,360:103-106.
[29]Hanks G,Pajak T,Porter A,et al.PhaseⅢtrial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate:The Radiation Therapy Oncology Group protocol 92-02[J].J Clin Oncol,2003,21:3972-3978.
[30]Bittner N,Merrick G,Butler W,et al.Long-term outcome for very high-risk prostate cancer treated primarily with a triple modality approach to include permanent interstitial brachytherapy[J].Brachytherapy,2012,11:250-255.
[31]Roach M.Editorial comment[J].BJU Int,2010,107: 232-233.
[32]Roach M,De Silvio M,Lawton C,et al.PhaseⅢtrial comparing whole-pelvic versus prostate-only radiotherapy and neoadjuvant versus adjuvant combined androgen suppression:Radiation Therapy Oncology Group 9413[J].J Clin Oncol,2003,21:1904-1911.
Effectiveness of Brachytherapy Combined with External Beam Radiation Therapy and Hormonal Therapy in Treating Localized High-risk Prostate Cancer
CHEN Jian,YAN Wei-gang,LI Han-zhong,JI Zhi-gang,ZHOU Yi,ZHOU Zhi-en,MAI Zhi-peng
Department of Urology,Peking Union Medical College Hospital,Chinese Academy of Medical Sciences&Peking Union Medical College,Beijing 100730,China
YAN Wei-gang Tel:010-69152510,E-mail:ywgemailbox@sina.com
Objective To evaluate the effectiveness of brachytherapy combined with external beam radiation therapy and hormonal therapy in treating localized high-risk prostate cancer patients.Methods We retrospectively analyzed 132 prostate cancer patients treated with brachytherapy from December 2003 to December 2007 in Department of Urology,Peking Union Medical College Hospital,including 97 localized high-risk patients,and 35 localized low-to intermediate-risk patients.Postoperative prostate specific antigen(PSA)level was monitored regularly in follow-up visits.Biochemical relapse,progression to castration-resistant prostate cancer(CRPC)or metastasis,and deaths were documented.Biochemical progression-free survival(bPFS),causespecific survival(CSS),and overall survival(OS)of the patients were evaluated.Results The bPFS,CSS,and OS of the 132 patients were 83.3%,91.7%,and 84.8%,respectively;those indexes of the 97 localized high-risk patients were 81.4%,88.7%,and 81.4%,respectively;and those of the 35 localized low-to inter-mediate-risk patients were 88.6%,100%,and 94.3%,respectively.No significant difference was observed in bPFS and OS between high-risk and low-to intermediate-risk patients(P=0.433,0.098),while CSS was significant higher in low-to intermediate-risk patients than in high-risk patients(P=0.037).After patients were grouped based on Gleason score,tumor-node-metastasis(TNM)clinical stage,or preoperative PSA levels,differences in bPFS among groups were not statistically significant(P=0.084,0.537,0.850).Conclusion Brachytherapy combined with external beam radiation therapy and hormonal therapy may effectively control PSA level and delay biochemical relapse in localized high-risk prostate cancer.
localized high-risk prostate cancer;brachytherapy;biochemical progression-free survival
严维刚 电话:010-69152510,E-mail:ywgemailbox@sina.com
R737.25
A
1674-9081(2016)02-0104-06
10.3969/j.issn.1674-9081.2016.02.005
2014-04-30)
